Abstract
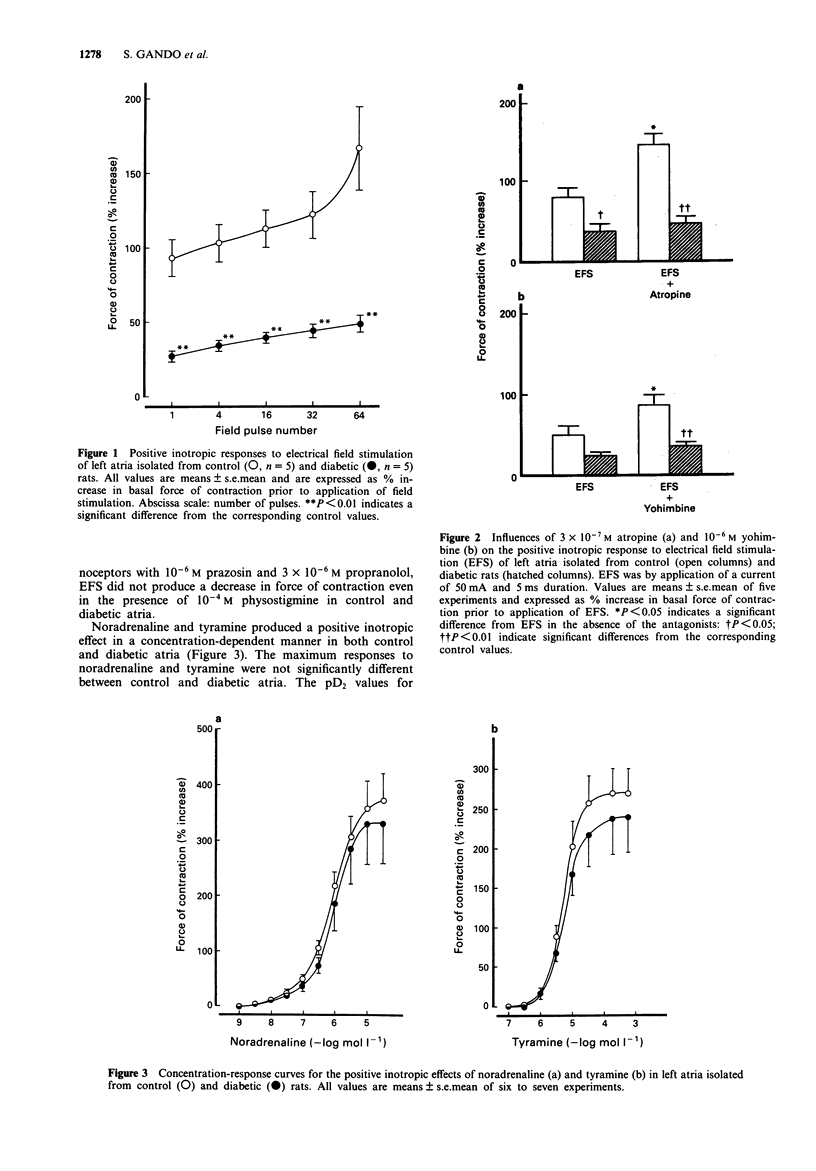
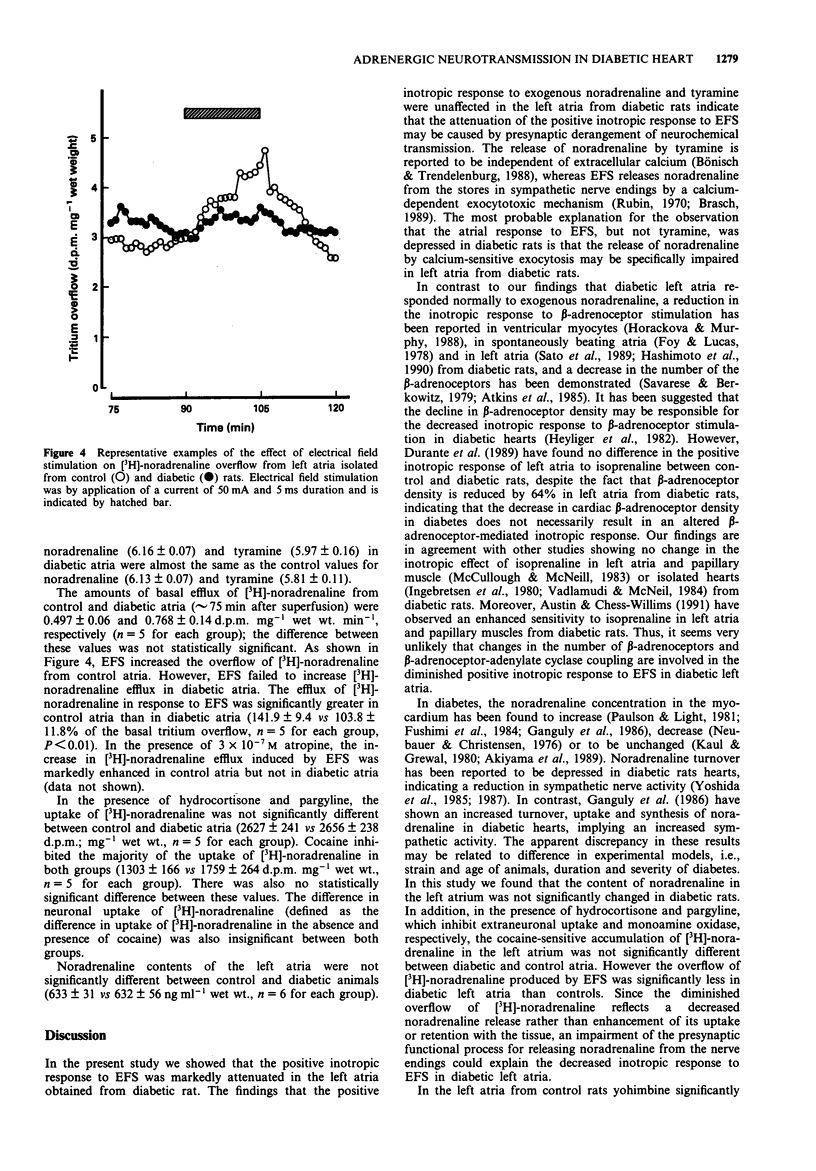
1. Functional alterations of the sympathetic neuroeffector junction of the left atria were studied in rats with streptozotocin-induced diabetes. 2. Eight to 12 weeks of diabetes resulted in a marked decrease in the positive inotropic response of left atria to electrical field stimulation (EFS). 3. The overflow of [3H]-noradrenaline from diabetic left atria caused by EFS was much less than that from control preparations. 4. The concentration-response curves showed no change in sensitivities of the left atria to exogenous noradrenaline and tyramine in diabetic rats. The maximum positive inotropic response to these agents were similar in diabetic and control animals. 5. The left atrial content of noradrenaline was not significantly changed in diabetic rats. The cocaine-sensitive uptake of [3H]-noradrenaline was also unaltered. 6. Atropine enhanced the positive inotropic response and [3H]-noradrenaline overflow induced by EFS in control left atria. Similarly, yohimbine caused an enhancement of EFS-evoked inotropic response in control atria. However, these effects of the antagonists were not observed in diabetic left atria. 7. It is concluded that the decrease in the positive inotropic response of the left atria to EFS in diabetic rats is caused by an impairment of noradrenaline release from the sympathetic nerve terminals through a calcium-dependent exocytotic mechanism. The present results also indicate that presynaptic alpha 2-adrenoceptors and muscarinic receptors that are linked to inhibition of the noradrenaline release during nerve stimulation may be functionally impaired in diabetic animals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama N., Okumura K., Watanabe Y., Hashimoto H., Ito T., Ogawa K., Satake T. Altered acetylcholine and norepinephrine concentrations in diabetic rat hearts. Role of parasympathetic nervous system in diabetic cardiomyopathy. Diabetes. 1989 Feb;38(2):231–236. doi: 10.2337/diab.38.2.231. [DOI] [PubMed] [Google Scholar]
- Atkins F. L., Dowell R. T., Love S. beta-Adrenergic receptors, adenylate cyclase activity, and cardiac dysfunction in the diabetic rat. J Cardiovasc Pharmacol. 1985 Jan-Feb;7(1):66–70. doi: 10.1097/00005344-198501000-00011. [DOI] [PubMed] [Google Scholar]
- Austin C. E., Chess-Williams R. Diabetes-induced changes in cardiac beta-adrenoceptor responsiveness: effects of aldose reductase inhibition with ponalrestat. Br J Pharmacol. 1991 Feb;102(2):478–482. doi: 10.1111/j.1476-5381.1991.tb12197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R. Field stimulation as a means of effecting the graded release of autonomic transmitters in isolated heart muscle. J Pharmacol Exp Ther. 1966 Feb;151(2):221–235. [PubMed] [Google Scholar]
- Brasch H. Pulse duration and alpha 2-adrenoceptors modify noradrenaline release from field-stimulated atria. Eur J Pharmacol. 1989 Nov 14;171(1):49–57. doi: 10.1016/0014-2999(89)90428-7. [DOI] [PubMed] [Google Scholar]
- Cryer P. E., Weiss S. Reduced plasma norepinephrine response to standing in autonomic dysfunction. Arch Neurol. 1976 Apr;33(4):275–277. doi: 10.1001/archneur.1976.00500040059009. [DOI] [PubMed] [Google Scholar]
- Durante W., Sunahara F. A., Sen A. K. Alterations in atrial reactivity in a strain of spontaneously diabetic rats. Br J Pharmacol. 1989 Aug;97(4):1137–1144. doi: 10.1111/j.1476-5381.1989.tb12571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M. E., Nicolaides K. H., Watkins P. J. Autonomic neuropathy and diabetic foot ulceration. Diabet Med. 1986 Jan;3(1):56–59. doi: 10.1111/j.1464-5491.1986.tb00708.x. [DOI] [PubMed] [Google Scholar]
- Foy J. M., Lucas P. D. Comparison between spontaneously beating atria from control and streptozocin-diabetic rats. J Pharm Pharmacol. 1978 Sep;30(9):558–562. doi: 10.1111/j.2042-7158.1978.tb13323.x. [DOI] [PubMed] [Google Scholar]
- Fushimi H., Inoue T., Kishino B., Nishikawa M., Tochino Y., Funakawa S., Yamatodani A., Wada H. Abnormalities in plasma catecholamine response and tissue catecholamine accumulation in streptozotocin diabetic rats: a possible role for diabetic autonomic neuropathy. Life Sci. 1984 Sep 3;35(10):1077–1081. doi: 10.1016/0024-3205(84)90072-9. [DOI] [PubMed] [Google Scholar]
- Ganguly P. K., Dhalla K. S., Innes I. R., Beamish R. E., Dhalla N. S. Altered norepinephrine turnover and metabolism in diabetic cardiomyopathy. Circ Res. 1986 Dec;59(6):684–693. doi: 10.1161/01.res.59.6.684. [DOI] [PubMed] [Google Scholar]
- Goebel F. D., Füessl H. S. Mönckeberg's sclerosis after sympathetic denervation in diabetic and non-diabetic subjects. Diabetologia. 1983 May;24(5):347–350. doi: 10.1007/BF00251822. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Satoh N., Takiguchi Y., Nakashima M. Effects of an aldose reductase inhibitor, ONO-2235, insulin and their combination on the altered responsiveness to the nerve stimulation and agonists of the isolated atria of diabetic rats. J Pharmacol Exp Ther. 1990 May;253(2):552–557. [PubMed] [Google Scholar]
- Hattori Y., Kanno M. Effect of Ni2+ on the multiphasic positive inotropic responses to histamine mediated by H1-receptors in left atria of guinea pigs. Naunyn Schmiedebergs Arch Pharmacol. 1985 Apr;329(2):188–194. doi: 10.1007/BF00501211. [DOI] [PubMed] [Google Scholar]
- Hattori Y., Sakuma I., Nakao Y., Kanno M. Pharmacological analysis of the cardiac actions of xamoterol, a beta adrenoceptor antagonist with partial agonistic activity, in guinea pig heart: evidence for involvement of adenylate cyclase system in its cardiac stimulant actions. J Pharmacol Exp Ther. 1987 Sep;242(3):1077–1085. [PubMed] [Google Scholar]
- Heyliger C. E., Pierce G. N., Singal P. K., Beamish R. E., Dhalla N. S. Cardiac alpha- and beta-adrenergic receptor alterations in diabetic cardiomyopathy. Basic Res Cardiol. 1982 Nov-Dec;77(6):610–618. doi: 10.1007/BF01908314. [DOI] [PubMed] [Google Scholar]
- Hilsted J. Pathophysiology in diabetic autonomic neuropathy: cardiovascular, hormonal, and metabolic studies. Diabetes. 1982 Aug;31(8 Pt 1):730–737. doi: 10.2337/diab.31.8.730. [DOI] [PubMed] [Google Scholar]
- Horackova M., Murphy M. G. Effects of chronic diabetes mellitus on the electrical and contractile activities, 45Ca2+ transport, fatty acid profiles and ultrastructure of isolated rat ventricular myocytes. Pflugers Arch. 1988 May;411(5):564–572. doi: 10.1007/BF00582379. [DOI] [PubMed] [Google Scholar]
- Ingebretsen C. G., Moreau P., Hawelu-Johnson C., Ingebretsen W. R., Jr Performance of diabetic rat hearts: effects of anoxia and increased work. Am J Physiol. 1980 Nov;239(5):H614–H620. doi: 10.1152/ajpheart.1980.239.5.H614. [DOI] [PubMed] [Google Scholar]
- Kaul C. L., Grewal R. S. Increased urinary excretion of catecholamines and their metabolites in streptozotocin diabetic rat. Pharmacology. 1980;21(3):223–228. doi: 10.1159/000137436. [DOI] [PubMed] [Google Scholar]
- Langer S. Z. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980 Dec;32(4):337–362. [PubMed] [Google Scholar]
- McCullough A. L., McNeill J. H. Chronic diabetes decreases the ouabain inotropic response in rat left atria and papillary muscles. Gen Pharmacol. 1983;14(4):401–405. doi: 10.1016/0306-3623(83)90022-8. [DOI] [PubMed] [Google Scholar]
- Moorhouse J. A., Carter S. A., Doupe J. Vascular responses in diabetic peripheral neuropathy. Br Med J. 1966 Apr 9;1(5492):883–888. doi: 10.1136/bmj.1.5492.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer B., Christensen N. J. Norepinephrine, epinephrine, and dopamine contents of the cardiovascular system in long-term diabetics. Diabetes. 1976 Jan;25(1):6–10. doi: 10.2337/diab.25.1.6. [DOI] [PubMed] [Google Scholar]
- Paulson D. J., Light K. E. Elevation of serum and ventricular norepinephrine content in the diabetic rat. Res Commun Chem Pathol Pharmacol. 1981 Sep;33(3):559–562. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SHARPEY-SCHAFER E. P., TAYLOR P. J. Absent circulatory reflexes in diabetic neuritis. Lancet. 1960 Mar 12;1(7124):559–562. doi: 10.1016/s0140-6736(60)92773-2. [DOI] [PubMed] [Google Scholar]
- Sato N., Hashimoto H., Takiguchi Y., Nakashima M. Altered responsiveness to sympathetic nerve stimulation and agonists of isolated left atria of diabetic rats: no evidence for involvement of hypothyroidism. J Pharmacol Exp Ther. 1989 Jan;248(1):367–371. [PubMed] [Google Scholar]
- Savarese J. J., Berkowitz B. A. beta-Adrenergic receptor decrease in diabetic rat hearts. Life Sci. 1979 Dec 10;25(24-25):2075–2078. doi: 10.1016/0024-3205(79)90200-5. [DOI] [PubMed] [Google Scholar]
- Smith C. I., Pierce G. N., Dhalla N. S. Alterations in adenylate cyclase activity due to streptozotocin-induced diabetic cardiomyopathy. Life Sci. 1984 Mar 26;34(13):1223–1230. doi: 10.1016/0024-3205(84)90544-7. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Tomlinson D. R., Yusof A. P. Autonomic neuropathy in the alloxan-diabetic rat. J Auton Pharmacol. 1983 Dec;3(4):257–263. doi: 10.1111/j.1474-8673.1983.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Vadlamudi R. V., McNeill J. H. Effect of experimental diabetes on isolated rat heart responsiveness to isoproterenol. Can J Physiol Pharmacol. 1984 Jan;62(1):124–131. doi: 10.1139/y84-020. [DOI] [PubMed] [Google Scholar]
- Williams P. B. Norepinephrine uptake and efflux from canine peripheral collateral arteries. J Cardiovasc Pharmacol. 1991 Aug;18(2):198–206. doi: 10.1097/00005344-199108000-00004. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nishioka H., Nakamura Y., Kondo M. Reduced noradrenaline turnover in streptozotocin-induced diabetic rats. Diabetologia. 1985 Sep;28(9):692–696. doi: 10.1007/BF00291978. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Nishioka H., Yoshioka K., Nakano K., Kondo M., Terashima H. Effect of aldose reductase inhibitor ONO 2235 on reduced sympathetic nervous system activity and peripheral nerve disorders in STZ-induced diabetic rats. Diabetes. 1987 Jan;36(1):6–13. doi: 10.2337/diab.36.1.6. [DOI] [PubMed] [Google Scholar]
- Yui Y., Itokawa Y., Kawai C. A rapid and highly sensitive method for determination of picogram levels of norepinephrine and epinephrine in tissues by high-performance liquid chromatography. Anal Biochem. 1980 Oct;108(1):11–15. doi: 10.1016/0003-2697(80)90687-9. [DOI] [PubMed] [Google Scholar]


