Abstract
A class of high-affinity inhibitors is disclosed that selectively target and irreversibly inactivate the epidermal growth factor receptor tyrosine kinase through specific, covalent modification of a cysteine residue present in the ATP binding pocket. A series of experiments employing MS, molecular modeling, site-directed mutagenesis, and 14C-labeling studies in viable cells unequivocally demonstrate that these compounds selectively bind to the catalytic domain of the epidermal growth factor receptor with a 1:1 stoichiometry and alkylate Cys-773. While the compounds are essentially nonreactive in solution, they are subject to rapid nucleophilic attack by this particular amino acid when bound in the ATP pocket. The molecular orientation and positioning of the acrylamide group in these inhibitors in relation to Cys-773 entirely support these results as determined from docking experiments in a homology-built molecular model of the ATP site. Evidence is also presented to indicate that the compounds interact in an analogous fashion with erbB2 but have no activity against the other receptor tyrosine kinases or intracellular tyrosine kinases that were tested in this study. Finally, a direct comparison between 6-acrylamido-4-anilinoquinazoline and an equally potent but reversible analog shows that the irreversible inhibitor has far superior in vivo antitumor activity in a human epidermoid carcinoma xenograft model with no overt toxicity at therapeutically active doses. The activity profile for this compound is prototypical of a generation of tyrosine kinase inhibitors with great promise for therapeutic significance in the treatment of proliferative disease.
Considerable evidence has emerged, both preclinically and clinically, over the last decade to implicate the epidermal growth factor (EGF) receptor (EGFr) and erbB2 in the development, progression, and severity of certain human cancers. More recently, however, it has become clear that these receptors can intensify the transforming signal in a synergistic manner through their ability to form both homo- and heterodimers (1–7). Coexpression of the EGFr and erbB2 to levels where either receptor alone had little effect was highly transforming (8–10). The association between overexpression and/or constitutive activation of members of the type 1 receptor TK family (11) as well as coexpression of their cognate ligands (EGF, the heregulin family, transforming growth factor-α, betacellulin) and transformation has been well established in many primary tumors. In particular, high expression levels of the EGFr and erbB2 have been frequently observed in breast, prostate, ovarian, and various squamous cell carcinomas in which overexpression positively correlates with shortened survival times and increased relapse rates (12–21).
Over the past decade drug discovery efforts have produced a wide variety of chemical structures, generated either by synthetic means or as fermentation products, that reportedly inhibit purified or partially purified preparations of the EGFr tyrosine kinase (TK). The results of this work have been summarized in a number of review articles (22–27). Recent studies, however, with 2′-thioadenosine (28) and N-ethylmaleimide (29, 30) combined with the current advances in high-affinity inhibitors of EGFr (31–34) have revealed an exploitable opportunity to design potent and specific irreversible inhibitors of EGFr TK. This strategy (35, 36) is founded on the critical observation that two prominent cysteines, Cys-751 and Cys-773, are uniquely positioned within the ATP binding pocket of EGFr and erbB2 to provide an immediate direction for a rational synthetic approach. By using a model for binding of the general class of 4-anilinoquinazolines within the ATP pocket (37), we have designed a class of EGFr TK inhibitors that specifically bind with high affinity to EGFr and erbB2 and irreversibly alkylate Cys-773.
MATERIALS AND METHODS
Antibodies.
Anti-phosphotyrosine, EGFr, and erbB2 antibodies were from Upstate Biotechnology (Lake Placid, NY). F4 EGFr antibodies were from Sigma. EGF was obtained from Chiron (Emeryville, CA). Platelet-derived growth factor and basic fibroblast growth factor (FGF) were from Intergen (Purchase, NY). The active fragment of heregulin-β was cloned, expressed, and purified as described earlier (33).
Tissue Culture.
The human breast carcinoma, MDA-MB-453, human epidermoid carcinoma, A431, and human fibroblast (HS-27) were obtained from the American Type Culture Collection (Manassas, VA). All cell lines were maintained as monolayers in DMEM/F12, 50:50 (GIBCO/BRL) containing 10% fetal bovine serum and grown in a humidified atmosphere containing 5% CO2 in air.
Recombinant Proteins, Purification of TKs, and Enzyme Assays.
EGFr cytoplasmic fusion protein was made by cloning the sequence coding for Arg-646 to Ala-1185 into the baculoviral vector pBlueBac4 (Invitrogen), using PCR. Oligonucleotide primers containing an epitope tag (FLAG; IBI) sequence at the N terminus were used to facilitate purification (38). A point mutation changing amino acid 773 from cysteine to serine was introduced by PCR using QuickChange SDM (Stratagene). The EGFr catalytic fusion protein was constructed by cloning the sequence coding for Ser-671 to Phe-973 into the baculoviral vector pETL by using PCR to also add an N-terminal FLAG sequence (38). DNA sequencing was used to confirm the correct sequence of the entire ORF of all constructs. Similar procedures were used to obtain recombinant full-length EGFr.
All tagged proteins were purified with anti-FLAG M2 affinity gel (Eastman Kodak) according to the manufacturer’s procedure. Fibroblast growth factor, platelet-derived growth factor, and insulin receptor TKs were obtained as described (38). Protein kinase C was obtained as a rat brain preparation (Promega). IC50 values were determined as described (33, 39).
Immunoprecipitation and Western Blot Analysis.
Inhibition of receptor autophosphorylation in viable cells and immunoprecipitation of individual proteins were assessed as described (33, 38).
MS.
A solution of PD 168393 or 174265 (in dimethyl sulfoxide) was added to 50 μg of EGFr TK catalytic domain in 20 mM Tris (pH 8.0), 150 mM NaCl, 1 mM DTT, 1 mM EDTA, and 1 μg/ml of the protease inhibitors (aprotinin and leupeptin), and diluted with 75 mM ammonium bicarbonate (pH 7.5). After 90 min, the reaction was quenched by addition of 5% (vol/vol) acetic acid. An aliquot of protein was analyzed by electrospray ionization (ESI)-MS on a Finnigan MAT 900Q mass spectrometer (Finnigan-MAT, San Jose, CA), equipped with a low-flow micro-ESI source operating at 150 nl/min. The remaining protein was reduced, alkylated, and digested with 0.5 μg of trypsin as described (40). Lyophilized tryptic peptides were suspended in 0.1% trifluoroacetic acid/2% CH3CN and analyzed by liquid chromatography-ESI-MS on a Michrom BioResources (Auburn, CA) Magic 2002 HPLC equipped with a 0.3 × 15 mm Vydac C18 column and coupled to a Finnigan LCQ quadruple ion trap mass spectrometer. Data were collected using the Finnigan navigator 1.0.1 data acquisition software set to the following default values for the automatic gain control for full scan MS, zoom scan MS, and MS/MS, respectively: 5 × 107, 1 × 107, and 2 × 107. A maximum injection time of 200 ms and three microscans were used to collect data in all modes. A relative collision energy of 35% was used for MS/MS fragmentation spectra.
RESULTS
Irreversible Inhibition by 6- or 7-Acrylamido-4-anilinoquinazolines.
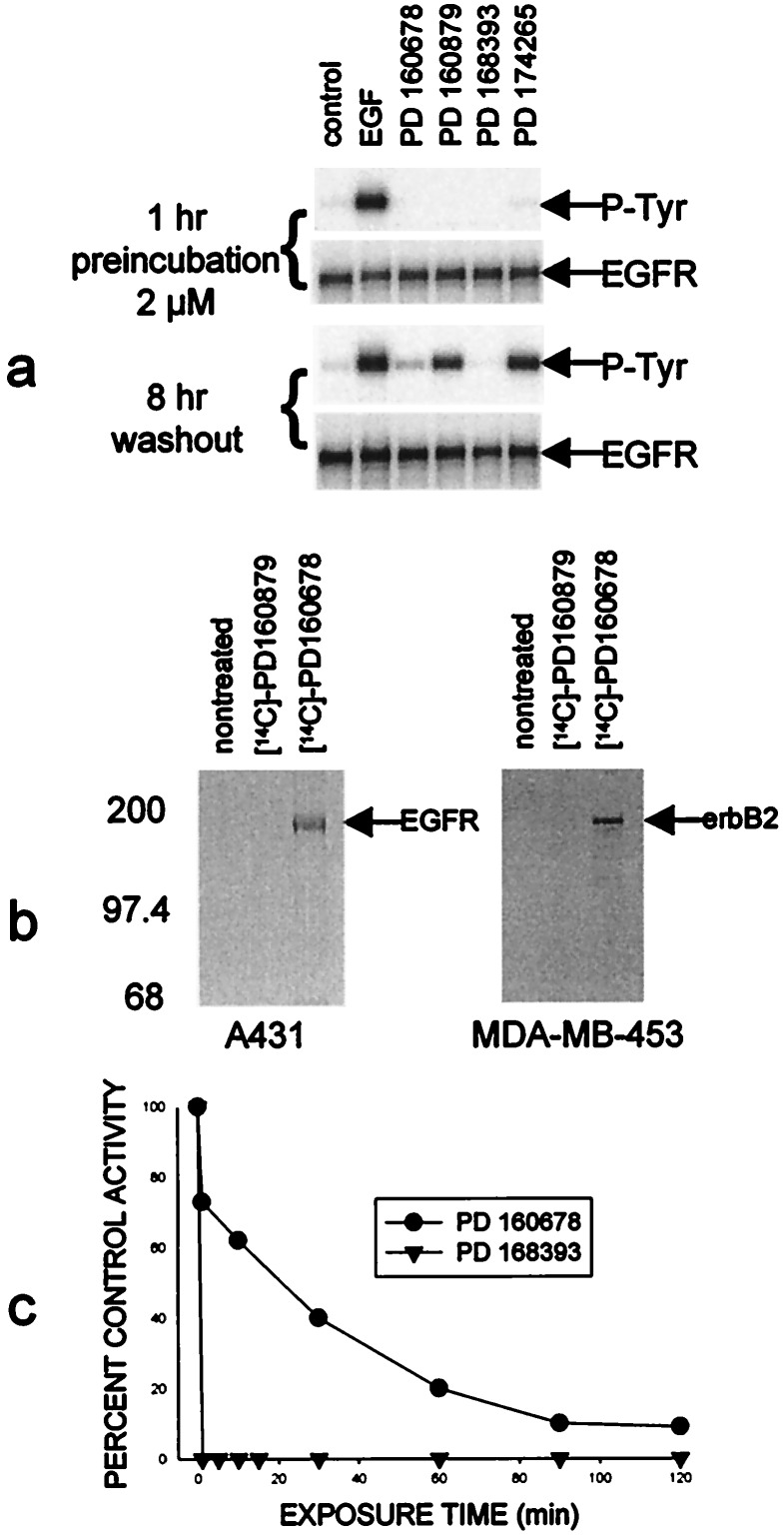
The four compounds used in this study are illustrated in Fig. 1 along with their IC50 values, which indicate that all possess comparable subnanomolar potency against purified EGFr TK activity. As expected, all four compounds completely suppressed EGF-dependent receptor autophosphorylation in A431 cells during continuous exposure (Fig. 2a). Only those cells treated with either PD 160678 or 168393, however, continued to have suppressed kinase activity after 8 hr in compound-free medium, despite the fact that all four compounds have similar IC50 values against the isolated enzyme. The reduction or absence of phosphotyrosine was not due to changes in protein content because receptor expression did not change during the pretreatment or the washout periods (Fig. 2a). More extended time frames showed that 50% of the autophosphorylation activity returns after ≈22 hr (data not shown), which corresponds to the half-life of new receptor synthesis in these cells (41). Evidence that these compounds permanently associate with certain members of the EGFr family was obtained by incubating A431 human epidermoid carcinoma or MDA-MB-453 human breast carcinoma cells with 14C-labeled PD 160879 or 160678. The cells were washed and then EGFr or erbB2 was immunoprecipitated from the cell extracts. Fig. 2b shows that radioactivity was permanently associated with either EGFr in A431 cells or erbB2 in MDA-MB-453 cells preincubated with the irreversible inhibitor PD 160678, but not with the reversible inhibitor PD 160879.
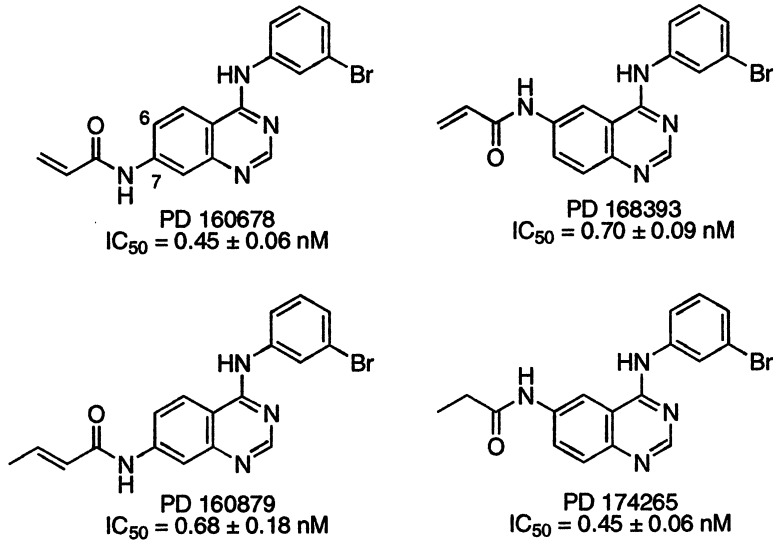
Figure 1.
Chemical structures for PD 160678, 160879, 168393, and 174265. The compounds were synthesized as described (35). The IC50 values represent the concentration of compound necessary to inhibit purified full-length EGFr TK activity by 50% ± SE and represent the average of three separate experiments.
Figure 2.
(a) A431 human epidermoid carcinoma cells were grown in 6-well plates to ≈90% confluence and then incubated in serum-free media for 18 hr. Duplicate sets of cells were treated with 2 μM designated compound for 1 hr. One set of cells was then stimulated with 100 ng/ml EGF for 5 min, and extracts were made as described (33). The other set of cells was washed free of the compound with warmed compound-free medium, incubated for 2 hr, washed again, incubated another 2 hr, washed again, and then incubated a further 4 hr and then stimulated with EGF. (b) 14C-labeled PD 160678 permanently associates with EGFr and erbB2, but not the reversible congener, PD 160879. A431 human epidermoid carcinoma cells or MDA-MB-453 human breast carcinoma cells were exposed to 2 μM 14C-labeled PD 160678 or 160879 for 2 hr and excess compound was washed away. Extracts were made and either the EGFr or erbB2 was immunoprecipitated as described (33). (c) Placement of the acrylamide side chain at the 6 position of the quinazoline allows a much more rapid irreversible interaction than at the 7 position. Cells were pretreated with the designated compound for the times shown, washed as described for a, and assayed for autophosphorylation activity 8 hr later.
The location of the acrylamide was critical not only for irreversibility to occur but also for efficient and rapid interaction with the enzyme. When cells were pretreated with PD 168393 (acrylamide in the 6 position) for various amounts of time, washed to remove compound, and assayed for autophosphorylation activity 8 hr later, 100% of the activity was irreversibly lost in 1 min or less (Fig. 2c). If the acrylamide was in the 7 position as is the case for PD 160678, nearly 2 hr were required to irreversibly inhibit the enzyme activity by >90%.
PD 168393 Specifically Interacts with Cys-773 in EGFr.
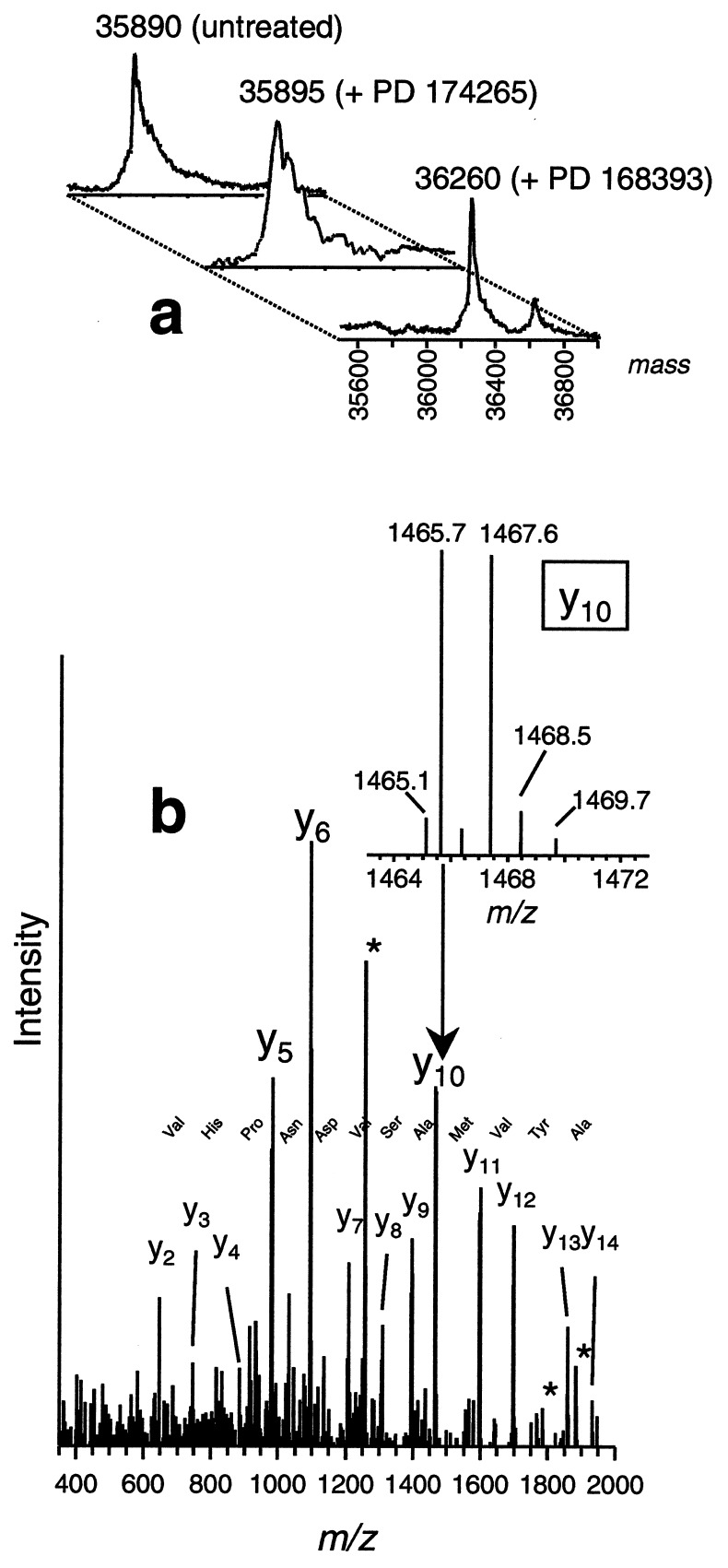
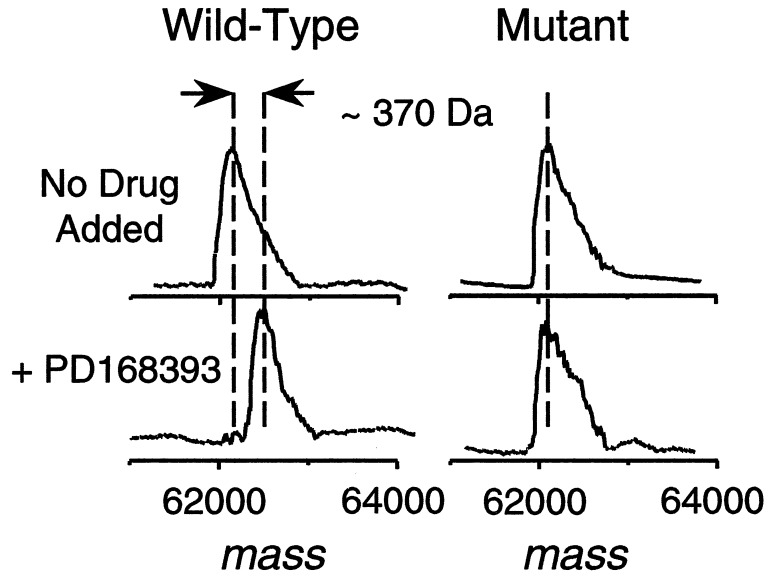
The binding stoichiometry between EGFr TK and PD 168393 and the site of interaction were determined through the use of MS by using an EGFr catalytic domain truncate Ser-671–Phe-973 (Fig. 3a). The molecular mass of the protein-compound complex, as measured by ESI-MS, was ≈370 Da higher than the apoprotein, and this mass difference is consistent with a covalent 1:1 protein–drug complex (Fig. 3a). A small amount (10–20% relative abundance) of 1:2 protein–drug complex was also observed. When the protein was incubated with PD 174265, no increase in mass occurred, indicating the lack of a covalent modification. Trypsin digestion of the drug-bound protein and analyses by liquid chromatography-ESI tandem MS (MS/MS) identified Cys-773 as the predominant site of interaction (Fig. 3b). The only other modified residue detected was Cys-926; however, this represented a relatively minor component. No other residues, including the four remaining cysteines, were found to be altered. As additional evidence that Cys-773 is the specific amino acid that combines with PD 168393, recombinant wild-type full cytoplasmic domain of human EGFr and a point mutant, in which Cys-773 was replaced with Ser (C773S), were expressed in baculovirus and purified from infected insect cells. Both wild-type and mutant protein exhibited comparable TK activity and were inhibited by both PD 168393 and 174265 at similar doses. Both proteins were incubated in the presence or absence of PD 168393 and molecular masses were measured by ESI-MS. As shown in Fig. 4, wild-type protein that was exposed to PD 168393 exhibited an increase in molecular mass of ≈370 Da, the precise molecular mass of the compound, whereas the same experiment utilizing the mutant protein showed no increase in molecular mass. These data provide proof that the nucleophilic sulfur atom of Cys-773 is absolutely required for PD 168393 to add to the EGFr and is consistent with its activity against erbB2, which has an analogous cysteine in its ATP binding pocket.
Figure 3.
MS analysis of PD 168393 binding to EGFr TK or intracellular domain. (a) Deconvoluted ESI mass spectra of EGFr TK protein and protein after addition of PD 174265 or 168393, with the latter showing a mass increase of ≈370 Da, consistent for a 1:1 protein–drug covalent binding stoichiometry. A small amount (≈15% abundance) of a 1:2 protein-drug species (molecular mass 36,626 Da) is also observed in the spectrum. (b) Fragmentation mass spectrum (MS/MS) of the selected doubly charged tryptic peptide at mass-to-charge m/z 1266 produces predominantly C-terminal containing y-ion series ions, using the nomenclature of Biemann (42). Tandem mass spectra were acquired from an on-line liquid chromatography-MS/MS experiment. The continuous y-ion series and the few N-terminally containing b-ions observed (marked with *) are consistent with the tryptic fragment EILDEAYVMASVDNPHVCR, with the drug attached to the cysteine residue. (Inset) Blowup of the isotope peak pattern for Y10 indicating the nearly equal abundant M and M+2 peak pattern, consistent for a Br-containing ion resulting from addition of a Br-containing compound.
Figure 4.
Deconvoluted ESI mass spectra of wild-type or mutant (C773S) EGFr intracellular domain as described in Fig. 3.
A lack of inhibitory activity against other protein kinases by PD 160678 and 168393 indicates that despite their alkylating potential, these inhibitors possess a high specificity toward the EGFr. Both compounds were inactive against insulin, platelet-derived growth factor, and basic FGF receptor TKs as well as protein kinase C at 50 μM, indicating a selectivity ratio of nearly 105-fold (Table 1). This high selectivity also occurred in viable cells, where both compounds inhibited EGF-mediated tyrosine phosphorylation in HS-27 human fibroblasts with IC50 values ranging from 1 to 6 nM but had little effect on FGF- or platelet-derived growth factor-mediated tyrosine phosphorylation at concentrations as high as 50 μM (Table 1).
Table 1.
Effect of irreversible inhibitors on purified protein kinase activities and growth factor-dependent receptor tyrosine phosphorylation in HS-27 human fibroblasts
| Activity affected | PD no. | IC50, nM
|
||||
|---|---|---|---|---|---|---|
| EGFr | Insulin receptor | PDGFr | Basic FGFr | PKC | ||
| Purified protein kinase | 160678 | 0.45 | >50,000 | >50,000 | >50,000 | >50,000 |
| 168393 | 0.70 | >50,000 | >50,000 | >50,000 | >50,000 | |
| Receptor phosphorylation in fibroblasts | 160678 | 6.4 | >50,000 | >50,000 | ||
| 168393 | 1.3 | >50,000 | >50,000 | |||
IC50 values represent the concentration of compound that inhibits the designated activity by 50%. PDGFr, platelet-derived growth factor receptor; PKC, protein kinase C.
Molecular Modeling.
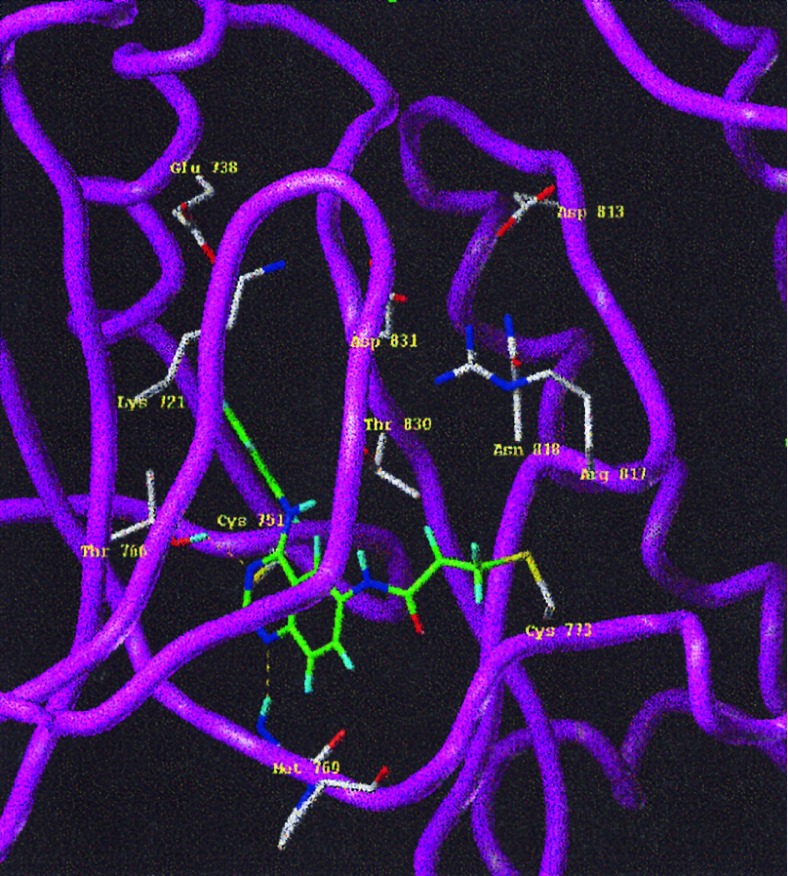
A binding mode for quinazolines, pyrazolo-, and pyrroloquinazolines has been proposed on the basis of the three-dimensional model of EGFr kinase and an extensive structure–activity relationship for this class of inhibitors, details of which have provided elsewhere (37). Employing a previously described molecular model (37), PD 168393 was docked into the ATP binding pocket of EGFr TK. The proposed binding mode brings the γ-carbon atom of the 6-acrylamide into close proximity (no greater than 2.8 Å) to the nucleophilic thiol atom of Cys-773, located on the extended coil stretch that facilitates rapid formation of the addition product shown in Fig. 5. In contrast, the corresponding Cγ–S distance for the 7-acrylamide analog, PD 160879, is greater than 7 Å. This model is consistent with the specific interaction of PD 168393 with Cys-773 and explains why analogs with 6-acrylamide alkylate this amino acid much more rapidly than the those possessing a 7-acrylamide.
Figure 5.
The reaction product of PD 168393 in the EGFr TK ATP pocket. The three-dimensional model was constructed by using the homology modeling module implemented in look (Molecular Applications Group, Palo Alto, CA ) with cAMP kinase as the template. The basis for the three-dimensional model was the sequence alignment recently published (43). PD 168393 was constructed in sybyl 6.2 (Trips E & S, St. Louis) and the charges were derived from the semi-empirical molecular orbital package mopac using the modified neglect of differential overlap (MNDO) approach (44). The inhibitor was docked manually into the ATP binding site and the geometry of the reaction product was derived by using structural information from the Cambridge crystallographic data bank (48). The geometry was then optimized with the Trips force field implemented in sybyl 6.2 to relieve unfavorable steric contacts.
In Vivo Efficacy.
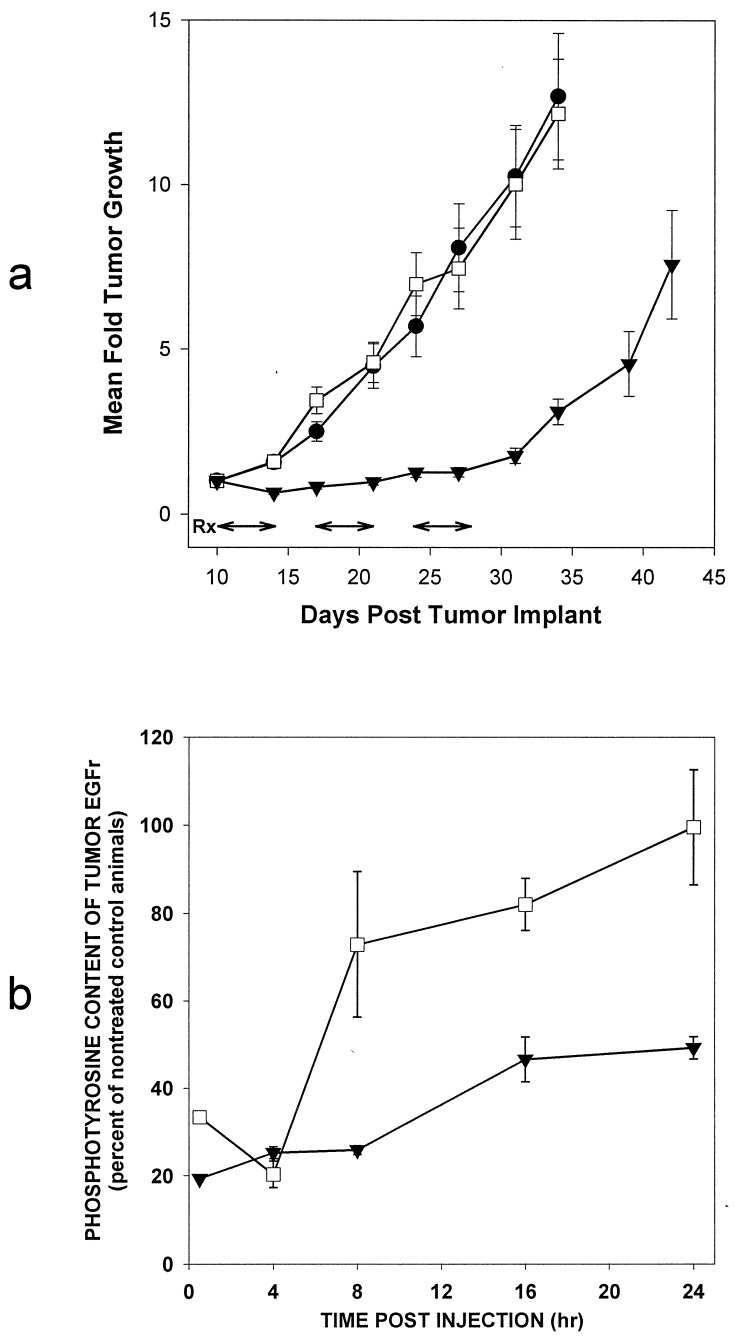
To illustrate the advantage of irreversibility, a direct comparison between PD 168393 (irreversible) and 174265 (reversible) for target modulation in viable cells is shown in Table 2. PD 168393 inhibited EGFr autophosphorylation in A431 human epidermoid carcinoma cells with >9-fold greater potency than PD 174265. An even greater difference was seen against heregulin-mediated tyrosine phosphorylation in MDA-MB-453 human breast carcinoma cells, where PD 168393 was >30-fold more potent. The therapeutic advantage of irreversible inhibition is illustrated quite dramatically in Fig. 6a, which shows a head-to-head comparison of in vivo activity for PD 168393 and 174265 against the A431 human epidermoid carcinoma grown as a xenograft in nude mice. PD 168393 was far superior to PD 174265 in maintaining suppression of tumor growth with once-daily i.p. dosing. PD 168393 produced tumor growth inhibition of 115%, which for this experiment is defined as the median time for treated tumors to reach three volume doublings minus the median time for control tumors to reach three volume doublings, expressed as a percent of treatment duration (15 days). PD 174265, in contrast, produced a tumor growth inhibition of only 13%. The antitumor activity of these two compounds correlated with their ability to suppress the phosphotyrosine content of the EGFr. Both compounds had reduced the phosphorylation status by ≈80%, 4 hr after injection (Fig. 6b). However, by 8 hr, phosphorylation had returned to 75% of controls in mice treated with the reversible compound, PD 174265, and to 100% after 24 hr. In contrast, the phosphotyrosine content of EGFr in animals receiving PD 168393 was still reduced by 50% 24 hr after injection. The therapeutic advantage of PD 168393 was maintained despite a lower plasma concentration than that of PD 174265 at all time points examined (data not shown).
Table 2.
Inhibition of ligand-dependent receptor phosphorylation in viable cells
| Phosphorylation | IC50, nM
|
|
|---|---|---|
| PD 168393 | PD 174265 | |
| Purified EGFr TK | 0.70 ± 0.09 | 0.45 ± 0.06 |
| EGF-induced tyrosine phosphorylation in A431 cells | 4.3 ± 0.6 | 39 ± 12 |
| Heregulin-induced tyrosine phosphorylation in MDA-MB-453 cells | 5.7 ± 0.8 | 220 ± 65 |
IC50 values represent the concentration of compound that inhibits activity by 50% ± SE and are the mean of three separate experiments.
Figure 6.
Comparative antitumor activity of PD 168393 and its reversible congener PD 174265 (a) and corresponding modulation of the EGFr phosphotyrosine content (b). •, Control; □, PD 174265; ▾, PD 168393. (a) Athymic nude mice housed in filtered cages were implanted s.c. with a fragment (≈30 mg) of A431 human epidermoid carcinoma and were randomized into treatment groups when the tumors were palpable. Animal dosing and tumor measurement were carried out essentially as described (45). The compounds were suspended in a vehicle containing 4% dimethylacetamide in aqueous 50 mM sodium lactate buffer (pH 4) and delivered i.p. to the mice at 58 mg/kg on days 10–14, 17–21, and 24–28 (shown with arrows) after tumor implant. Bars = mean ± SE. (b) Nude mice bearing 300- to 500-mg A431 tumors were treated with a single 58 mg/kg i.p. dose of either inhibitor or vehicle as described above. At various times after treatment the tumors were excised and ground to a frozen powder with a mortar and pestle chilled with liquid nitrogen. EGFr was extracted (33) and phosphotyrosine content was determined by ELISA (Calbiochem).
DISCUSSION
The present report describes a class of EGFr and erbB2 TK inhibitor that builds on previous work and extends this field to yet another level. Further to the already demonstrated potency and specificity of the quinazolines, the present series of compounds incorporate a mild Michael acceptor in the quinazoline ring, which allows very specific alkylation of Cys-773 in the ATP pocket of the EGFr. This reaction becomes even more extraordinary when it is realized that these molecules are virtually unreactive with any other nucleophile and are active only when bound to the enzyme. The process of binding into the ATP pocket places the acrylamide in an optimal position to react with the target cysteine, an orientation that is accurately predicted by the current molecular modeling studies.
The ability of the currently described class of molecules to bind irreversibly to both EGFr and erbB2 is a distinguishing property that may give these inhibitors a therapeutic advantage over existing compounds. Although suppression of the EGFr alone has been efficacious (46, 47), most data imply that erbB2 and EGFr are equally involved in the progression of certain tumors and the outcome of the disease. Furthermore, both preclinical and clinical evidence continue to indicate that the highest transforming potential and the most aggressive tumors are those that express both of these receptors. Inactivation of both receptors provides the greatest opportunity for the irreversible compounds to provide meaningful antitumor activity in a larger spectrum of tumors driven by either EGFr or erbB2 or by both.
The irreversible nature of these compounds may offer other potential advantages in terms of target suppression and in vivo pharmacokinetics. Prolonged suppression of the kinase target(s) will likely be necessary for maximum antitumor activity, and an irreversible inhibitor will provide an advantage in this respect by permanently eliminating existing kinase activity, which will return only when new receptor is synthesized. The present compounds would require that plasma concentrations be attained only long enough to briefly expose the receptors to drug, which would irreversibly suppress their kinase activity. Plasma levels could then rapidly decline while kinase activity would remain inactivated. This has the potential advantage of lowering the minimal plasma concentration at which activity occurs, minimizing multiple dosing requirements and eliminating the requirement for long plasma half-lives without compromising efficacy. All of these considerations could reduce toxicity due to any nonspecific interactions that may occur at high or prolonged plasma levels. These pharmacokinetic factors may be contributing in part to the data in Fig. 6, where it is clear that under the dosing schedule given in this experiment, PD 168393 was markedly superior to the reversible compound, PD 174265.
These studies then have demonstrated that the enzyme activity of two relevant cancer chemotherapeutic targets, EGFr and erbB2, can be specifically targeted and irreversibly suppressed through very selective covalent modification of an individual cysteine located within the ATP binding site. Furthermore, the targeting nature of these molecules was possible through exploiting specific high-affinity binding to precisely position the electrophile. In addition, we have shown that the property of irreversibility confers a significant therapeutic advantage over equally potent reversible analogs in terms of in vivo tumor suppression.
Footnotes
Abbreviaitons: EGF, epidermal growth factor; EGFr, EGF receptor; FGF, fibroblast growth factor; ESI, electrospray ionization; TK, tyrosine kinase.
References
- 1. Carraway K L, Cantley L C. Cell. 1994;78:5–8. doi: 10.1016/0092-8674(94)90564-9. [DOI] [PubMed] [Google Scholar]
- 2.Pinkas-Kramarski R, Soussan L, Waterman H, Levkowitz G, Alroy I, Klapper L, Lavi S, Seger R, Ratzkin B J, Sela M, Yarden Y. EMBO J. 1996;15:2452–2467. [PMC free article] [PubMed] [Google Scholar]
- 3.Reese D M, Slamon D J. Stem Cells. 1997;15:1–8. doi: 10.1002/stem.150001. [DOI] [PubMed] [Google Scholar]
- 4.Burke C L, Lemmon M A, Coren B A, Engelman D M, Stern D F. Oncogene. 1997;14:687–696. doi: 10.1038/sj.onc.1200873. [DOI] [PubMed] [Google Scholar]
- 5.Graus-Porta D, Beerli R R, Daly J M, Hynes N E. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzahar E, Pinkas-Kramarski R, Moyer J D, Kapper L N, Alroy I, Levkowitz G, Shelly M, Henis S, Eisenstein M, Ratzkin B J, et al. EMBO J. 1997;16:4938–4950. doi: 10.1093/emboj/16.16.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riese D J, II, Stern D F. BioEssays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 8.Dougall W C, Qian X, Greene M I. J Cell Biochem. 1993;53:61–73. doi: 10.1002/jcb.240530108. [DOI] [PubMed] [Google Scholar]
- 9.Qian X L, Dougall W C, Fei Z Z, Greene M I. Oncogene. 1995;10:211–219. [PubMed] [Google Scholar]
- 10.Muller W J, Arteaga C L, Muthuswamy S K, Siegel P M, Webster M A, Cardiff R D, Meise K S, Li F, Halter S A, Coffey R J. Mol Cell Biol. 1996;16:5726–5736. doi: 10.1128/mcb.16.10.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajkumar T, Gullick J. Breast Cancer Res Treat. 1994;29:3–9. doi: 10.1007/BF00666177. [DOI] [PubMed] [Google Scholar]
- 12.Klijn J G M, Berns P M J J, Schmitz P I M, Foekens J A. Endocr Rev. 1992;13:3–17. doi: 10.1210/edrv-13-1-3. [DOI] [PubMed] [Google Scholar]
- 13.Rajkumar T, Gullick W J. Cancer Breast Cancer Res Treat. 1994;29:3–9. doi: 10.1007/BF00666177. [DOI] [PubMed] [Google Scholar]
- 14.Bucci B, D’Agnano I, Botti C, Mottolese M, Carico E, Zupi G, Vecchione A. Anticancer Res. 1997;17:769–774. [PubMed] [Google Scholar]
- 15.Klijn J G, Look M P, Portengen H, Alexieva-Figusch J, van Putten W L, Foekens J A. Breast Cancer Res Treat. 1994;29:73–83. doi: 10.1007/BF00666183. [DOI] [PubMed] [Google Scholar]
- 16.Toi M, Tominaga T, Osaki A, Toge T. Breast Cancer Res Treat. 1994;29:51–58. doi: 10.1007/BF00666181. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett J M, Langdon S P, Simpson B J, Stewart M, Datsaros D, Sismondi P, Love S, Scott W N, Williams A R, Lessells A M, et al. Br J Cancer. 1996;73:301–306. doi: 10.1038/bjc.1996.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scambia G, Benedetti-Panici P, Ferrandina G, Distefano M, Salerno G, Romanini M E, Fagotti A, Mancuso S. Br J Cancer. 1995;72:361–366. doi: 10.1038/bjc.1995.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meden H, Marx D, Raab T, Schauer K M, Kuhn W. J Obstet Gynaecol Br Emp. 1995;21:167–178. doi: 10.1111/j.1447-0756.1995.tb01090.x. [DOI] [PubMed] [Google Scholar]
- 20.Maurizi M, Almadori G, Ferrandina G, Distefano M, Romanini M E, Cadoni G, Benedetti-Panici P, Paludetti G, Scambia G, Mancuso S. Br J Cancer. 1996;74:1253–1257. doi: 10.1038/bjc.1996.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbertson R J, Perry R H, Kelly P J, Pearson D J, Lunec J. Cancer Res. 1997;57:3272–3280. [PubMed] [Google Scholar]
- 22.Levitzki A, Gazit A. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 23.Fry D W. Exp Opin Invest Drugs. 1994;3:577–595. [Google Scholar]
- 24.Fry D W, Bridges A J. Curr Opin Biotechnol. 1995;6:662–667. doi: 10.1016/0958-1669(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 25.Fry D W. Annu Rev Med Chem. 1996;31:151–160. [Google Scholar]
- 26.Lawrence D S, Niu J K. Pharmacol Ther. 1998;77:81–114. doi: 10.1016/s0163-7258(97)00052-1. [DOI] [PubMed] [Google Scholar]
- 27.Spada A P, Myers M R. Exp Opin Ther Patents. 1995;5:805–817. [Google Scholar]
- 28.Singh J, Dobrusin E M, Fry D W, Haske T, Whitty A, McNamara D J. J Med Chem. 1997;40:1130–1135. doi: 10.1021/jm960380s. [DOI] [PubMed] [Google Scholar]
- 29.Clark S, Konstantopoulos N. Biochem J. 1993;292:217–223. doi: 10.1042/bj2920217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woltjer R L, Staros J V. Biochemistry. 1997;36:9911–9916. doi: 10.1021/bi963007v. [DOI] [PubMed] [Google Scholar]
- 31.Fry D W, Kraker A J, McMichael A, Ambroso L A, Nelson J M, Leopold W R, Connors R W, Bridges A J. Science. 1994;265:1093–1095. doi: 10.1126/science.8066447. [DOI] [PubMed] [Google Scholar]
- 32.Bridges A J, Zhou H, Cody D R, Rewcastle G W, McMichael A, Showalter H D H, Fry D W, Kraker A J, Denny W A. J Med Chem. 1996;39:267–276. doi: 10.1021/jm9503613. [DOI] [PubMed] [Google Scholar]
- 33.Fry D W, Nelson J M, Slintak V, Keller P R, Rewcastle G W, Denny W A, Zhou H, Bridges A J. Biochem Pharmacol. 1997;54:877–887. doi: 10.1016/s0006-2952(97)00242-6. [DOI] [PubMed] [Google Scholar]
- 34.Rewcastle G W, Murray D K, Elliott W L, Fry D W, Winters R T, Showalter H D H, Denny W A. J Med Chem. 1998;41:742–751. doi: 10.1021/jm970641d. [DOI] [PubMed] [Google Scholar]
- 35.Bridges, A. J., Denny, W. A., Dobrusin, E. M., Doherty, A. M., Fry, D. W., McNamara, D. J., Showalter, H. D. H., Smaill, J. B. & Zhou, H. (1997) World Patent Appl. WO 9738983.
- 36.Wissner, A., Johnson, B. D., Floyd, M. B. & Kitchen, D. B (1997) Eur. Patent Appl. EP 0 787 722 A1.
- 37.Palmer B D, Trumpp-Kallmeyer S, Fry D W, Nelson J M, Showalter H D H, Denny W A. J Med Chem. 1997;40:1519–1529. doi: 10.1021/jm960789h. [DOI] [PubMed] [Google Scholar]
- 38.Fry D W, Kraker A J, Connors R C, Elliott W L, Nelson J M, Showalter H D H, Leopold W R. Anticancer Drug Design. 1994;9:331–351. [PubMed] [Google Scholar]
- 39.Martiny-Baron G, Kazanietz M G, Mischak H, Blumberg P M, Kochs G, Hug H, Marme D, Schachtele C. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 40.Greis K D, Zhu S, Matalon S. Arch Biochem Biophys. 1996;335:396–402. doi: 10.1006/abbi.1996.0522. [DOI] [PubMed] [Google Scholar]
- 41.Decker S J. Mol Cell Biol. 1984;4:571–575. doi: 10.1128/mcb.4.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biemann K. Biomed Environ Mass Spectrom. 1988;16:99–111. doi: 10.1002/bms.1200160119. [DOI] [PubMed] [Google Scholar]
- 43.Knighton D R, Cadena D L, Zheng J, Ten Eyck L F, Taylor S S, Sowadski J M, Gill G N. Proc Natl Acad Sci USA. 1993;90:5001–5005. doi: 10.1073/pnas.90.11.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dewar M J S, Thiel W. J Am Chem Soc. 1977;99:4907. [Google Scholar]
- 45.Leopold W R, Nelson J M, Plowman J, Jackson R C. Cancer Res. 1985;45:5532–5539. [PubMed] [Google Scholar]
- 46.Gibson K H, Grundy W, Godfrey A A, Woodburn J R, Ashton S E, Curry B J, Scarlett L, Barkder A J, Brown D S. Bioorg Med Chem. 1997;7:2723–2728. [Google Scholar]
- 47.Moyer J D, Barbacci E G, Iwata K K, Arnold L, Boman B, Cunningham A, DiOrio C, Doty J, Morin M J, Moyer M P, et al. Cancer Res. 1997;57:4838–4848. [PubMed] [Google Scholar]
- 48.Allen F H, Davies J E, Galloy J J, Johnson O, Kennard O, Macrea C F, Mitchell E M, Mitchell G F, Smith J M, Watson D G. J Chem Inf Comput Sci. 1991;31:187–195. [Google Scholar]