Abstract
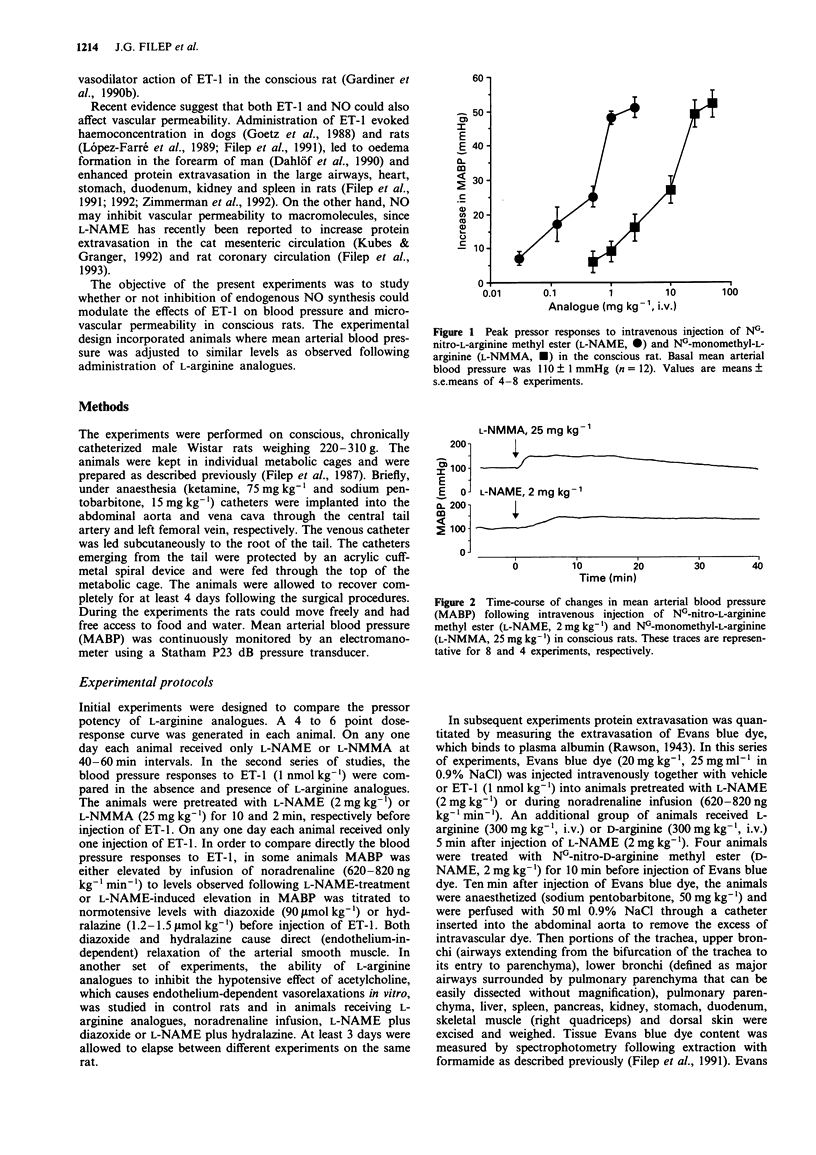
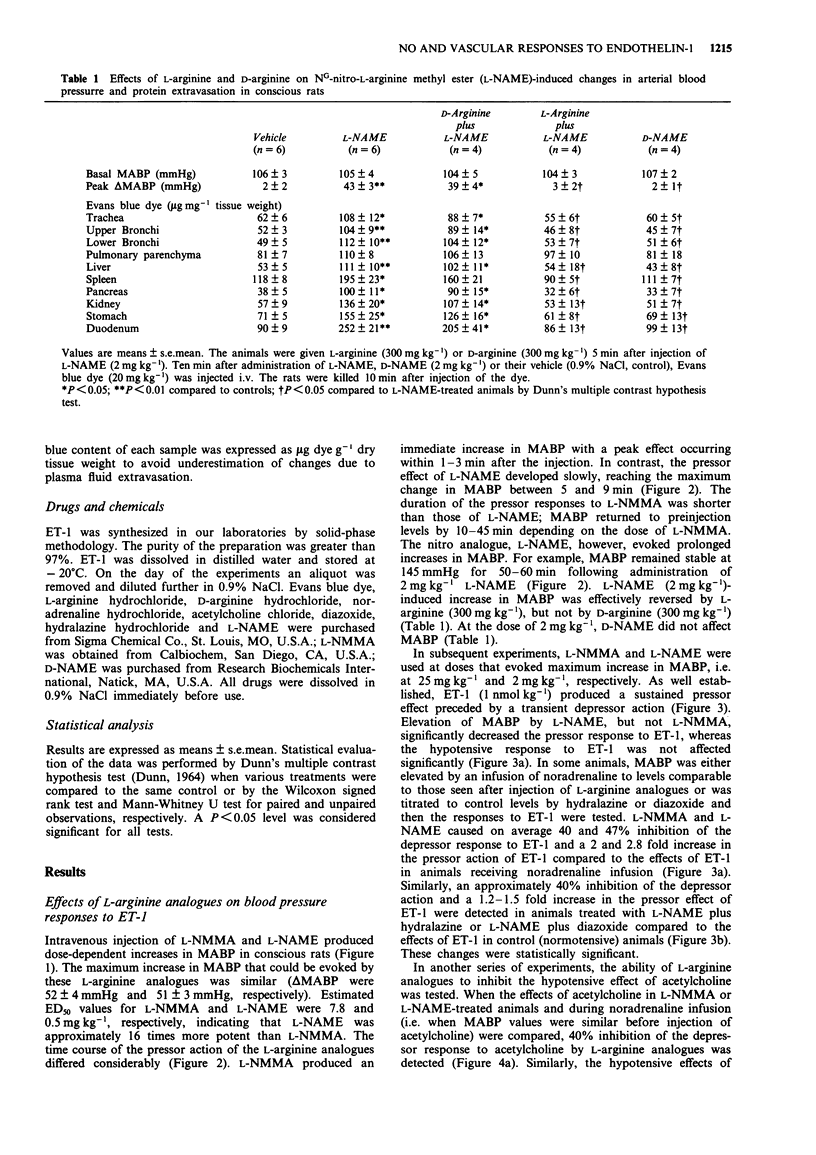
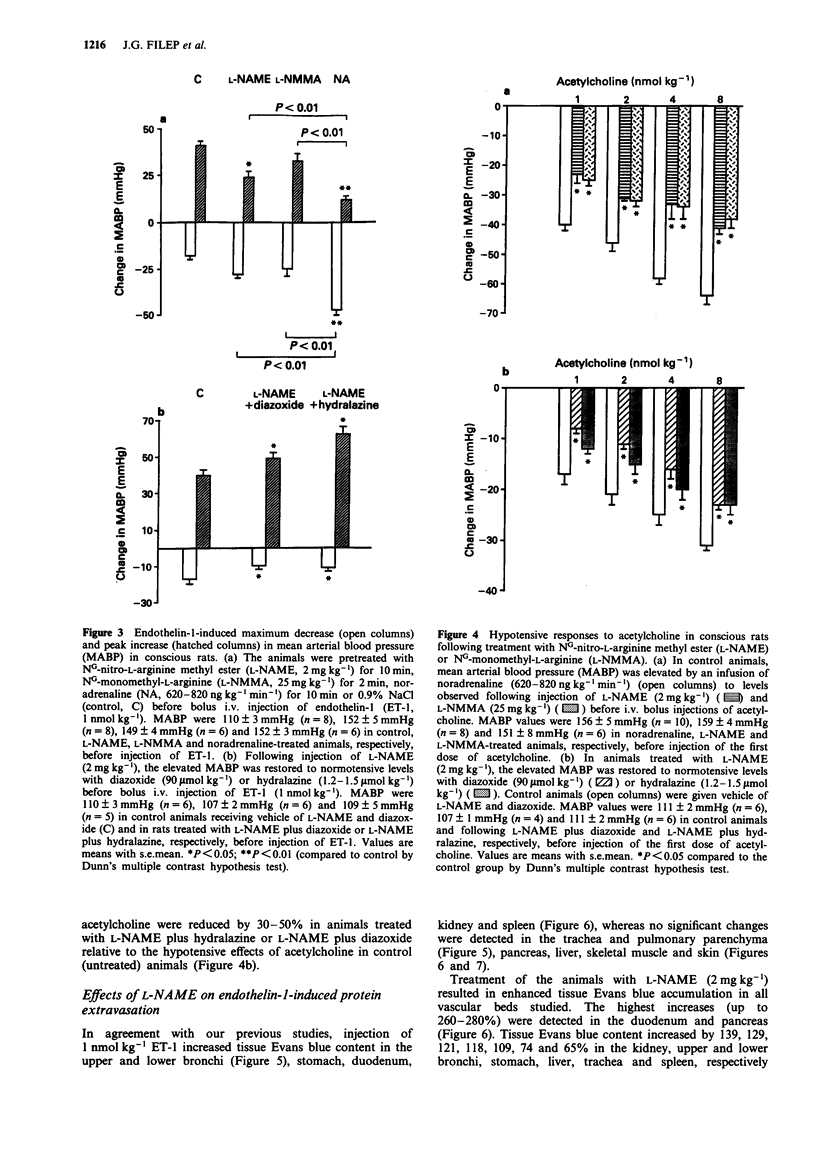
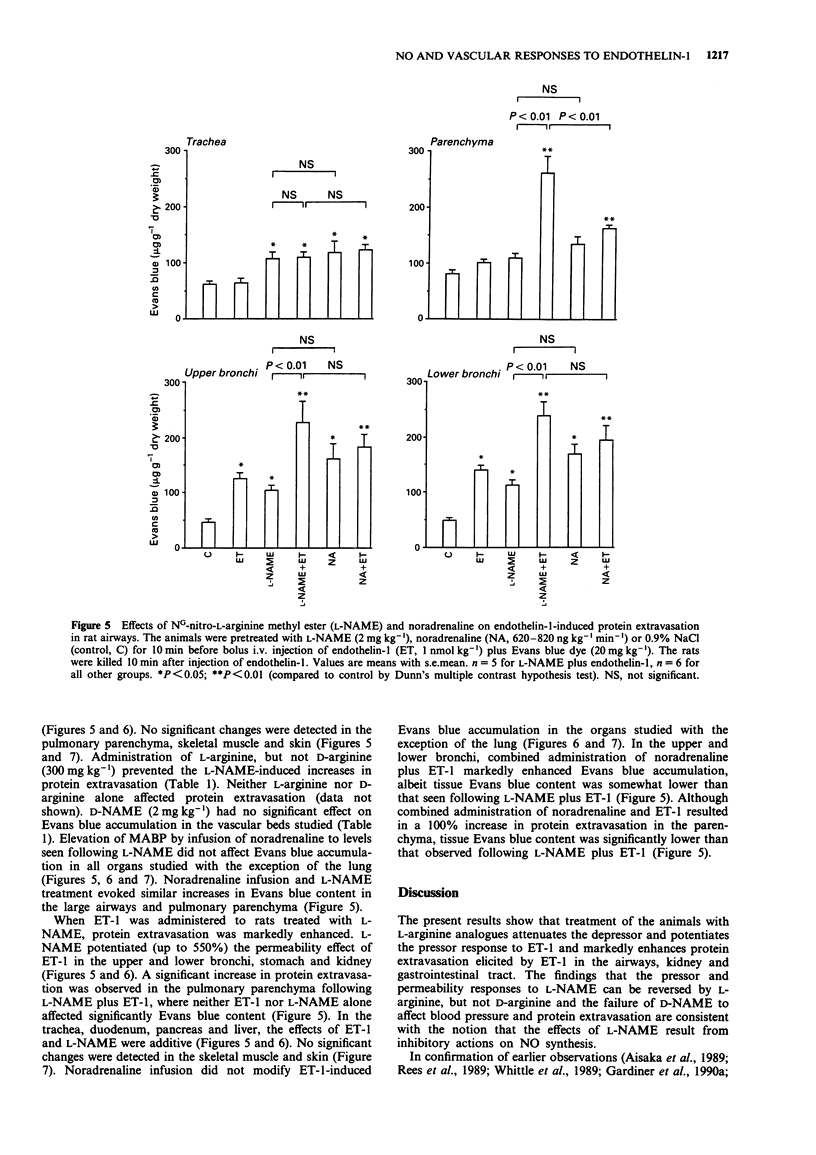
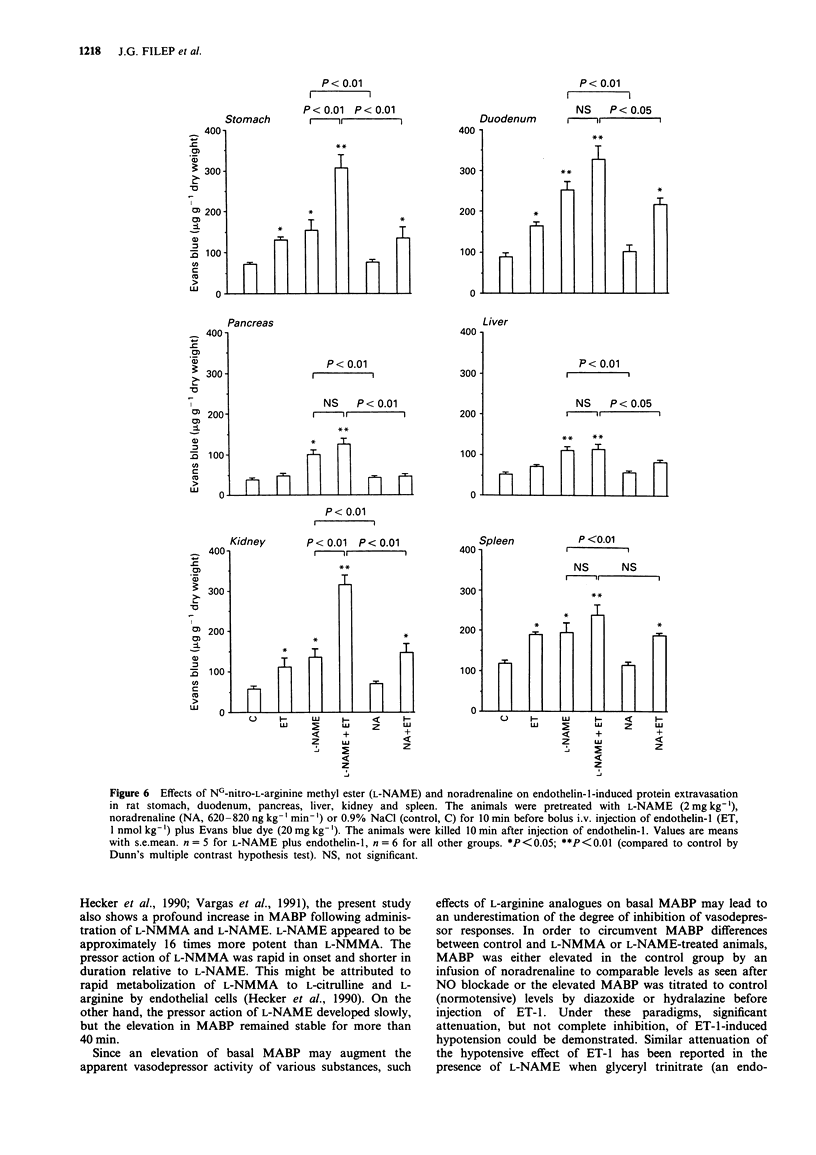
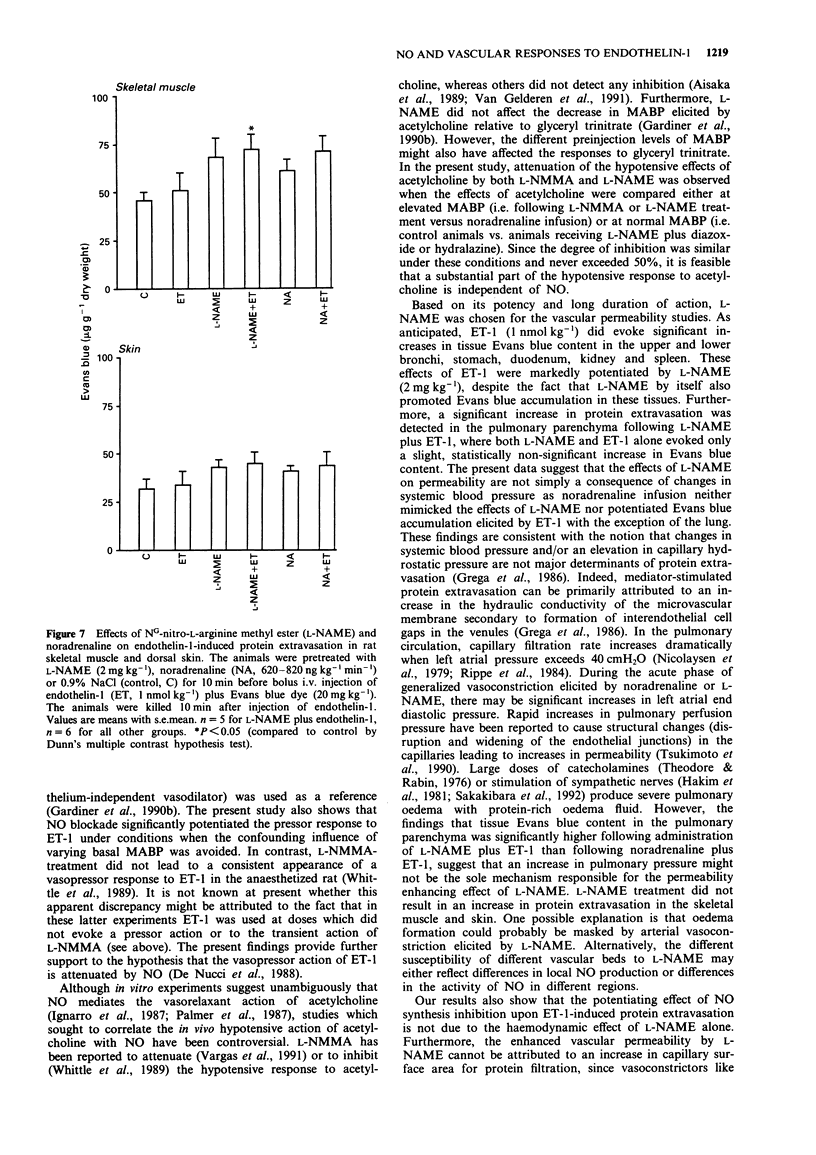
1. The objectives of the present experiments were to assess the role of endogenous nitric oxide (NO) in mediating and/or modulating the effects of endothelin-1 (ET-1) on blood pressure and microvascular permeability in conscious rats. 2. Intravenous administration of the NO synthesis inhibitors, NG-monomethyl-L-arginine (L-NMMA) or NG-nitro-L-arginine methyl ester (L-NAME) at a dose (25 mg kg-1 or 2 mg kg-1, respectively) which evoked maximum increase in mean arterial blood pressure (MABP) significantly attenuated (by about 40%) the vasodepressor response and potentiated (by 100-180%) the pressor response to ET-1 (1 nmol kg-1, i.v.) compared to the effects of ET-1 in animals where the peripheral vasoconstrictor effects of L-arginine analogues were mimicked by an infusion of noradrenaline (620-820 ng kg-1 min-1). Similar inhibition of the depressor and potentiation of the pressor actions of ET-1 were observed when the MABP which had been elevated by L-NMMA or L-NAME was titrated to normotensive levels with hydralazine or diazoxide before injection of ET-1. 3. L-NAME (2 mg kg-1) increased the vascular permeability of the large airways, stomach, duodenum, pancreas, liver, kidney and spleen (up to 280%) as measured by the extravasation of Evans blue dye. The permeability of pulmonary parenchyma, skeletal muscle and skin was not affected significantly by L-NAME treatment. Elevation of MABP by noradrenaline infusion did not evoke protein extravasation in the vascular beds studied with the exception of the lung.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisaka K., Gross S. S., Griffith O. W., Levi R. L-arginine availability determines the duration of acetylcholine-induced systemic vasodilation in vivo. Biochem Biophys Res Commun. 1989 Sep 15;163(2):710–717. doi: 10.1016/0006-291x(89)92281-x. [DOI] [PubMed] [Google Scholar]
- Aoki N., Johnson G., 3rd, Lefer A. M. Beneficial effects of two forms of NO administration in feline splanchnic artery occlusion shock. Am J Physiol. 1990 Feb;258(2 Pt 1):G275–G281. doi: 10.1152/ajpgi.1990.258.2.G275. [DOI] [PubMed] [Google Scholar]
- Boulanger C., Lüscher T. F. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990 Feb;85(2):587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlöf B., Gustafsson D., Hedner T., Jern S., Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J Hypertens. 1990 Sep;8(9):811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- Del Maestro R. F., Björk J., Arfors K. E. Increase in microvascular permeability induced by enzymatically generated free radicals. I. In vivo study. Microvasc Res. 1981 Nov;22(3):239–254. doi: 10.1016/0026-2862(81)90095-9. [DOI] [PubMed] [Google Scholar]
- Filep J. G., Földes-Filep E., Rousseau A., Fournier A., Sirois P., Yano M. Endothelin-1 enhances vascular permeability in the rat heart through the ETA receptor. Eur J Pharmacol. 1992 Aug 25;219(2):343–344. doi: 10.1016/0014-2999(92)90318-x. [DOI] [PubMed] [Google Scholar]
- Filep J. G., Földes-Filep E., Sirois P. Nitric oxide modulates vascular permeability in the rat coronary circulation. Br J Pharmacol. 1993 Feb;108(2):323–326. doi: 10.1111/j.1476-5381.1993.tb12803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filep J. G., Sirois M. G., Rousseau A., Fournier A., Sirois P. Effects of endothelin-1 on vascular permeability in the conscious rat: interactions with platelet-activating factor. Br J Pharmacol. 1991 Dec;104(4):797–804. doi: 10.1111/j.1476-5381.1991.tb12509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filep J., Földes-Filep E., Frölich J. C. Vascular responses to leukotriene B4, C4 and D4 following FPL 55712, indomethacin, saralasin, phentolamine and verapamil in the conscious rat. Br J Pharmacol. 1987 Feb;90(2):431–439. doi: 10.1111/j.1476-5381.1987.tb08973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard J. R., Part M. L. The role of nitric oxide in the regional vasodilator effects of endothelin-1 in the rat. Br J Pharmacol. 1992 Mar;105(3):744–750. doi: 10.1111/j.1476-5381.1992.tb09049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990 May;15(5):486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Bennett T., Palmer R. M., Moncada S. NG-monomethyl-L-arginine does not inhibit the hindquarters vasodilator action of endothelin-1 in conscious rats. Eur J Pharmacol. 1989 Nov 21;171(2-3):237–240. doi: 10.1016/0014-2999(89)90113-1. [DOI] [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz K. L., Wang B. C., Madwed J. B., Zhu J. L., Leadley R. J., Jr Cardiovascular, renal, and endocrine responses to intravenous endothelin in conscious dogs. Am J Physiol. 1988 Dec;255(6 Pt 2):R1064–R1068. doi: 10.1152/ajpregu.1988.255.6.R1064. [DOI] [PubMed] [Google Scholar]
- Grega G. J., Adamski S. W., Dobbins D. E. Physiological and pharmacological evidence for the regulation of permeability. Fed Proc. 1986 Feb;45(2):96–100. [PubMed] [Google Scholar]
- Hakim T. S., Minnear F. L., van der Zee H., Barie P. S., Malik A. B. Adrenoceptor control of lung fluid and protein exchange. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jul;51(1):68–72. doi: 10.1152/jappl.1981.51.1.68. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberthal W., Wolf E. F., Rennke H. G., Valeri C. R., Levinsky N. G. Renal ischemia and reperfusion impair endothelium-dependent vascular relaxation. Am J Physiol. 1989 May;256(5 Pt 2):F894–F900. doi: 10.1152/ajprenal.1989.256.5.F894. [DOI] [PubMed] [Google Scholar]
- López-Farré A., Montañs I., Millás I., López-Novoa J. M. Effect of endothelin on renal function in rats. Eur J Pharmacol. 1989 Apr 12;163(1):187–189. doi: 10.1016/0014-2999(89)90417-2. [DOI] [PubMed] [Google Scholar]
- Marcel van Gelderen E., Heiligers J. P., Saxena P. R. Haemodynamic changes and acetylcholine-induced hypotensive responses after NG-nitro-L-arginine methyl ester in rats and cats. Br J Pharmacol. 1991 Aug;103(4):1899–1904. doi: 10.1111/j.1476-5381.1991.tb12349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaysen G., Waaler B. A., Aarseth P. On the existence of stretchable pores in the exchange vessels of the isolated rabbit lung preparation. Lymphology. 1979 Sep;12(3):201–207. [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe B., Townsley M., Thigpen J., Parker J. C., Korthuis R. J., Taylor A. E. Effects of vascular pressure on the pulmonary microvasculature in isolated dog lungs. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jul;57(1):233–239. doi: 10.1152/jappl.1984.57.1.233. [DOI] [PubMed] [Google Scholar]
- Saijonmaa O., Ristimäki A., Fyhrquist F. Atrial natriuretic peptide, nitroglycerine, and nitroprusside reduce basal and stimulated endothelin production from cultured endothelial cells. Biochem Biophys Res Commun. 1990 Dec 14;173(2):514–520. doi: 10.1016/s0006-291x(05)80064-6. [DOI] [PubMed] [Google Scholar]
- Sakakibara H., Hashiba Y., Taki K., Kawanishi M., Shimada Y., Ishikawa N. Effect of sympathetic nerve stimulation on lung vascular permeability in the rat. Am Rev Respir Dis. 1992 Mar;145(3):685–692. doi: 10.1164/ajrccm/145.3.685. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore J., Robin E. D. Speculations on neurogenic pulmonary edema (NPE). Am Rev Respir Dis. 1976 Apr;113(4):405–411. doi: 10.1164/arrd.1976.113.4.405. [DOI] [PubMed] [Google Scholar]
- Vargas H. M., Cuevas J. M., Ignarro L. J., Chaudhuri G. Comparison of the inhibitory potencies of N(G)-methyl-, N(G)-nitro- and N(G)-amino-L-arginine on EDRF function in the rat: evidence for continuous basal EDRF release. J Pharmacol Exp Ther. 1991 Jun;257(3):1208–1215. [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. S., Martinez A. J., Maymind M., Barbee R. W. Effect of endothelin on plasma volume and albumin escape. Circ Res. 1992 May;70(5):1027–1034. doi: 10.1161/01.res.70.5.1027. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]


