Abstract
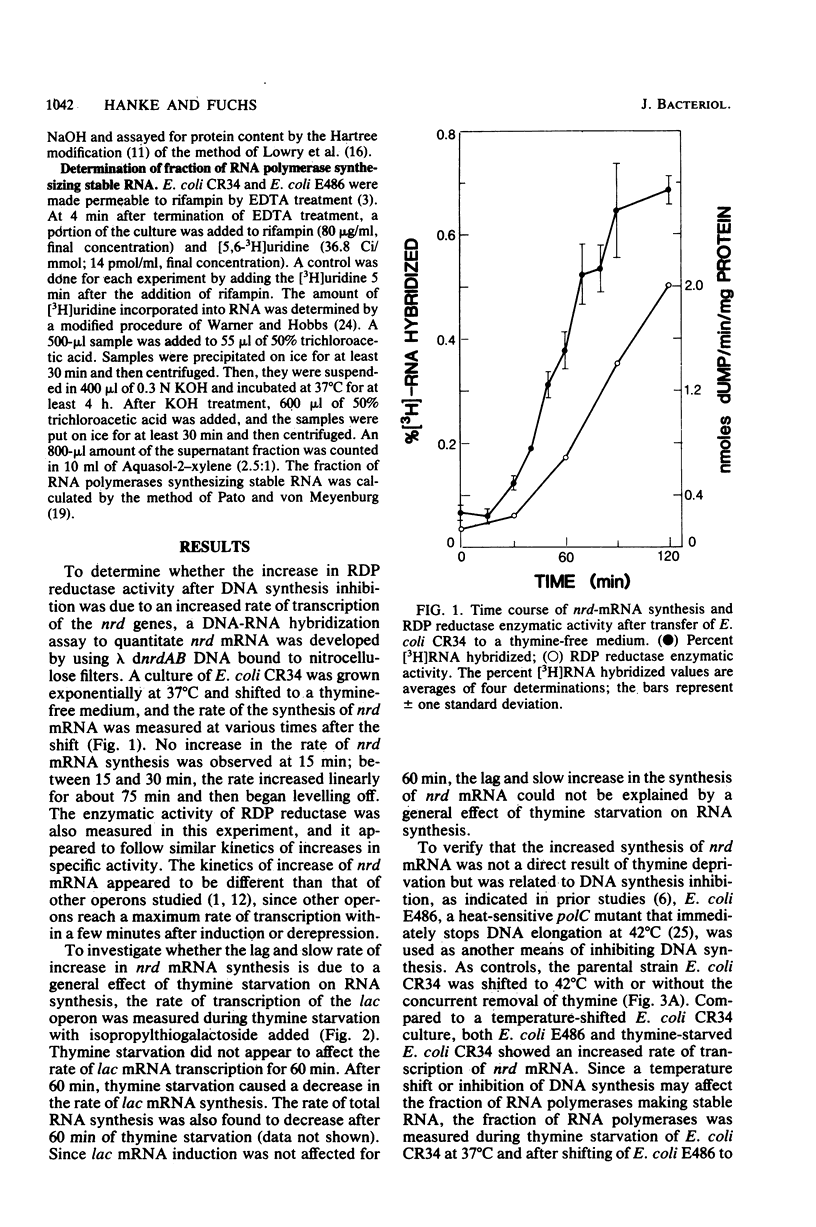
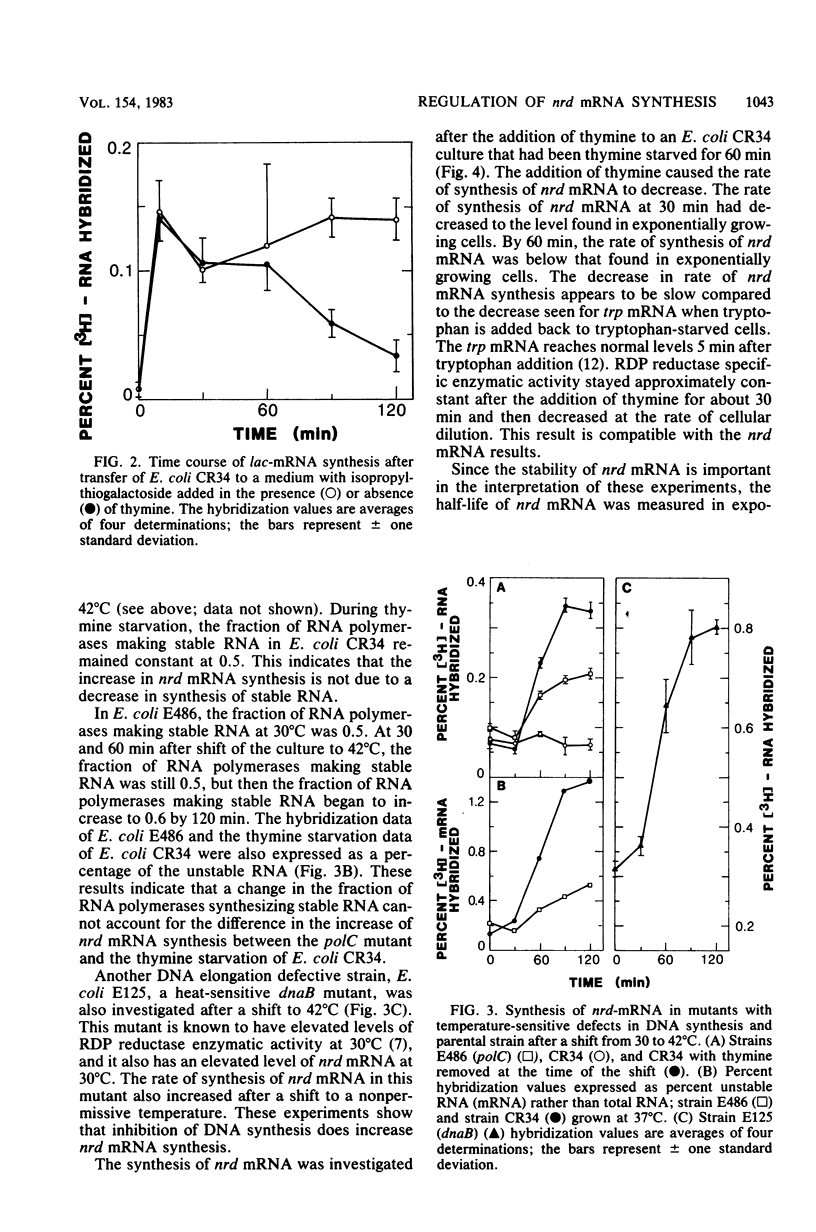
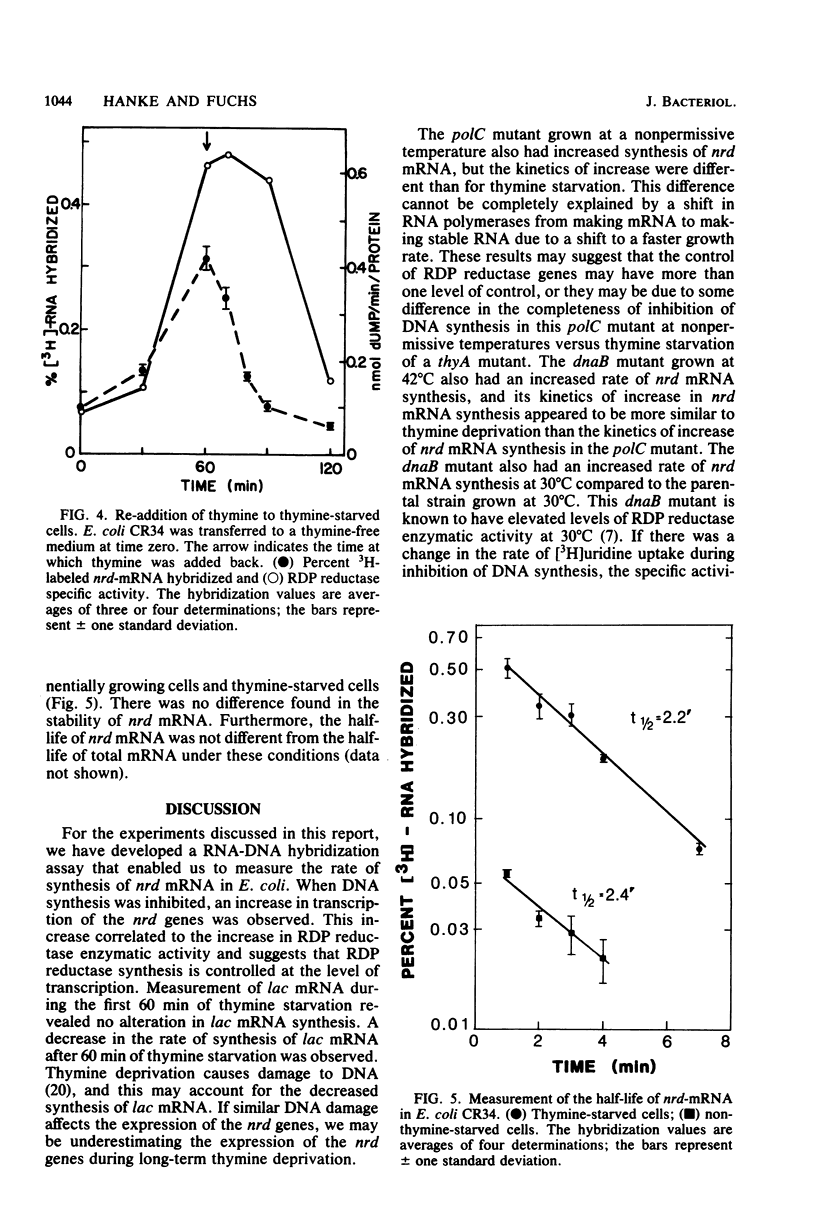
A RNA-DNA hybridization assay for ribonucleoside diphosphate reductase (RDP reductase) mRNA was used to determine whether control of RDP reductase synthesis in Escherichia coli is at the level of RNA transcription. The correlation observed between the level of RDP reductase enzymatic activity and the rate of RDP reductase mRNA synthesis suggested that the control is at the level of RNA transcription. No increase in RDP reductase enzymatic activity or RDP reductase mRNA was observed during the first 15 min after removal of thymine from a thymine-requiring culture. Thereafter, the rate of RDP reductase mRNA synthesis increased linearly for approximately 75 min before beginning to level off. The addition of thymine to a culture starved for thymine resulted in a decreasing rate of RDP reductase mRNA synthesis. However, 30 min of growth in the presence of thymine was required before the rate of RDP reductase mRNA synthesis dropped to the rate observed in an exponentially growing culture. Inhibition of DNA synthesis caused by shifting a culture of a polC mutant or a dnaB mutant to nonpermissive growth conditions resulted in an increase in the rate of RDP reductase mRNA synthesis similar to that observed for a thymine-starved culture.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouvier F., Sicard N. Interference of dna ts mutations of Escherichia coli with thymineless death. J Bacteriol. 1975 Dec;124(3):1198–1204. doi: 10.1128/jb.124.3.1198-1204.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Berry L., Dennis P. P. Regulation of ribonucleic acid synthesis in Escherichia coli B-r: an analysis of a shift-up. II. Fraction of RNA polymerase engaged in the synthesis of stable RNA at different steady-state growth rates. J Mol Biol. 1973 Mar 25;75(1):161–179. doi: 10.1016/0022-2836(73)90536-6. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Eriksson S., Sjöberg B. M., Hahne S. Ribonucleoside diphosphate reductase from Escherichia coli. An immunological assay and a novel purification from an overproducing strain lysogenic for phage lambdadnrd. J Biol Chem. 1977 Sep 10;252(17):6132–6138. [PubMed] [Google Scholar]
- Filpula D., Fuchs J. A. Regulation of ribonucleoside diphosphate reductase synthesis in Escherichia coli: increased enzyme synthesis as a result of inhibition of deoxyribonucleic acid synthesis. J Bacteriol. 1977 Apr;130(1):107–113. doi: 10.1128/jb.130.1.107-113.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filpula D., Fuchs J. A. Regulation of the synthesis of ribonucleoside diphosphate reductase in Escherichia coli: specific activity of the enzyme in relationship to perturbations of DNA replication. J Bacteriol. 1978 Aug;135(2):429–435. doi: 10.1128/jb.135.2.429-435.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A. Coordinate control of the synthesis of ribonucleoside diphosphate reductase components in Escherichia coli. J Bacteriol. 1977 May;130(2):957–959. doi: 10.1128/jb.130.2.957-959.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs J. A., Karlström H. O. Mapping of nrdA and nrdB in Escherichia coli K-12. J Bacteriol. 1976 Dec;128(3):810–814. doi: 10.1128/jb.128.3.810-814.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Morikawa N., Sato K., Mishima S., Nishimura T. On the transcription of the tryptophan operon in Escherichia coli. II. Production of the specific messenger RNA. J Mol Biol. 1965 Aug;13(1):157–168. doi: 10.1016/s0022-2836(65)80086-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsson A., Reichard P. Enzymatic synthesis of deoxyribonucleotides. IX. Allosteric effects in the reduction of pyrimidine ribonucleotides by the ribonucleoside diphosphate reductase system of Escherichia coli. J Biol Chem. 1966 Jun 10;241(11):2533–2539. [PubMed] [Google Scholar]
- Larsson A., Reichard P. Enzymatic synthesis of deoxyribonucleotides. X. Reduction of purine ribonucleotides; allosteric behavior and substrate specificity of the enzyme system from Escherichia coli B. J Biol Chem. 1966 Jun 10;241(11):2540–2549. [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- OKAMOTO K., SUGINO Y., NOMURA M. Synthesis and turnover of phage messenger RNA in E. coli infected with bacteriophage T4 in the presence of chloromycetin. J Mol Biol. 1962 Nov;5:527–534. doi: 10.1016/s0022-2836(62)80126-0. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Oishi M. Early events and mechanisms in the induction of bacterial SOS functions: analysis of the phage repressor inactivation process in vivo. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1657–1661. doi: 10.1073/pnas.75.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs J. D., Hall B. D. Level of tryptophan messenger RNA in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):289–302. doi: 10.1016/0022-2836(68)90268-4. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Warner H. R., Hobbs M. D. Incorporation of uracil-14C into nucleic acids in Escherichia coli infected with bacteriophage T4 and T4 amber mutants. Virology. 1967 Nov;33(3):376–384. doi: 10.1016/0042-6822(67)90113-4. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]



