Abstract
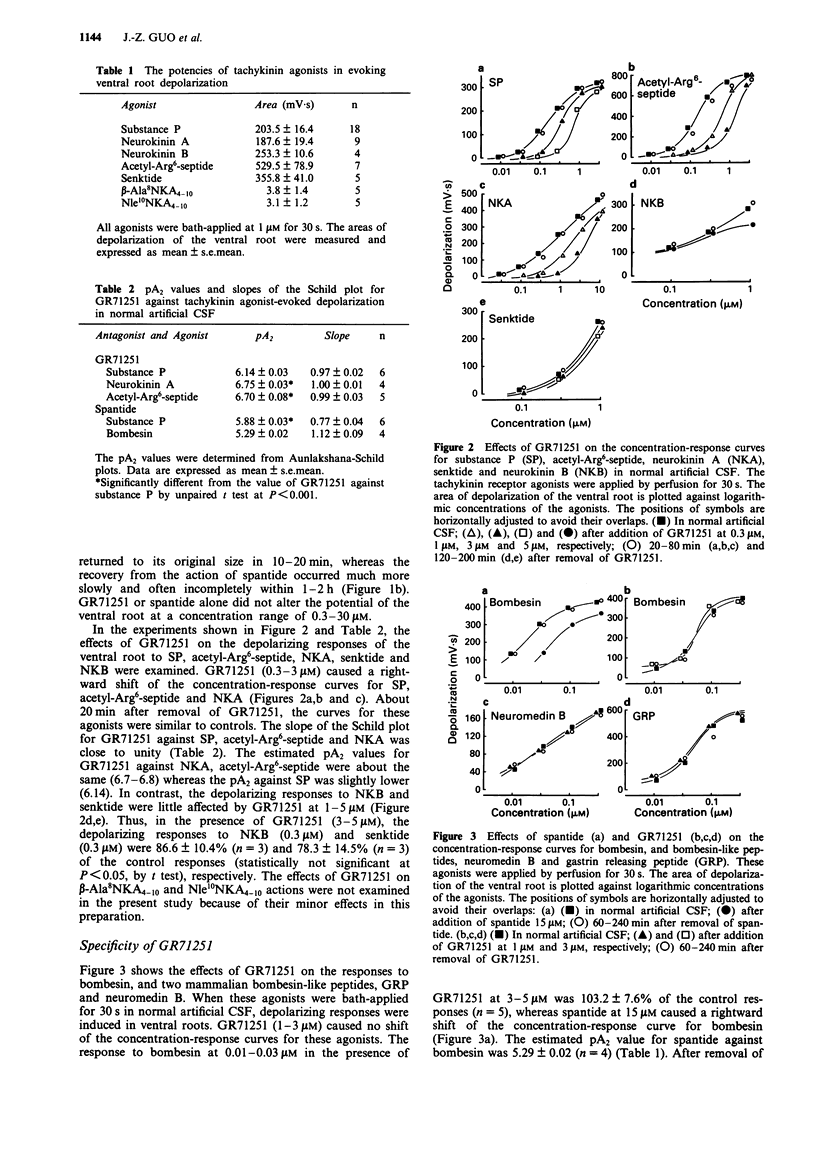
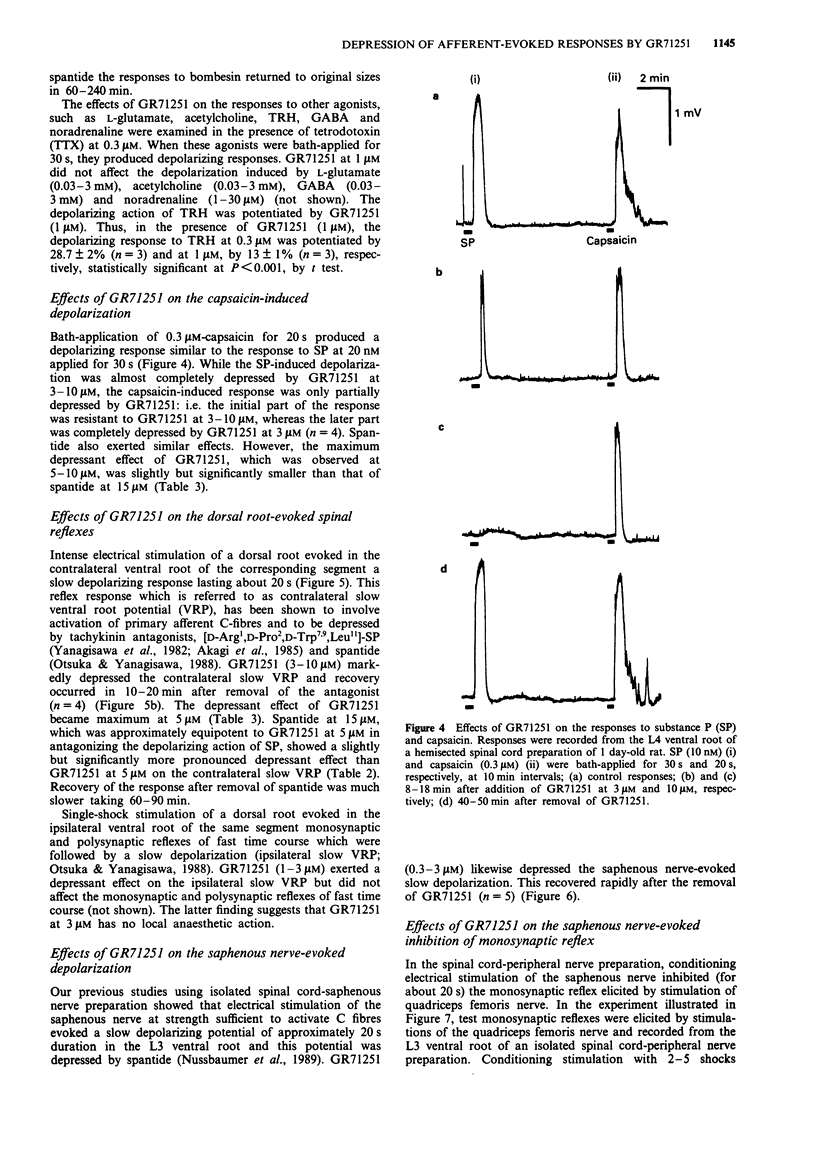
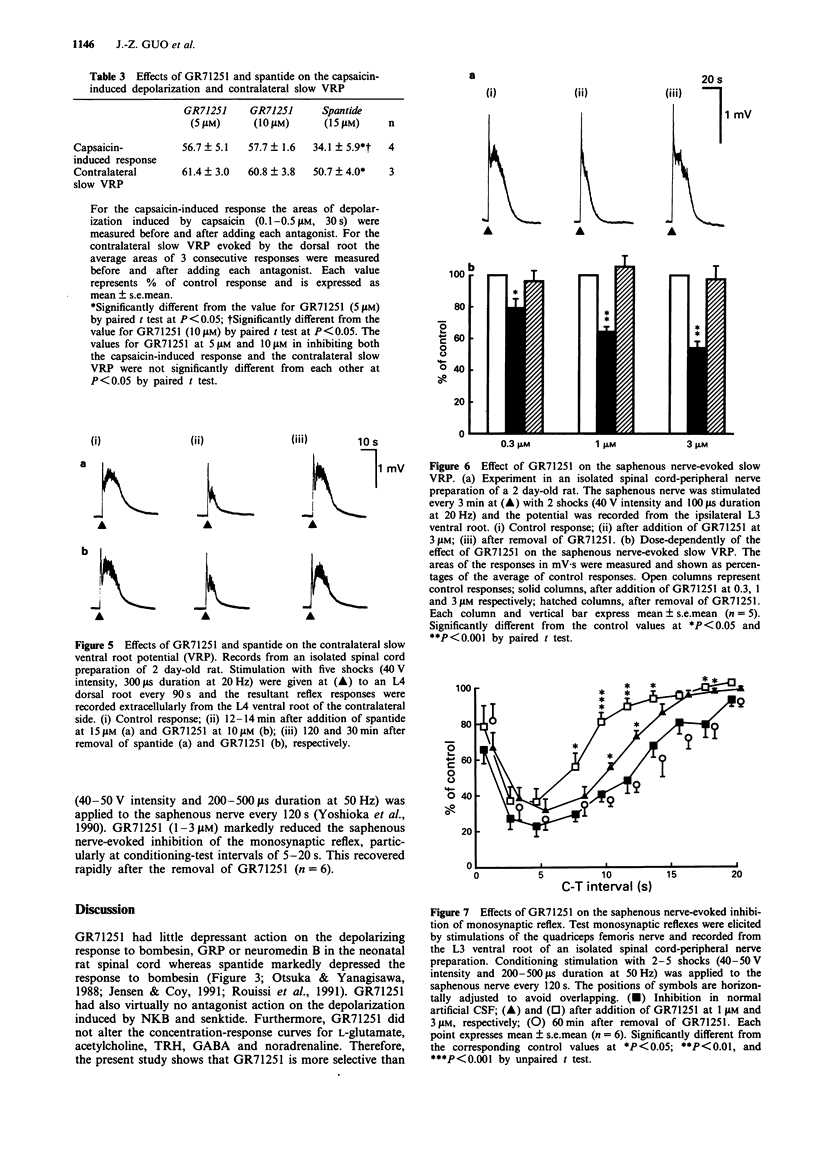
1. The pharmacological profile of GR71251, a new tachykinin receptor antagonist, and its effect on the responses evoked by stimulation of primary afferent fibres were studied in isolated spinal cord preparations of neonatal rats. Potential changes were recorded extracellularly from a lumbar ventral root (L3-L5). 2. Bath-application of substance P (SP), neurokinin A (NKA) and neurokinin B (NKB) at 0.01-3 microM to the spinal cord induced depolarization of the ventral root in normal artificial cerebrospinal fluid (CSF). The NK1 agonist, acetyl-Arg6-septide, and the NK3 agonist, senktide, at 0.01-3 microM, also had potent depolarizing actions whereas two NK2 agonists, beta-Ala8NKA4-10 and Nle10NKA4-10, showed little depolarizing effects at 1 microM. 3. GR71251 (0.3-3 microM) caused a rightward shift of the concentration-response curves for SP, acetyl-Arg6-septide and NKA with pA2 values of 6.14, 6.75 or 6.70, respectively. The effects of GR71251 were readily reversible within 15-30 min after its removal. By contrast, GR71251 (1-5 microM) had little effect on the depolarizing responses to NKB and senktide. 4. GR71251 (1-3 microM) did not depress the depolarizing responses to bombesin, neuromedin B and gastrin-releasing peptide in normal artificial CSF. 5. Application of capsaicin to the spinal cord induced a depolarizing response, which was partially depressed by GR71251 (3-10 microM). 6. In the isolated spinal cord preparation, intense electrical stimulation of a dorsal root evoked a slow depolarizing response of the contralateral ventral root of the same segment.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi H., Konishi S., Otsuka M., Yanagisawa M. The role of substance P as a neurotransmitter in the reflexes of slow time courses in the neonatal rat spinal cord. Br J Pharmacol. 1985 Mar;84(3):663–673. doi: 10.1111/j.1476-5381.1985.tb16148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. A., Leah J. D., Snow P. J. The coexistence of neuropeptides in feline sensory neurons. Neuroscience. 1988 Dec;27(3):969–979. doi: 10.1016/0306-4522(88)90200-x. [DOI] [PubMed] [Google Scholar]
- Folkers K., Håkanson R., Hörig J., Xu J. C., Leander S. Biological evaluation of substance P antagonists. Br J Pharmacol. 1984 Oct;83(2):449–456. doi: 10.1111/j.1476-5381.1984.tb16506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Agnati L. F., McDonald T., Locatelli V., Hökfelt T., Dalsgaard C. J., Battistini N., Yanaihara N., Mutt V., Cuello A. C. Immunohistochemical indications of gastrin releasing peptide--bombesin-like immunoreactivity in the nervous system of the rat. Codistribution with substance P-like immunoreactive nerve terminal systems and coexistence with substance P-like immunoreactivity in dorsal root ganglion cell bodies. Neurosci Lett. 1983 May 27;37(1):17–22. doi: 10.1016/0304-3940(83)90498-6. [DOI] [PubMed] [Google Scholar]
- Guo J. Z., Yoshioka K., Yanagisawa M., Hagan R. M., Otsuka M. Blockade of cutaneous nerve-evoked responses by GR71251 in the isolated spinal cord preparation of newborn rat. Regul Pept. 1993 Jul 2;46(1-2):309–310. doi: 10.1016/0167-0115(93)90069-k. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Morton I. K. Novel selective agonists and antagonists confirm neurokinin NK1 receptors in guinea-pig vas deferens. Br J Pharmacol. 1991 Feb;102(2):511–517. doi: 10.1111/j.1476-5381.1991.tb12202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland S. J., Bailey F., Cook A., Hagan R. M., Jordan C. C., Stephens-Smith M. L. Receptors mediating tachykinin-induced contractile responses in guinea-pig trachea. Br J Pharmacol. 1991 Jun;103(2):1463–1469. doi: 10.1111/j.1476-5381.1991.tb09812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. T., Coy D. H. Progress in the development of potent bombesin receptor antagonists. Trends Pharmacol Sci. 1991 Jan;12(1):13–19. doi: 10.1016/0165-6147(91)90483-9. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Heinz-Erian P., Mantey S., Jones S. W., Gardner J. D. Characterization of ability of various substance P antagonists to inhibit action of bombesin. Am J Physiol. 1988 Jun;254(6 Pt 1):G883–G890. doi: 10.1152/ajpgi.1988.254.6.G883. [DOI] [PubMed] [Google Scholar]
- Jensen R. T., Jones S. W., Folkers K., Gardner J. D. A synthetic peptide that is a bombesin receptor antagonist. Nature. 1984 May 3;309(5963):61–63. doi: 10.1038/309061a0. [DOI] [PubMed] [Google Scholar]
- Leah J., Menétrey D., de Pommery J. neuropeptides in long ascending spinal tract cells in the rat: evidence for parallel processing of ascending information. Neuroscience. 1988 Jan;24(1):195–207. doi: 10.1016/0306-4522(88)90323-5. [DOI] [PubMed] [Google Scholar]
- McGregor G. P., Gibson S. J., Sabate I. M., Blank M. A., Christofides N. D., Wall P. D., Polak J. M., Bloom S. R. Effect of peripheral nerve section and nerve crush on spinal cord neuropeptides in the rat; increased VIP and PHI in the dorsal horn. Neuroscience. 1984 Sep;13(1):207–216. doi: 10.1016/0306-4522(84)90270-7. [DOI] [PubMed] [Google Scholar]
- Mizrahi J., Dion S., D'Orléans-Juste P., Regoli D. Activities and antagonism of bombesin on urinary smooth muscles. Eur J Pharmacol. 1985 May 20;111(3):339–345. doi: 10.1016/0014-2999(85)90640-5. [DOI] [PubMed] [Google Scholar]
- Nussbaumer J. C., Yanagisawa M., Otsuka M. Pharmacological properties of a C-fibre response evoked by saphenous nerve stimulation in an isolated spinal cord-nerve preparation of the newborn rat. Br J Pharmacol. 1989 Oct;98(2):373–382. doi: 10.1111/j.1476-5381.1989.tb12607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Yanagisawa M. Effect of a tachykinin antagonist on a nociceptive reflex in the isolated spinal cord-tail preparation of the newborn rat. J Physiol. 1988 Jan;395:255–270. doi: 10.1113/jphysiol.1988.sp016917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P., Hadjiconstantinou M., Yang H. Y., Costa E. Immunohistochemical localization of bombesin/gastrin-releasing peptide and substance P in primary sensory neurons. J Neurosci. 1983 Oct;3(10):2021–2029. doi: 10.1523/JNEUROSCI.03-10-02021.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papir-Kricheli D., Frey J., Laufer R., Gilon C., Chorev M., Selinger Z., Devor M. Behavioural effects of receptor-specific substance P agonists. Pain. 1987 Nov;31(2):263–276. doi: 10.1016/0304-3959(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Post C., Butterworth J. F., Strichartz G. R., Karlsson J. A., Persson C. G. Tachykinin antagonists have potent local anaesthetic actions. Eur J Pharmacol. 1985 Nov 19;117(3):347–354. doi: 10.1016/0014-2999(85)90008-1. [DOI] [PubMed] [Google Scholar]
- Regoli D., Rhaleb N. E., Dion S., Tousignant C., Rouissi N., Jukic D., Drapeau G. Neurokinin A. A pharmacological study. Pharmacol Res. 1990 Jan-Feb;22(1):1–14. doi: 10.1016/1043-6618(90)90738-y. [DOI] [PubMed] [Google Scholar]
- Rouissi N., Rhaleb N. E., Nantel F., Dion S., Drapeau G., Regoli D. Characterization of bombesin receptors in peripheral contractile organs. Br J Pharmacol. 1991 May;103(1):1141–1147. doi: 10.1111/j.1476-5381.1991.tb12314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovero P., Pestellini V., Patacchini R., Giuliani S., Santicioli P., Maggi C. A., Meli A., Giachetti A. A potent and selective agonist for NK-2 tachykinin receptor. Peptides. 1989 May-Jun;10(3):593–595. doi: 10.1016/0196-9781(89)90148-4. [DOI] [PubMed] [Google Scholar]
- Urban L., Dray A. Synaptic activation of dorsal horn neurons by selective C-fibre excitation with capsaicin in the mouse spinal cord in vitro. Neuroscience. 1992;47(3):693–702. doi: 10.1016/0306-4522(92)90177-4. [DOI] [PubMed] [Google Scholar]
- Wada E., Way J., Lebacq-Verheyden A. M., Battey J. F. Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. J Neurosci. 1990 Sep;10(9):2917–2930. doi: 10.1523/JNEUROSCI.10-09-02917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P., Ewan G. B., Jordan C. C., Ireland S. J., Hagan R. M., Brown J. R. Potent and highly selective neurokinin antagonists. J Med Chem. 1990 Jul;33(7):1848–1851. doi: 10.1021/jm00169a003. [DOI] [PubMed] [Google Scholar]
- Yachnis A. T., Crawley J. N., Jensen R. T., McGrane M. M., Moody T. W. The antagonism of bombesin in the CNS by substance P analogues. Life Sci. 1984 Nov 5;35(19):1963–1969. doi: 10.1016/0024-3205(84)90477-6. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Michener S. R., Bailey J. E., Harty G. J., Lucas D. L., Nelson D. K., Roddy D. R., Go V. L. Survey of distribution of substance P, vasoactive intestinal polypeptide, cholecystokinin, neurotensin, Met-enkephalin, bombesin and PHI in the spinal cord of cat, dog, sloth and monkey. Peptides. 1988 Mar-Apr;9(2):357–372. doi: 10.1016/0196-9781(88)90272-0. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Otsuka M., Konishi S., Akagi H., Folkers K., Rosell S. A substance P antagonist inhibits a slow reflex response in the spinal cord of the newborn rat. Acta Physiol Scand. 1982 Sep;116(1):109–112. doi: 10.1111/j.1748-1716.1982.tb10608.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Otsuka M. Pharmacological profile of a tachykinin antagonist, spantide, as examined on rat spinal motoneurones. Br J Pharmacol. 1990 Aug;100(4):711–716. doi: 10.1111/j.1476-5381.1990.tb14080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Sakuma M., Otsuka M. Cutaneous nerve-evoked cholinergic inhibition of monosynaptic reflex in the neonatal rat spinal cord: involvement on M2 receptors and tachykininergic primary afferents. Neuroscience. 1990;38(1):195–203. doi: 10.1016/0306-4522(90)90385-h. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T., Kanazawa I., Nakanishi T. Substance P antagonist (spantide) suppresses the compound action potentials of the rat sciatic nerve in vitro. Neuropeptides. 1987 Aug-Sep;10(2):181–188. doi: 10.1016/0143-4179(87)90020-5. [DOI] [PubMed] [Google Scholar]


