Abstract
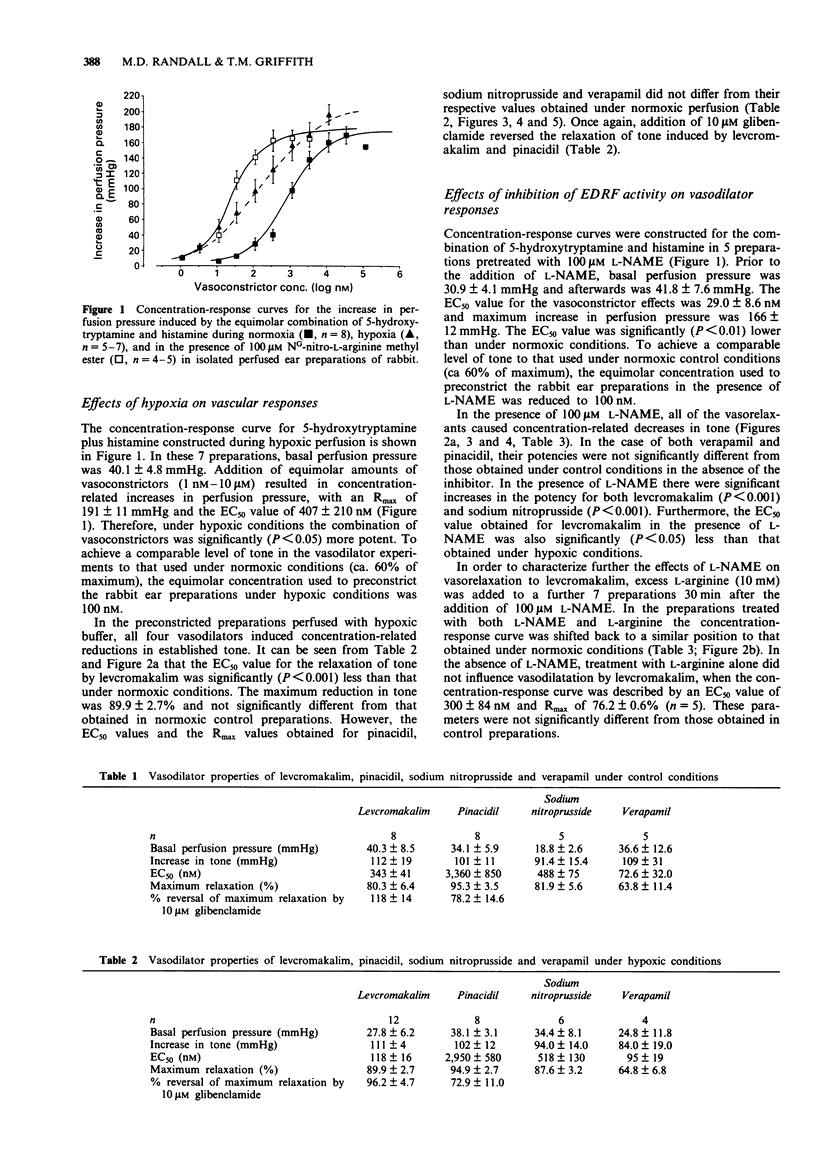
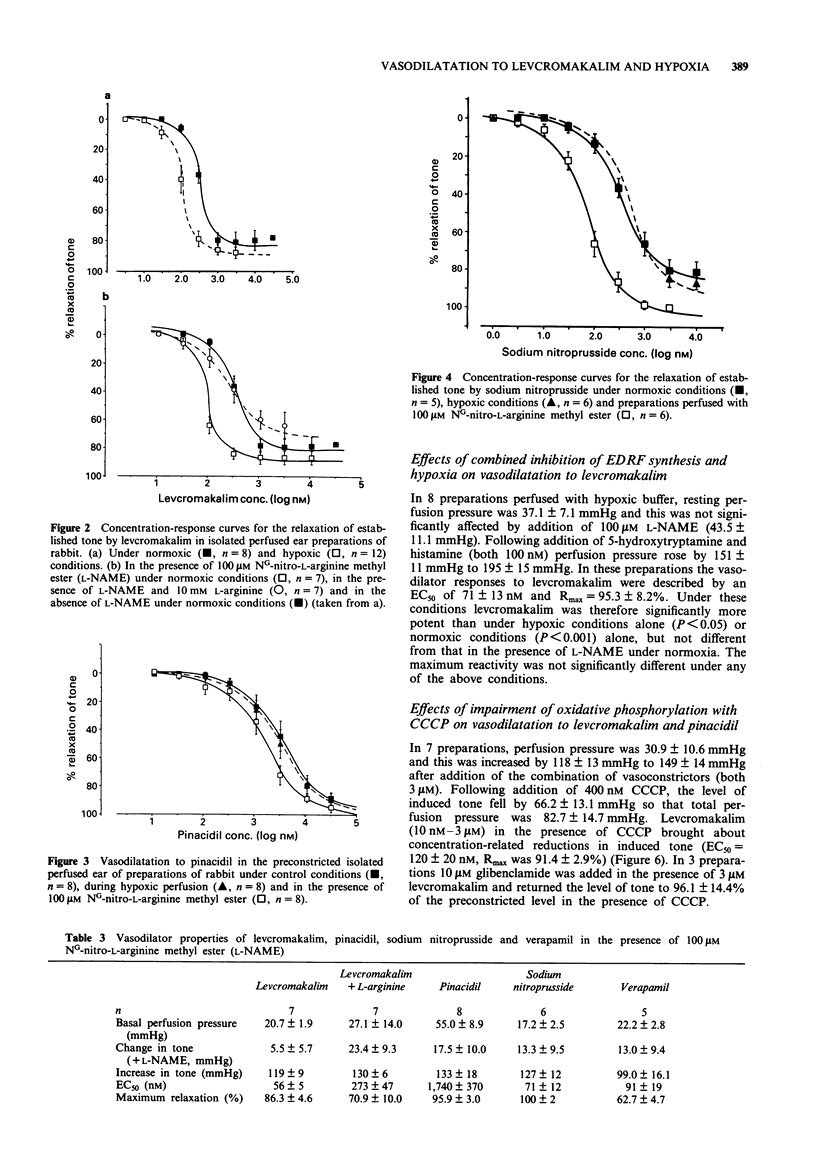
1. We have used an isolated buffer-perfused preparation of the rabbit ear to investigate the effects of hypoxia and inhibition of endothelium-derived relaxing factor (EDRF) synthesis on the vasodilator responses to the potassium channel opener, levcromakalim (the active (-)-enantiomer of cromakalim). The results obtained with levcromakalim have been compared with those for pinacidil, sodium nitroprusside and verapamil. 2. Levcromakalim relaxed preconstricted preparations with an EC50 = 343 +/- 41 nM and Rmax = 80.3 +/- 6.4%. Under hypoxic conditions the concentration-response curve was significantly (P < 0.01) shifted to the left with an EC50 = 118 +/- 16 nM and Rmax = 89.9 +/- 2.7%. Hypoxia did not influence relaxation to either pinacidil, sodium nitroprusside or verapamil. 3. Inhibition of EDRF synthesis with 100 microM NG-nitro-L-arginine methyl ester (L-NAME) also significantly (P < 0.001) increased the vasodilator potency of levcromakalim (EC50 = 56 +/- 5 nM), and caused a similar shift in the concentration-response curve to sodium nitroprusside. It did not influence vasodilation to either verapamil or pinacidil. The potentiation of vasodilator responses to levcromakalim by L-NAME was reversed by an excess of L-arginine. 4. Impairment of oxidative phosphorylation with 400 nM carbonyl cyanide m-chlorophenylhydrazone significantly (P < 0.05) increased the potency of levcromakalim (EC50 = 120 +/- 20 nM) but did not influence vasodilation to pinacidil or endothelium-dependent relaxations to acetylcholine. 5. Vasodilatation to levcromakalim was augmented both by hypoxia and by inhibition of EDRF activity.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

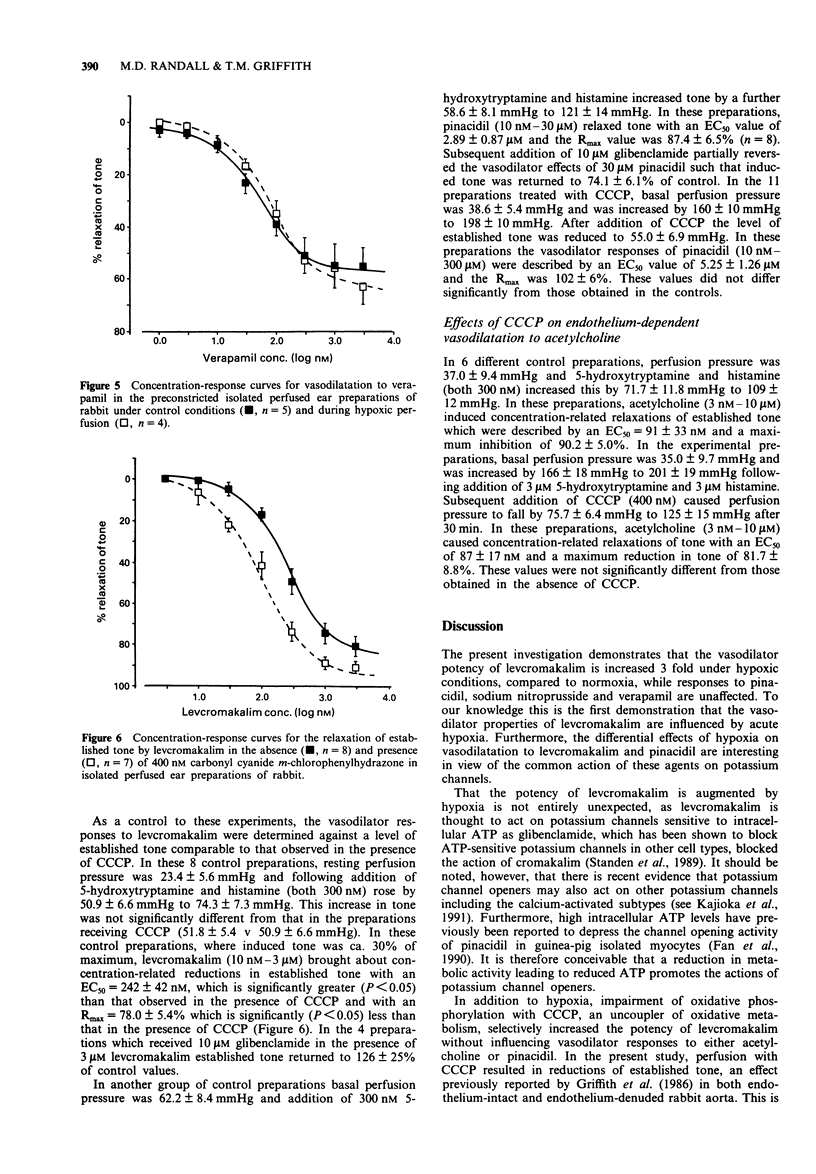



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerbach D., Nicholson C. D. Enhancement of muscle blood cell flux and pO2 by cromakalim (BRL 34915) and other compounds enhancing membrane K+ conductance, but not by Ca2+ antagonists or hydralazine, in an animal model of occlusive arterial disease. Naunyn Schmiedebergs Arch Pharmacol. 1988 Mar;337(3):341–346. doi: 10.1007/BF00168848. [DOI] [PubMed] [Google Scholar]
- Buckingham R. E., Clapham J. C., Hamilton T. C., Longman S. D., Norton J., Poyser R. H. BRL 34915, a novel antihypertensive agent: comparison of effects on blood pressure and other haemodynamic parameters with those of nifedipine in animal models. J Cardiovasc Pharmacol. 1986 Jul-Aug;8(4):798–804. doi: 10.1097/00005344-198709010-00022. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- De Mey J. G., Vanhoutte P. M. Anoxia and endothelium-dependent reactivity of the canine femoral artery. J Physiol. 1983 Feb;335:65–74. doi: 10.1113/jphysiol.1983.sp014519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Potassium channel openers and vascular smooth muscle relaxation. Pharmacol Ther. 1990;48(2):237–258. doi: 10.1016/0163-7258(90)90082-d. [DOI] [PubMed] [Google Scholar]
- Fan Z., Nakayama K., Hiraoka M. Multiple actions of pinacidil on adenosine triphosphate-sensitive potassium channels in guinea-pig ventricular myocytes. J Physiol. 1990 Nov;430:273–295. doi: 10.1113/jphysiol.1990.sp018291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K., Nakaya S., Wakatsuki T., Miyoshi Y., Nakaya Y., Mori H., Inoue I. Effects of nitroglycerin on ATP-induced Ca(++)-mobilization, Ca(++)-activated K channels and contraction of cultured smooth muscle cells of porcine coronary artery. J Pharmacol Exp Ther. 1991 Jan;256(1):371–377. [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Henderson A. H. Unstimulated release of endothelium derived relaxing factor is independent of mitochondrial ATP generation. Cardiovasc Res. 1987 Aug;21(8):565–568. doi: 10.1093/cvr/21.8.565. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Newby A. C., Lewis M. J., Henderson A. H. Production of endothelium derived relaxant factor is dependent on oxidative phosphorylation and extracellular calcium. Cardiovasc Res. 1986 Jan;20(1):7–12. doi: 10.1093/cvr/20.1.7. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns R. A., Linden J. M., Peach M. J. Endothelium-dependent relaxation and cyclic GMP accumulation in rabbit pulmonary artery are selectively impaired by moderate hypoxia. Circ Res. 1989 Dec;65(6):1508–1515. doi: 10.1161/01.res.65.6.1508. [DOI] [PubMed] [Google Scholar]
- Kajioka S., Nakashima M., Kitamura K., Kuriyama H. Mechanisms of vasodilatation induced by potassium-channel activators. Clin Sci (Lond) 1991 Aug;81(2):129–139. doi: 10.1042/cs0810129. [DOI] [PubMed] [Google Scholar]
- Komori K., Lorenz R. R., Vanhoutte P. M. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. Am J Physiol. 1988 Jul;255(1 Pt 2):H207–H212. doi: 10.1152/ajpheart.1988.255.1.H207. [DOI] [PubMed] [Google Scholar]
- Lawson K., Barras M., Zazzi-Sudriez E., Martin D. J., Armstrong J. M., Hicks P. E. Differential effects of endothelin-1 on the vasorelaxant properties of benzopyran and non-benzopyran potassium channel openers. Br J Pharmacol. 1992 Sep;107(1):58–65. doi: 10.1111/j.1476-5381.1992.tb14463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longman S. D., Clapham J. C., Wilson C., Hamilton T. C. Cromakalim, a potassium channel activator: a comparison of its cardiovascular haemodynamic profile and tissue specificity with those of pinacidil and nicorandil. J Cardiovasc Pharmacol. 1988;12(5):535–542. [PubMed] [Google Scholar]
- Lüscher T. F., Vanhoutte P. M. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension. 1986 Apr;8(4):344–348. doi: 10.1161/01.hyp.8.4.344. [DOI] [PubMed] [Google Scholar]
- McPherson G. A., Angus J. A. Characterization of responses to cromakalim and pinacidil in smooth and cardiac muscle by use of selective antagonists. Br J Pharmacol. 1990 Jun;100(2):201–206. doi: 10.1111/j.1476-5381.1990.tb15782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misler S., Falke L. C., Gillis K., McDaniel M. L. A metabolite-regulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7119–7123. doi: 10.1073/pnas.83.18.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Rees D. D., Schulz R., Palmer R. M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991 Dec;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Rakugi H., Tabuchi Y., Nakamaru M., Nagano M., Higashimori K., Mikami H., Ogihara T., Suzuki N. Evidence for endothelin-1 release from resistance vessels of rats in response to hypoxia. Biochem Biophys Res Commun. 1990 Jun 29;169(3):973–977. doi: 10.1016/0006-291x(90)91989-6. [DOI] [PubMed] [Google Scholar]
- Randall M. D., Edwards D. H., Griffith T. M. Activities of endothelin-1 in the vascular network of the rabbit ear: a microangiographic study. Br J Pharmacol. 1990 Dec;101(4):781–788. doi: 10.1111/j.1476-5381.1990.tb14157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. D., Griffith T. M. Differential effects of L-arginine on the inhibition by NG-nitro-L-arginine methyl ester of basal and agonist-stimulated EDRF activity. Br J Pharmacol. 1991 Nov;104(3):743–749. doi: 10.1111/j.1476-5381.1991.tb12498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall M. D., Griffith T. M. Effects of BRL 38227, sodium nitroprusside and verapamil on collateral perfusion following acute arterial occlusion in the rabbit isolated ear. Br J Pharmacol. 1992 Jun;106(2):315–323. doi: 10.1111/j.1476-5381.1992.tb14334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. M., Gibson I. F., Martin W. Effects of hypoxia and metabolic inhibitors on production of prostacyclin and endothelium-derived relaxing factor by pig aortic endothelial cells. Br J Pharmacol. 1991 Jan;102(1):203–209. doi: 10.1111/j.1476-5381.1991.tb12154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki Y., Su C. Endothelium removal augments vasodilation by sodium nitroprusside and sodium nitrite. Eur J Pharmacol. 1985 Aug 7;114(1):93–96. doi: 10.1016/0014-2999(85)90527-8. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Sturgess N. C., Ashford M. L., Cook D. L., Hales C. N. The sulphonylurea receptor may be an ATP-sensitive potassium channel. Lancet. 1985 Aug 31;2(8453):474–475. doi: 10.1016/s0140-6736(85)90403-9. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Southerton J. S., Weston A. H., Baker J. R. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol. 1988 Jul;94(3):853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Maltby N. H., MacCormack D., Barnes P. J. Pulmonary endothelium-derived relaxing factor is impaired in hypoxia. Clin Sci (Lond) 1989 Dec;77(6):671–676. doi: 10.1042/cs0770671. [DOI] [PubMed] [Google Scholar]
- White D. G., Lewis M. J., Griffith T. M., Edwards D. H., Henderson A. H. Influence of endothelium on drug-induced relaxation of the rabbit aorta. Eur J Pharmacol. 1986 Feb 11;121(1):19–23. doi: 10.1016/0014-2999(86)90387-0. [DOI] [PubMed] [Google Scholar]


