Abstract
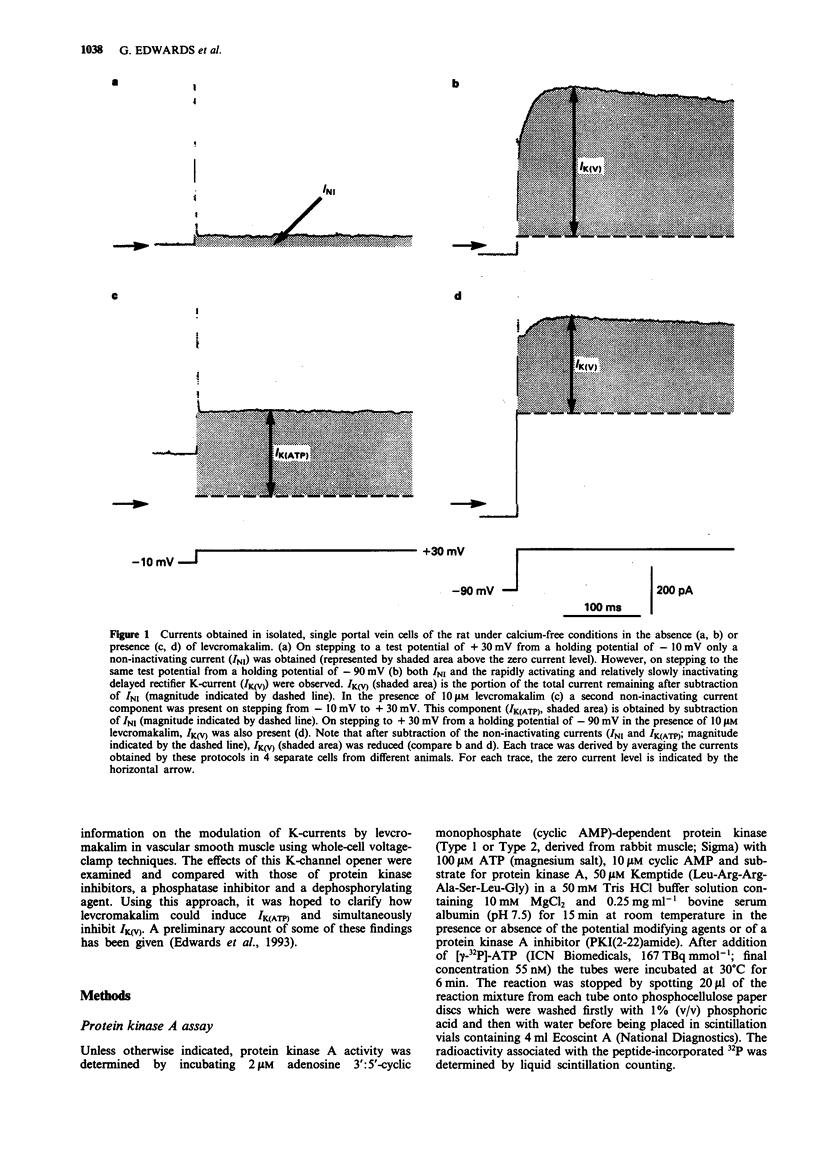
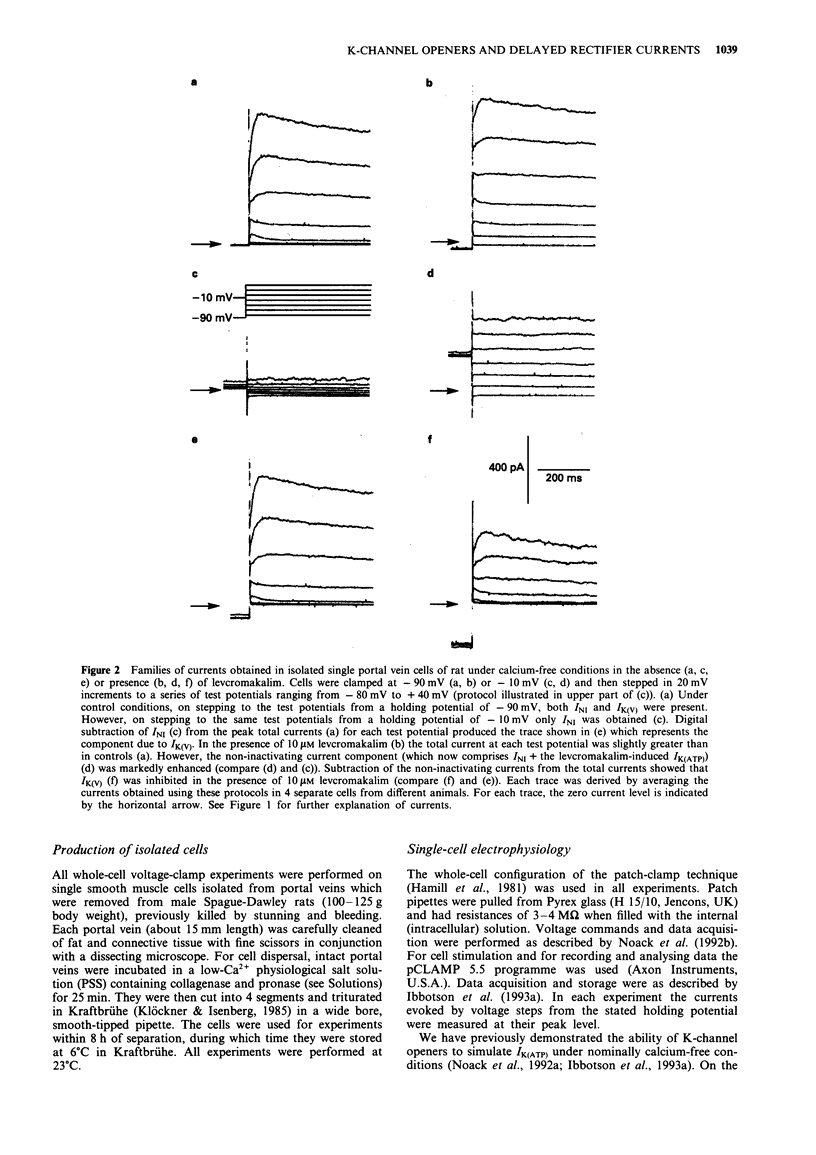
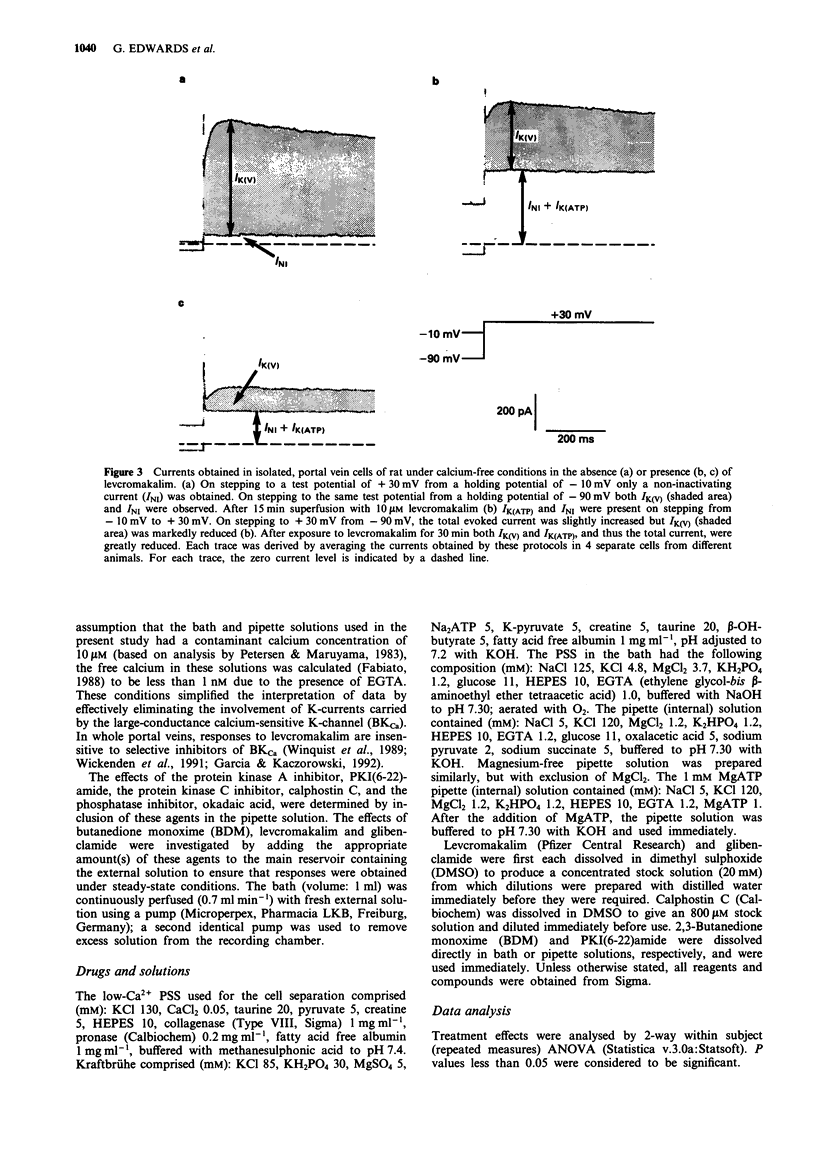
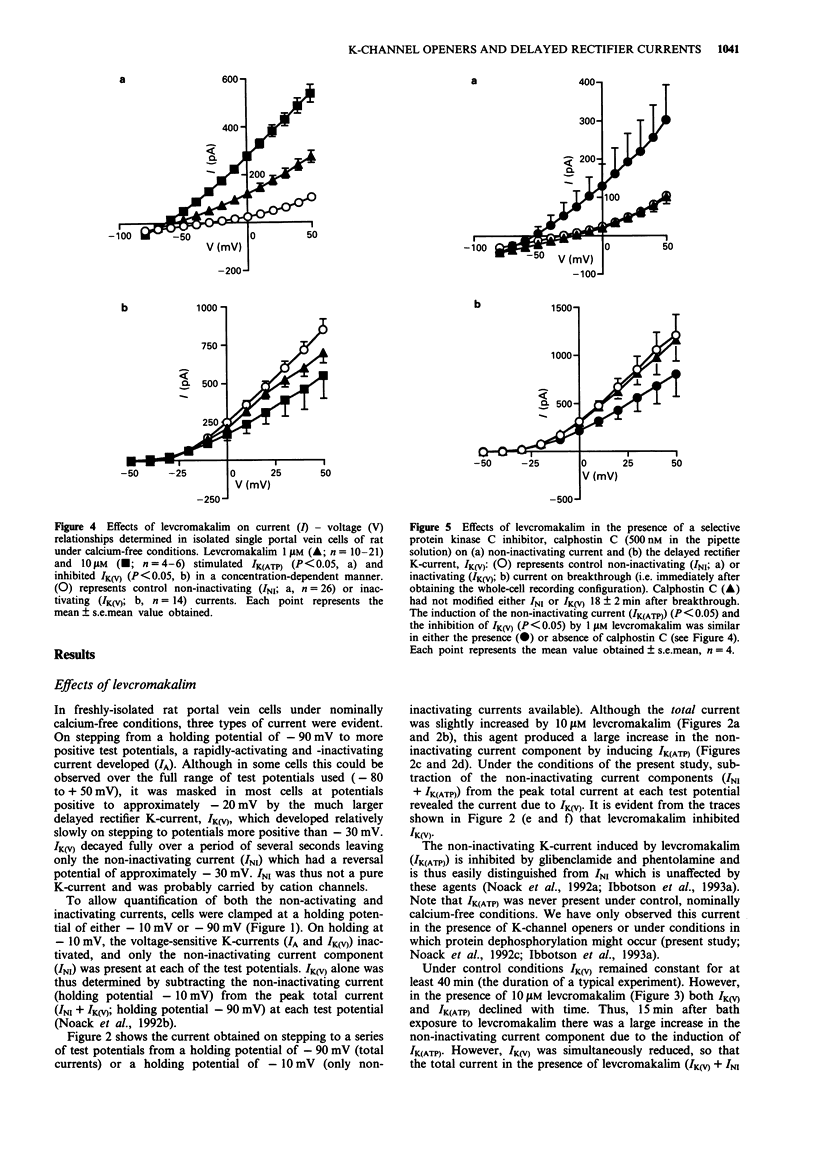
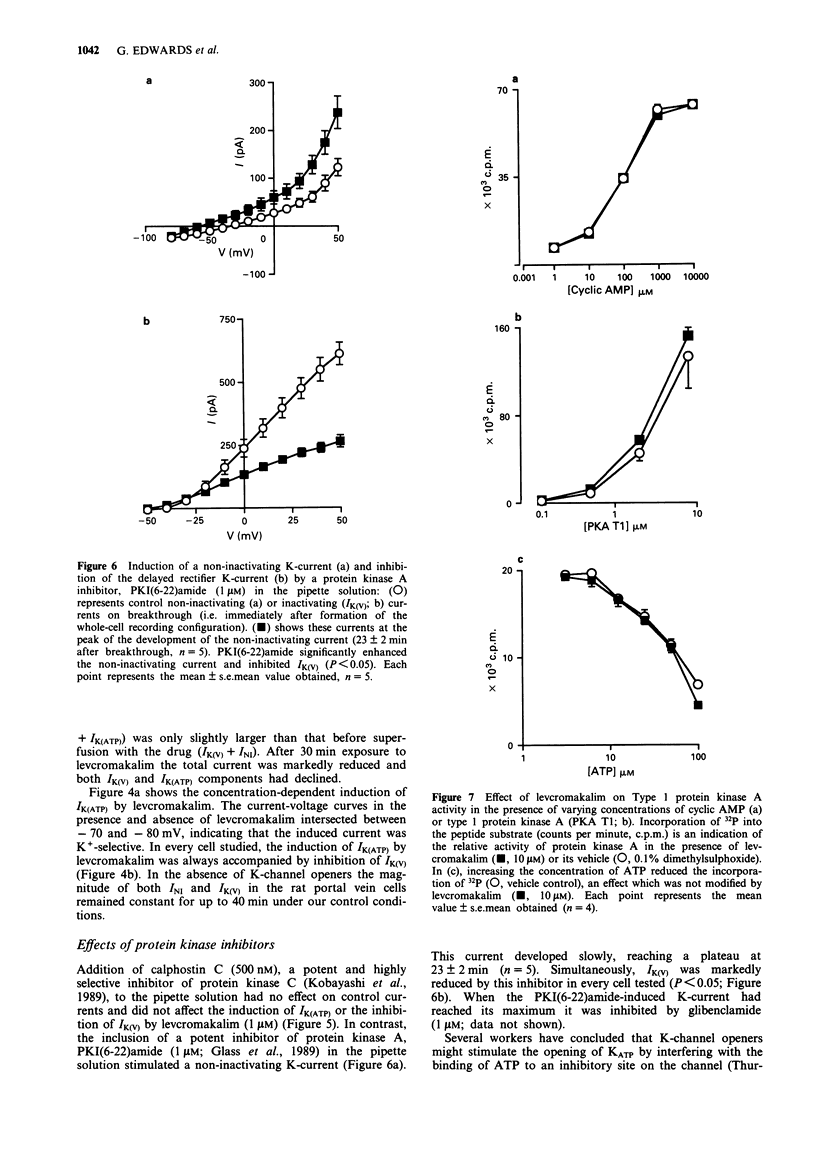
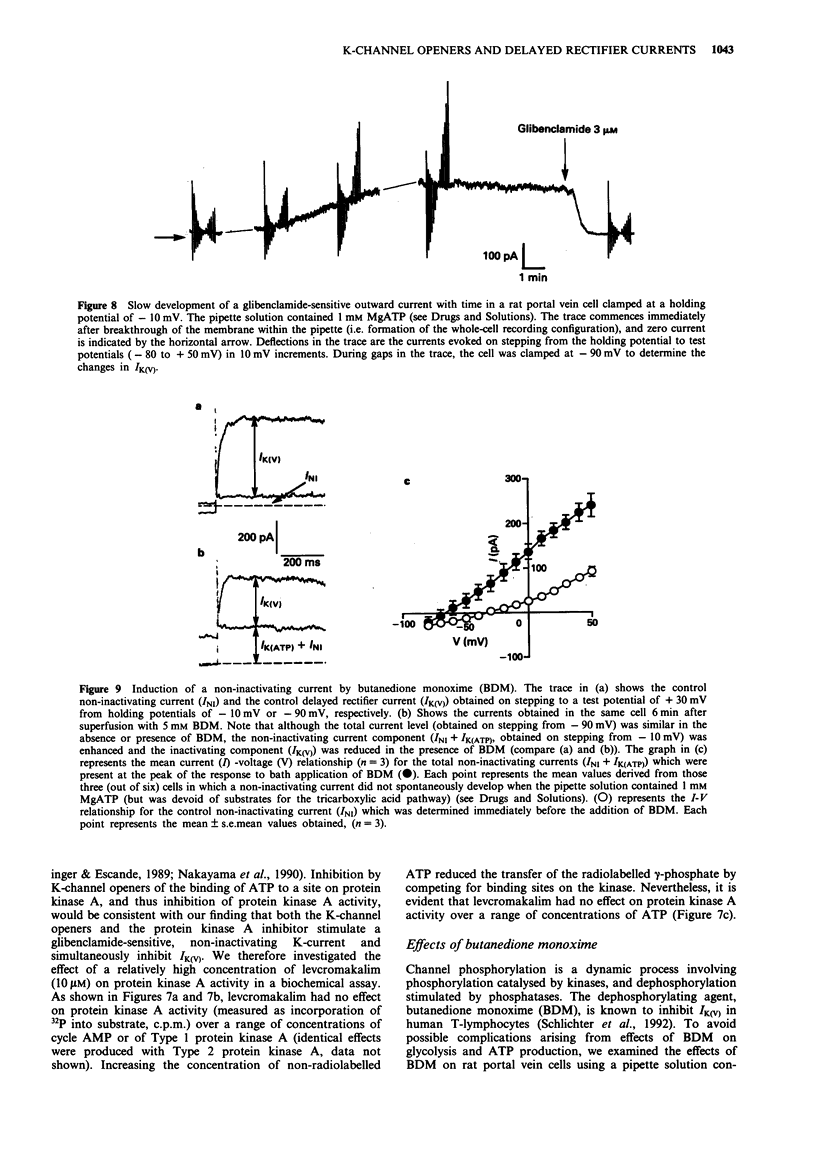
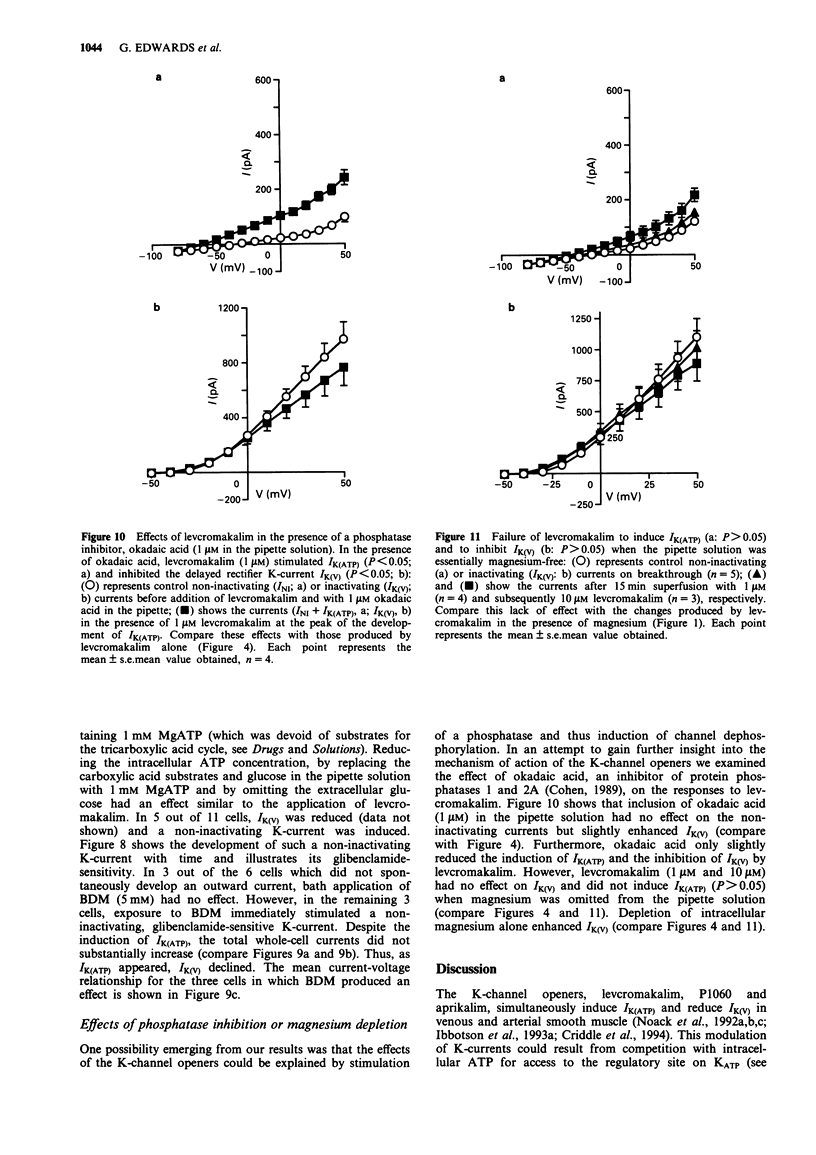
1. Smooth muscle cells of the rat portal vein were dispersed by enzymatic treatment and recordings of whole-cell currents under calcium-free conditions were made by the voltage-clamp technique. The effects of the potassium (K)-channel opener, levcromakalim, on K-currents were compared with those of agents which modify protein phosphorylation. 2. Levcromakalim (1-10 microM) added to the extracellular (bath) fluid caused the development of a non-inactivating current (IK(ATP)) and simultaneously inhibited the delayed rectifier current (IK(V)) in a concentration-dependent manner. On prolonged exposure to levcromakalim (10 microM), IK(ATP) declined and IK(V) was further diminished. 3. Addition to the pipette (intracellular) solution of the selective inhibitor of protein kinase C, calphostin C, itself had no effect on K-currents and did not modify the induction of IK(ATP) or the simultaneous inhibition of IK(V) produced by 1 microM levcromakalim. 4. Addition of the protein kinase inhibitor (PKI(6-22)amide, 1 microM) to the pipette solution caused the production of a glibenclamide-sensitive, non-inactivating current and inhibited IK(V). 5. In an assay system, levcromakalim (10 microM) did not inhibit the activity of purified protein kinase A (Type 1 or Type 2). 6. Addition to the pipette solution of the phosphatase inhibitor, okadaic acid (1 microM), did not itself modify K-currents and had little effect on the simultaneous induction of IK(ATP) and inhibition of IK(V) by levcromakalim (1 microM). 7. When the pipette solution contained 1 mM MgATP (but was depleted of substrates for ATP production), a non-inactivating, glibenclamide-sensitive K-current developed spontaneously in 5 out of 11 cells with the simultaneous reduction of IK(V). In 3 of the 6 remaining cells, addition of the dephosphorylating agent, butanedione monoxime (5 mM) to the bath inhibited IK(V) and stimulated a glibenclamide-sensitive non-inactivating current. 8. Depletion of intracellular Mg2+ slightly enhanced IK(V). Under these conditions, levcromakalim (1 microM and 10 microM) did not significantly induce IK(ATP) or inhibit IK(V). 9. It is concluded that the effects of levcromakalim on K-currents can be mimicked by procedures designed to reduce channel phosphorylation. The results are consistent with the view that levcromkalim dephosphorylates the delayed rectifier channel, KV, which becomes converted into a voltage-independent, non-inactivating form known as KATP. The possible mechanisms which underlie this interconversion are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrea J. E., Walsh M. P. Protein kinase C of smooth muscle. Hypertension. 1992 Nov;20(5):585–595. doi: 10.1161/01.hyp.20.5.585. [DOI] [PubMed] [Google Scholar]
- Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Two components of potassium current activated by depolarization of single smooth muscle cells from the rabbit portal vein. J Physiol. 1989 Nov;418:293–309. doi: 10.1113/jphysiol.1989.sp017841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. A., Travis S. M., Welsh M. J. Regulation of the cystic fibrosis transmembrane conductance regulator Cl- channel by specific protein kinases and protein phosphatases. J Biol Chem. 1993 Jan 25;268(3):2037–2047. [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. P., Tomasic M., Kotlikoff M. I. Delayed rectifier potassium channels in canine and porcine airway smooth muscle cells. J Physiol. 1992 Feb;447:329–350. doi: 10.1113/jphysiol.1992.sp019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray K., Quast U. Differential inhibition by tedisamil (KC 8857) and glibenclamide of the responses to cromakalim and minoxidil sulphate in rat isolated aorta. Naunyn Schmiedebergs Arch Pharmacol. 1992 Feb;345(2):244–250. doi: 10.1007/BF00165744. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992 Mar;262(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Davis P. D., Elliott L. H., Harris W., Hill C. H., Hurst S. A., Keech E., Kumar M. K., Lawton G., Nixon J. S., Wilkinson S. E. Inhibitors of protein kinase C. 2. Substituted bisindolylmaleimides with improved potency and selectivity. J Med Chem. 1992 Mar 20;35(6):994–1001. doi: 10.1021/jm00084a004. [DOI] [PubMed] [Google Scholar]
- Duchatelle-Gourdon I., Lagrutta A. A., Hartzell H. C. Effects of Mg2+ on basal and beta-adrenergic-stimulated delayed rectifier potassium current in frog atrial myocytes. J Physiol. 1991 Apr;435:333–347. doi: 10.1113/jphysiol.1991.sp018513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne M. J. Effects of pinacidil, RP 49356 and nicorandil on ATP-sensitive potassium channels in insulin-secreting cells. Br J Pharmacol. 1990 Mar;99(3):487–492. doi: 10.1111/j.1476-5381.1990.tb12955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fink R., Wettwer E. Modified K-channel gating by exhaustion and the block by internally applied TEA+ and 4-aminopyridine in muscle. Pflugers Arch. 1978 May 31;374(3):289–292. doi: 10.1007/BF00585607. [DOI] [PubMed] [Google Scholar]
- Garrino M. G., Plant T. D., Henquin J. C. Effects of putative activators of K+ channels in mouse pancreatic beta-cells. Br J Pharmacol. 1989 Nov;98(3):957–965. doi: 10.1111/j.1476-5381.1989.tb14626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D. B., Cheng H. C., Mende-Mueller L., Reed J., Walsh D. A. Primary structural determinants essential for potent inhibition of cAMP-dependent protein kinase by inhibitory peptides corresponding to the active portion of the heat-stable inhibitor protein. J Biol Chem. 1989 May 25;264(15):8802–8810. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamilton T. C., Weir S. W., Weston A. H. Comparison of the effects of BRL 34915 and verapamil on electrical and mechanical activity in rat portal vein. Br J Pharmacol. 1986 May;88(1):103–111. doi: 10.1111/j.1476-5381.1986.tb09476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Mieskes G., Rüegg J. C., Takai A., Trautwein W. Effects of a protein phosphatase inhibitor, okadaic acid, on membrane currents of isolated guinea-pig cardiac myocytes. Pflugers Arch. 1988 Aug;412(3):248–252. doi: 10.1007/BF00582504. [DOI] [PubMed] [Google Scholar]
- Ibbotson T., Edwards G., Noack T., Weston A. H. Effects of P1060 and aprikalim on whole-cell currents in rat portal vein; inhibition by glibenclamide and phentolamine. Br J Pharmacol. 1993 Apr;108(4):991–998. doi: 10.1111/j.1476-5381.1993.tb13496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajioka S., Kitamura K., Kuriyama H. Guanosine diphosphate activates an adenosine 5'-triphosphate-sensitive K+ channel in the rabbit portal vein. J Physiol. 1991 Dec;444:397–418. doi: 10.1113/jphysiol.1991.sp018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajioka S., Oike M., Kitamura K. Nicorandil opens a calcium-dependent potassium channel in smooth muscle cells of the rat portal vein. J Pharmacol Exp Ther. 1990 Sep;254(3):905–913. [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Action potentials and net membrane currents of isolated smooth muscle cells (urinary bladder of the guinea-pig). Pflugers Arch. 1985 Dec;405(4):329–339. doi: 10.1007/BF00595685. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Kozlowski R. Z., Hales C. N., Ashford M. L. Dual effects of diazoxide on ATP-K+ currents recorded from an insulin-secreting cell line. Br J Pharmacol. 1989 Aug;97(4):1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge N. J., Colatsky T. J., Cullinan C. A., Follmer C. H. Electromechanical effects of the putative potassium channel activator celikalim (WAY-120,491) on feline atrial and ventricular muscle. J Pharmacol Exp Ther. 1992 Jun;261(3):1153–1159. [PubMed] [Google Scholar]
- Nakayama K., Fan Z., Marumo F., Hiraoka M. Interrelation between pinacidil and intracellular ATP concentrations on activation of the ATP-sensitive K+ current in guinea pig ventricular myocytes. Circ Res. 1990 Nov;67(5):1124–1133. doi: 10.1161/01.res.67.5.1124. [DOI] [PubMed] [Google Scholar]
- Noack T., Deitmer P., Edwards G., Weston A. H. Characterization of potassium currents modulated by BRL 38227 in rat portal vein. Br J Pharmacol. 1992 Jul;106(3):717–726. doi: 10.1111/j.1476-5381.1992.tb14400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack T., Edwards G., Deitmer P., Greengrass P., Morita T., Andersson P. O., Criddle D., Wyllie M. G., Weston A. H. The involvement of potassium channels in the action of ciclazindol in rat portal vein. Br J Pharmacol. 1992 May;106(1):17–24. doi: 10.1111/j.1476-5381.1992.tb14286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack T., Edwards G., Deitmer P., Weston A. H. Potassium channel modulation in rat portal vein by ATP depletion: a comparison with the effects of levcromakalim (BRL 38227). Br J Pharmacol. 1992 Dec;107(4):945–955. doi: 10.1111/j.1476-5381.1992.tb13390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe K., Kajioka S., Nakao K., Kitamura K., Kuriyama H., Weston A. H. Actions of cromakalim on ionic currents recorded from single smooth muscle cells of the rat portal vein. J Pharmacol Exp Ther. 1990 Feb;252(2):832–839. [PubMed] [Google Scholar]
- Perozo E., Bezanilla F. Phosphorylation affects voltage gating of the delayed rectifier K+ channel by electrostatic interactions. Neuron. 1990 Nov;5(5):685–690. doi: 10.1016/0896-6273(90)90222-2. [DOI] [PubMed] [Google Scholar]
- Pfründer D., Kreye V. A. Tedisamil inhibits the delayed rectifier K+ current in single smooth muscle cells of the guinea-pig portal vein. Pflugers Arch. 1992 May;421(1):22–25. doi: 10.1007/BF00374728. [DOI] [PubMed] [Google Scholar]
- Russell S. N., Smirnov S. V., Aaronson P. I. Effects of BRL 38227 on potassium currents in smooth muscle cells isolated from rabbit portal vein and human mesenteric artery. Br J Pharmacol. 1992 Mar;105(3):549–556. doi: 10.1111/j.1476-5381.1992.tb09017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichter L. C., Pahapill P. A., Chung I. Dual action of 2,3-butanedione monoxime (BDM) on K+ current in human T lymphocytes. J Pharmacol Exp Ther. 1992 May;261(2):438–446. [PubMed] [Google Scholar]
- Schwanstecher M., Löser S., Rietze I., Panten U. Phosphate and thiophosphate group donating adenine and guanine nucleotides inhibit glibenclamide binding to membranes from pancreatic islets. Naunyn Schmiedebergs Arch Pharmacol. 1991 Jan;343(1):83–89. doi: 10.1007/BF00180681. [DOI] [PubMed] [Google Scholar]
- Scott J. D. Cyclic nucleotide-dependent protein kinases. Pharmacol Ther. 1991;50(1):123–145. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- Shenolikar S., Nairn A. C. Protein phosphatases: recent progress. Adv Second Messenger Phosphoprotein Res. 1991;23:1–121. [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg S. D., van Breemen C. A potassium current activated by lemakalim and metabolic inhibition in rabbit mesenteric artery. Pflugers Arch. 1992 Jan;420(1):118–120. doi: 10.1007/BF00378653. [DOI] [PubMed] [Google Scholar]
- Thuringer D., Escande D. Apparent competition between ATP and the potassium channel opener RP 49356 on ATP-sensitive K+ channels of cardiac myocytes. Mol Pharmacol. 1989 Dec;36(6):897–902. [PubMed] [Google Scholar]
- Volk K. A., Shibata E. F. Single delayed rectifier potassium channels from rabbit coronary artery myocytes. Am J Physiol. 1993 Apr;264(4 Pt 2):H1146–H1153. doi: 10.1152/ajpheart.1993.264.4.H1146. [DOI] [PubMed] [Google Scholar]
- White R. E., Lee A. B., Shcherbatko A. D., Lincoln T. M., Schonbrunn A., Armstrong D. L. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 1993 Jan 21;361(6409):263–266. doi: 10.1038/361263a0. [DOI] [PubMed] [Google Scholar]
- White R. E., Schonbrunn A., Armstrong D. L. Somatostatin stimulates Ca(2+)-activated K+ channels through protein dephosphorylation. Nature. 1991 Jun 13;351(6327):570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- Wickenden A. D., Grimwood S., Grant T. L., Todd M. H. Comparison of the effects of the K(+)-channel openers cromakalim and minoxidil sulphate on vascular smooth muscle. Br J Pharmacol. 1991 May;103(1):1148–1152. doi: 10.1111/j.1476-5381.1991.tb12315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist R. J., Heaney L. A., Wallace A. A., Baskin E. P., Stein R. B., Garcia M. L., Kaczorowski G. J. Glyburide blocks the relaxation response to BRL 34915 (cromakalim), minoxidil sulfate and diazoxide in vascular smooth muscle. J Pharmacol Exp Ther. 1989 Jan;248(1):149–156. [PubMed] [Google Scholar]
- de Weille J. R., Schmid-Antomarchi H., Fosset M., Lazdunski M. Regulation of ATP-sensitive K+ channels in insulinoma cells: activation by somatostatin and protein kinase C and the role of cAMP. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2971–2975. doi: 10.1073/pnas.86.8.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]



