Abstract
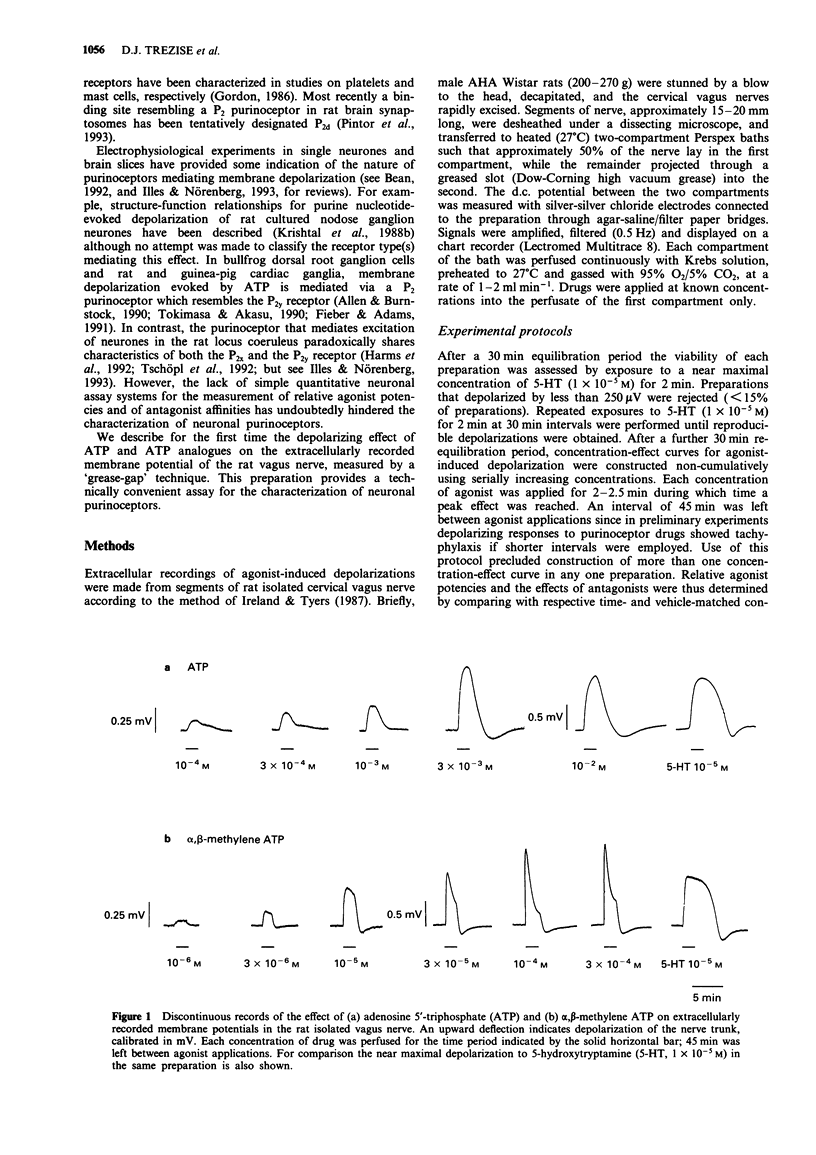
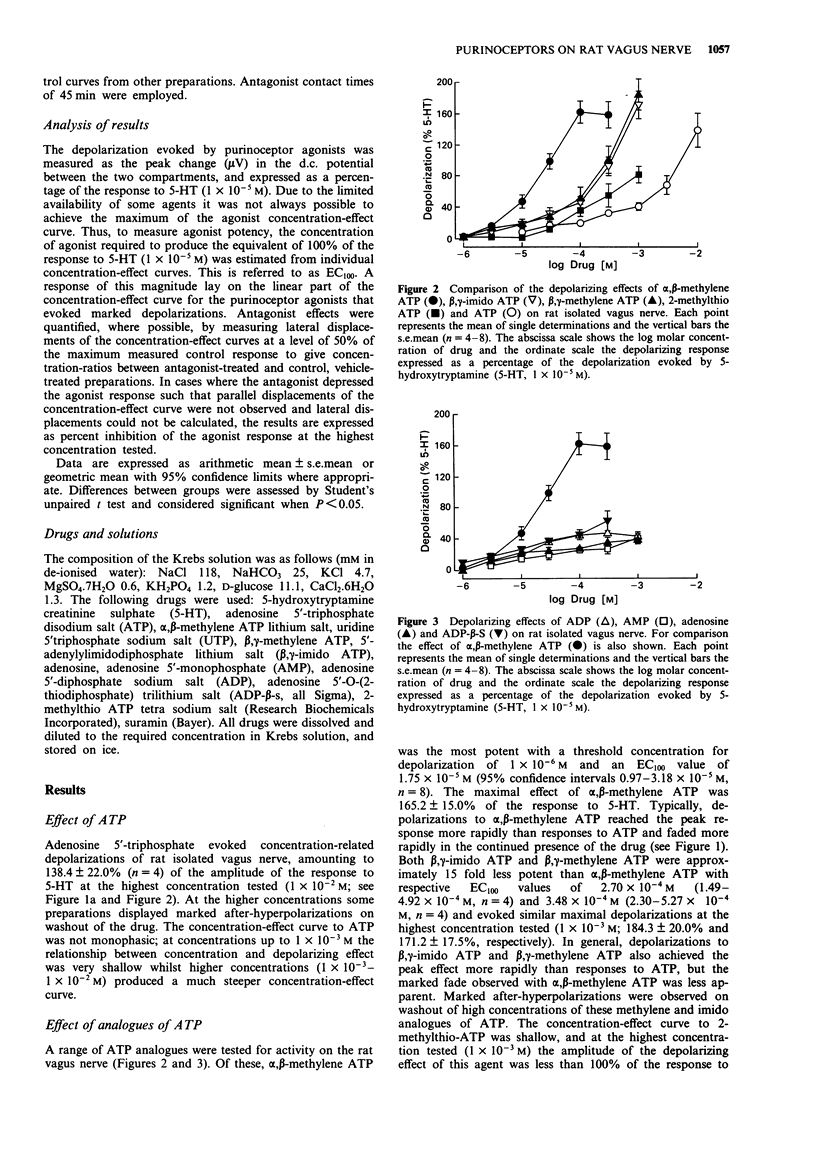
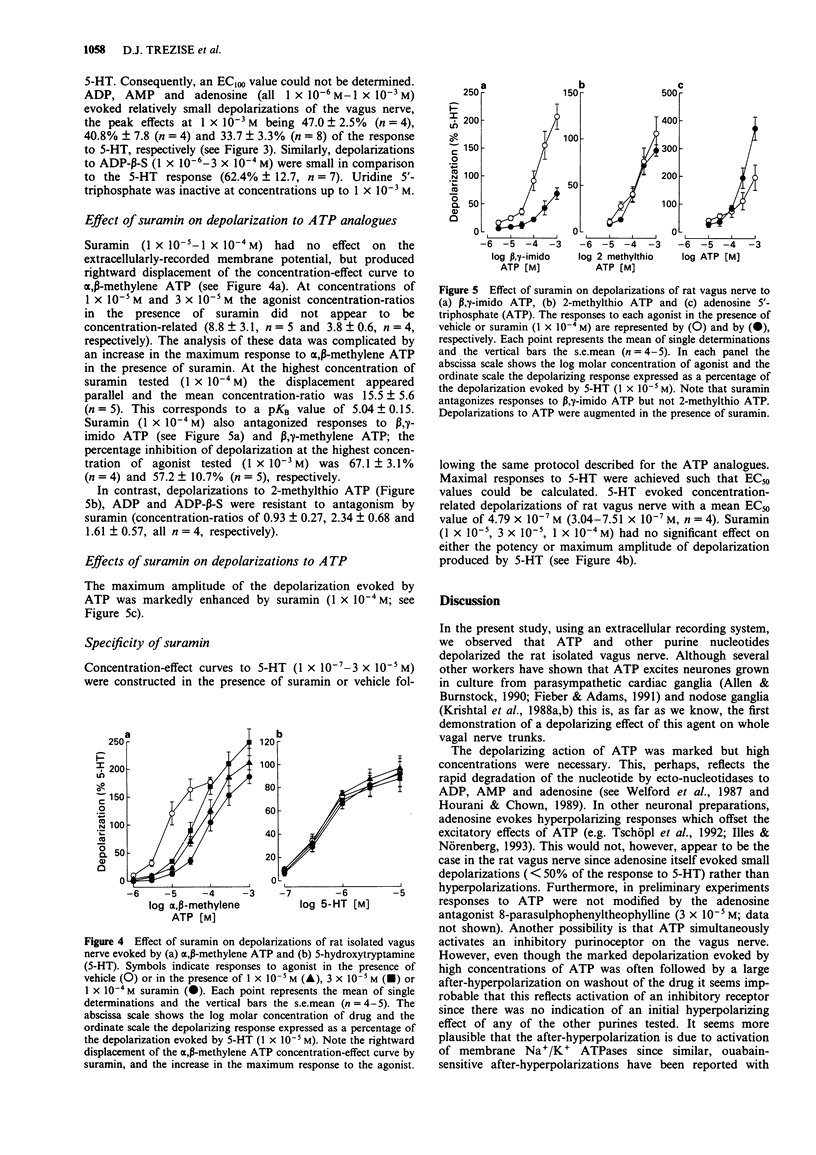
1. As part of a broader study to characterize neuronal purinoceptors, the effects of adenosine 5'-triphosphate (ATP) and a range of ATP analogues were investigated on the extracellularly recorded membrane potential of the rat isolated vagus nerve, using a 'grease-gap' technique. 2. ATP evoked depolarization of the rat vagus nerve. The concentration-effect curve to ATP was not monophasic: at the lower concentrations (1 x 10(-5)-1 x 10(-3) M) the curve was shallow (< 50% of the near maximal response to 5-hydroxytryptamine (5-HT)) whilst at higher concentrations the relationship between concentration and amplitude of depolarization was steeper (> 135% of the response to 5-HT at the highest concentration tested, 1 x 10(-2) M). On washout of the high drug concentrations large after-hyperpolarizations were often observed. 3. alpha,beta-methylene ATP (1 x 10(-6)-3 x 10(-4) M), beta,gamma-methylene ATP (1 x 10(-6)-1 x 10(-3) M), and 5'-adenylylimidodiphosphate (beta,gamma-imido ATP; 1 x 10(-6)-1 x 10(-3) M) were all more potent than ATP and produced large depolarizations of the rat vagus nerve at the highest concentrations tested (> 150% of the response to 5-HT). The overall rank order of potency was alpha,beta-methylene ATP > beta,gamma-methylene ATP = beta,gamma-imido ATP > ATP. 4. In contrast, 2-methylthio ATP (1 x 10(-6)-1 x 10(-3) M) produced relatively small depolarizations (< 100% of the response to 5-HT). As was the case with low concentrations of ATP, the concentration-effect curve to 2-methylthio ATP was very shallow.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. G., Burnstock G. The actions of adenosine 5'-triphosphate on guinea-pig intracardiac neurones in culture. Br J Pharmacol. 1990 Jun;100(2):269–276. doi: 10.1111/j.1476-5381.1990.tb15794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Pharmacology and electrophysiology of ATP-activated ion channels. Trends Pharmacol Sci. 1992 Mar;13(3):87–90. doi: 10.1016/0165-6147(92)90032-2. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. Origin of the after-hyperpolarization that follows removal of depolarizing agents from the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1972 Apr;44(4):651–671. doi: 10.1111/j.1476-5381.1972.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M. Some pharmacological and biochemical interactions of the enantiomers of adenylyl 5'-(beta, gamma-methylene)-diphosphonate with the guinea-pig urinary bladder. Br J Pharmacol. 1984 May;82(1):155–159. doi: 10.1111/j.1476-5381.1984.tb16453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn P. M., Blakeley A. G. Suramin: a reversible P2-purinoceptor antagonist in the mouse vas deferens. Br J Pharmacol. 1988 Feb;93(2):243–245. doi: 10.1111/j.1476-5381.1988.tb11427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. J., Derkach V., Surprenant A. ATP mediates fast synaptic transmission in mammalian neurons. Nature. 1992 Jun 11;357(6378):503–505. doi: 10.1038/357503a0. [DOI] [PubMed] [Google Scholar]
- Fedan J. S., Lamport S. J. P2-purinoceptor antagonists. Ann N Y Acad Sci. 1990;603:182–197. doi: 10.1111/j.1749-6632.1990.tb37672.x. [DOI] [PubMed] [Google Scholar]
- Fieber L. A., Adams D. J. Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991 Mar;434:239–256. doi: 10.1113/jphysiol.1991.sp018467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms L., Finta E. P., Tschöpl M., Illes P. Depolarization of rat locus coeruleus neurons by adenosine 5'-triphosphate. Neuroscience. 1992 Jun;48(4):941–952. doi: 10.1016/0306-4522(92)90282-7. [DOI] [PubMed] [Google Scholar]
- Henning R. H., Nelemans A., Scaf A. H., Van Eekeren J., Agoston S., Den Hertog A. Suramin reverses non-depolarizing neuromuscular blockade in rat diaphragm. Eur J Pharmacol. 1992 May 27;216(1):73–79. doi: 10.1016/0014-2999(92)90211-l. [DOI] [PubMed] [Google Scholar]
- Hourani S. M., Chown J. A. The effects of some possible inhibitors of ectonucleotidases on the breakdown and pharmacological effects of ATP in the guinea-pig urinary bladder. Gen Pharmacol. 1989;20(4):413–416. doi: 10.1016/0306-3623(89)90188-2. [DOI] [PubMed] [Google Scholar]
- Hoyle C. H., Knight G. E., Burnstock G. Suramin antagonizes responses to P2-purinoceptor agonists and purinergic nerve stimulation in the guinea-pig urinary bladder and taenia coli. Br J Pharmacol. 1990 Mar;99(3):617–621. doi: 10.1111/j.1476-5381.1990.tb12979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci. 1993 Feb;14(2):50–54. doi: 10.1016/0165-6147(93)90030-n. [DOI] [PubMed] [Google Scholar]
- Ireland S. J., Tyers M. B. Pharmacological characterization of 5-hydroxytryptamine-induced depolarization of the rat isolated vagus nerve. Br J Pharmacol. 1987 Jan;90(1):229–238. doi: 10.1111/j.1476-5381.1987.tb16844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasakov L., Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur J Pharmacol. 1982 Dec 24;86(2):291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988 Dec;27(3):995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G., Volkova T. M. Receptors for ATP in rat sensory neurones: the structure-function relationship for ligands. Br J Pharmacol. 1988 Dec;95(4):1057–1062. doi: 10.1111/j.1476-5381.1988.tb11739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff P., Wood B. E., O'Connor S. E. Suramin is a slowly-equilibrating but competitive antagonist at P2x-receptors in the rabbit isolated ear artery. Br J Pharmacol. 1990 Nov;101(3):645–649. doi: 10.1111/j.1476-5381.1990.tb14134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S. E., Wood B. E., Leff P. Characterization of P2x-receptors in rabbit isolated ear artery. Br J Pharmacol. 1990 Nov;101(3):640–644. doi: 10.1111/j.1476-5381.1990.tb14133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J., Díaz-Rey M. A., Miras-Portugal M. T. Ap4A and ADP-beta-S binding to P2 purinoceptors present on rat brain synaptic terminals. Br J Pharmacol. 1993 Apr;108(4):1094–1099. doi: 10.1111/j.1476-5381.1993.tb13510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M., Gerzanich V., Vanner S. M. ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol. 1992 Aug;106(4):762–763. doi: 10.1111/j.1476-5381.1992.tb14408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M. K., Wahlestedt C., Reis D. J. Action of externally applied ATP on rat reticulospinal vasomotor neurons. Eur J Pharmacol. 1992 Nov 24;224(1):93–96. doi: 10.1016/0014-2999(92)94824-f. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. ATP regulates muscarine-sensitive potassium current in dissociated bull-frog primary afferent neurones. J Physiol. 1990 Jul;426:241–264. doi: 10.1113/jphysiol.1990.sp018136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschöpl M., Harms L., Nörenberg W., Illes P. Excitatory effects of adenosine 5'-triphosphate on rat locus coeruleus neurones. Eur J Pharmacol. 1992 Mar 17;213(1):71–77. doi: 10.1016/0014-2999(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. The structure-activity relationships of ectonucleotidases and of excitatory P2-purinoceptors: evidence that dephosphorylation of ATP analogues reduces pharmacological potency. Eur J Pharmacol. 1987 Sep 2;141(1):123–130. doi: 10.1016/0014-2999(87)90418-3. [DOI] [PubMed] [Google Scholar]


