Abstract
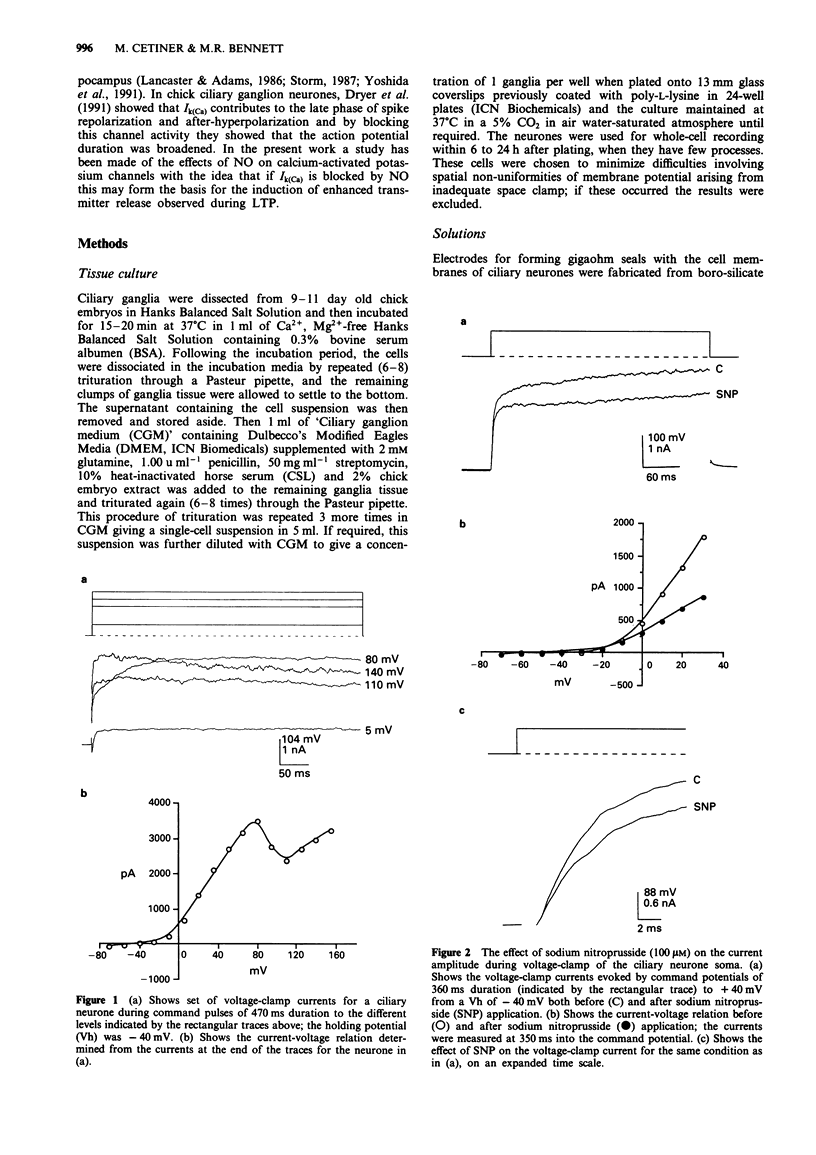
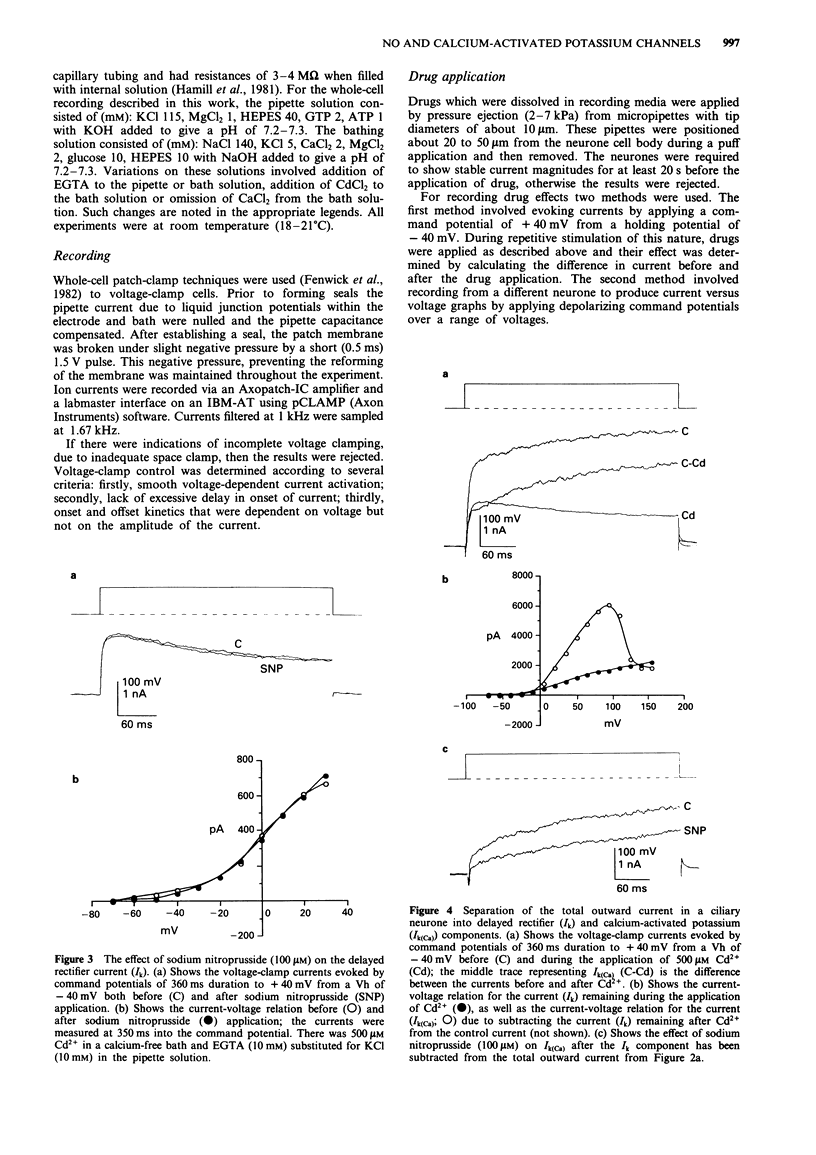
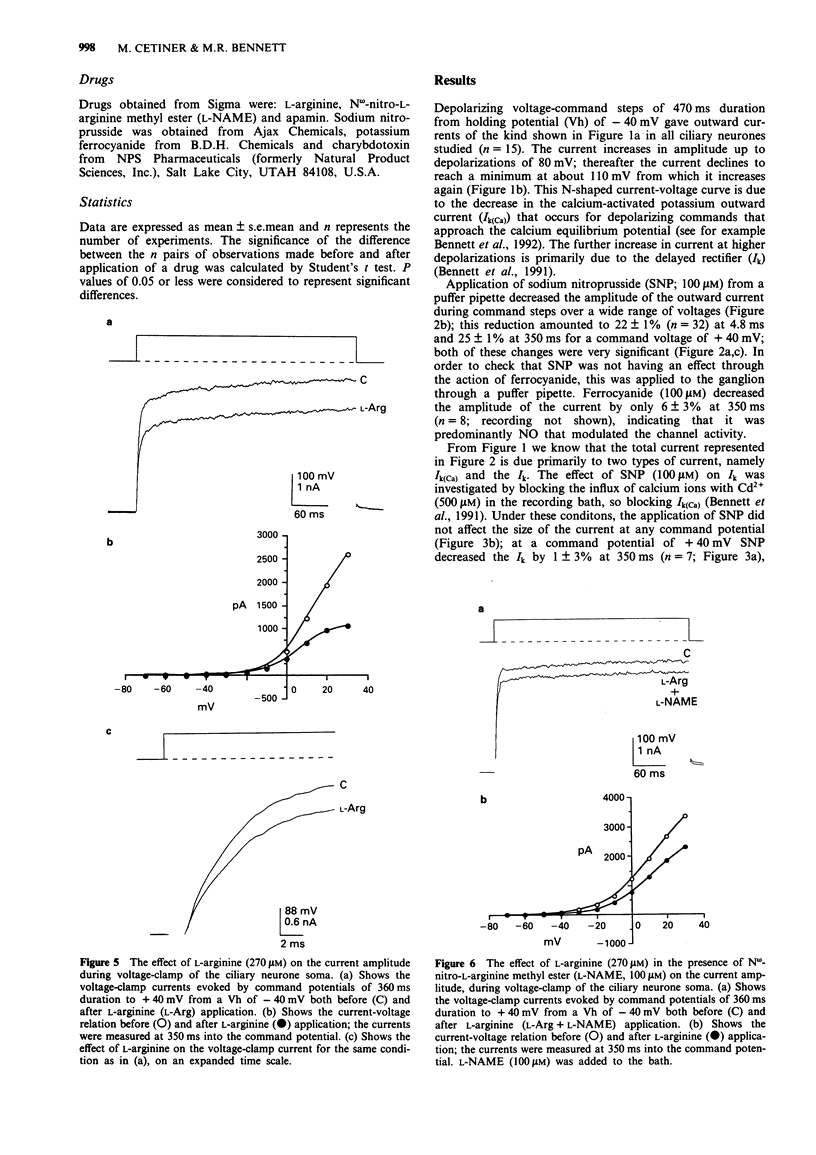
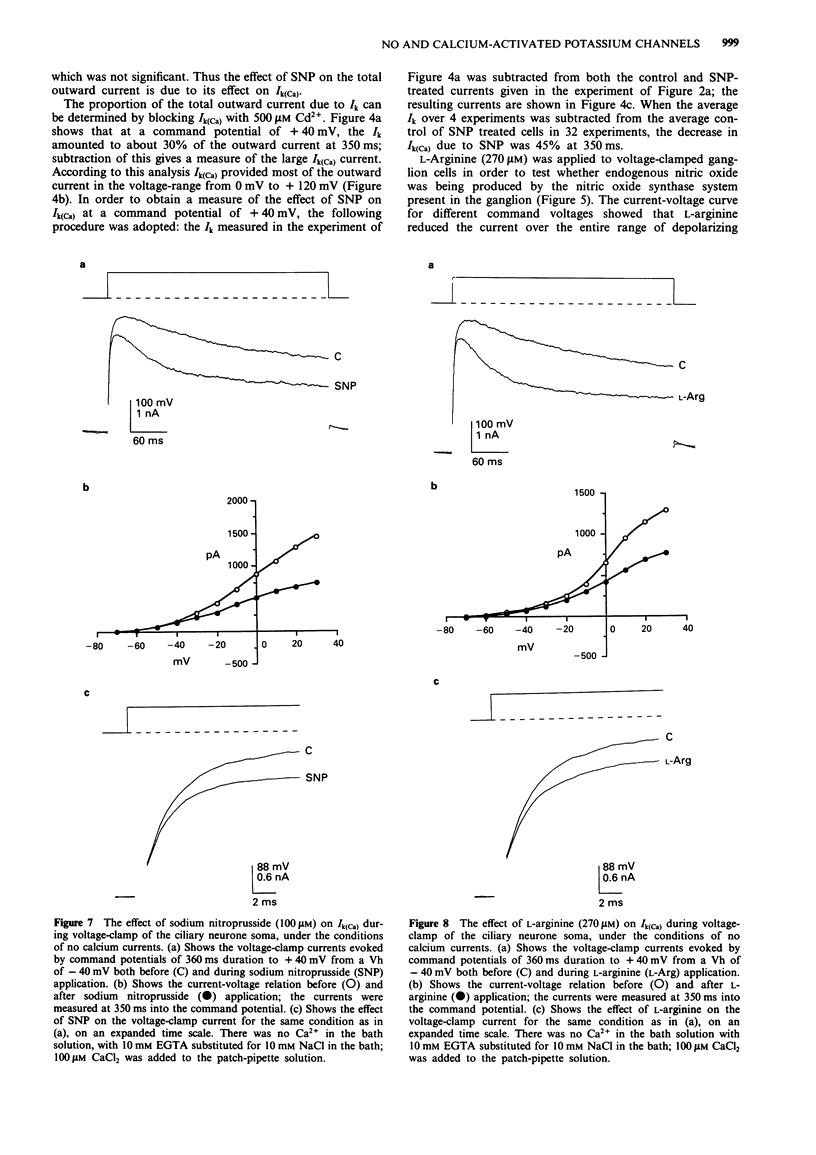
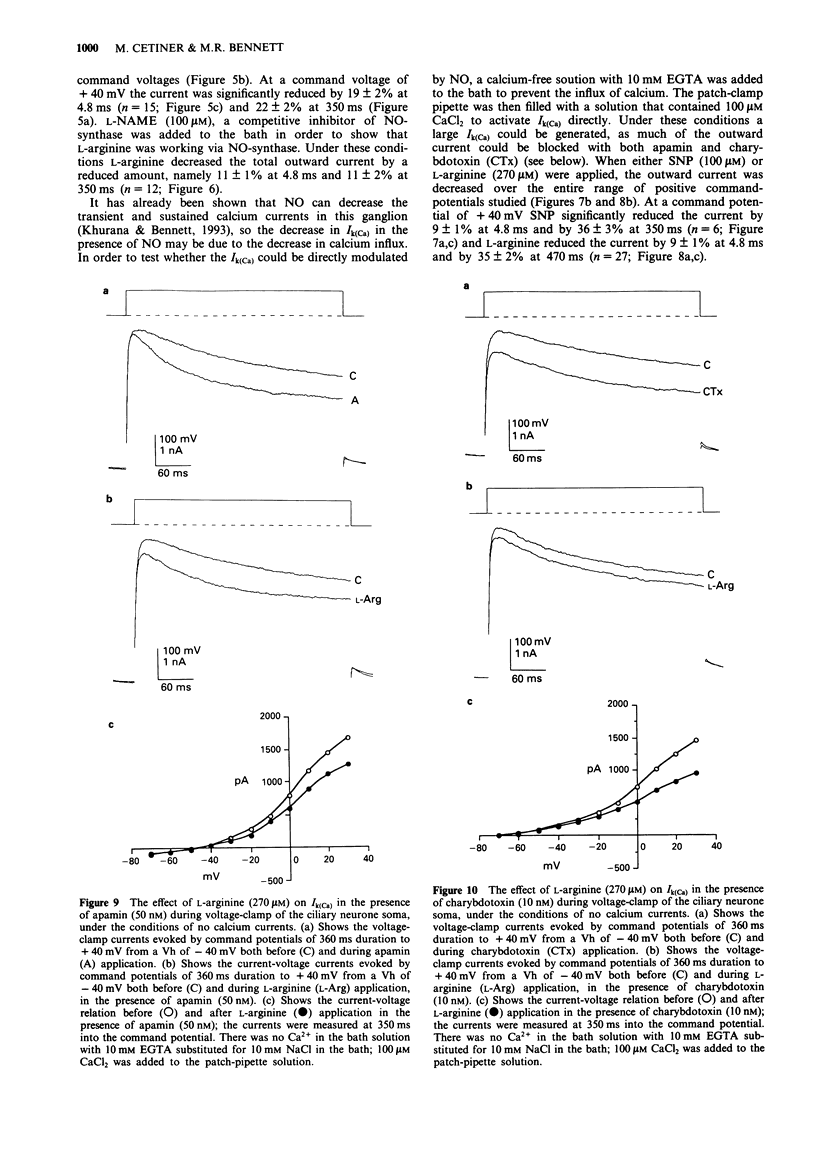
1. A study has been made of the modulation of calcium-activated potassium channels in cultured neurones of avian ciliary ganglia by sodium nitroprusside and L-arginine. 2. Sodium nitroprusside (100 microM) reduced the net outward current by 22 +/- 1% at 4.8 ms (mean +/- s.e. mean) and 25 +/- 1% at 350 ms during a test depolarization to +40 mV from a holding potential of -40 mV. The outward current remained reduced for the duration of the recording following a single application of sodium nitroprusside. These effects did not occur if the influx of calcium ions was first blocked with Cd2+ (500 microM). Application of ferrocyanide (100 microM) reduced the net outward current by only 6 +/- 3% at 350 ms during a test depolarization to +40 mV. 3. L-Arginine (270 microM) reduced the net outward current on average by 19 +/- 2% at 4.8 ms and 22 +/- 2% at 350 ms during a test depolarization to +40 mV. The current remained in this reduced state for the duration of the recording following a single application of L-arginine. These effects were reduced to 11 +/- 1% at 4.8 ms and 11 +/- 2% at 350 ms in the presence of N omega-nitro-L-arginine methyl ester (L-NAME, 100 microM). 4. In order to alleviate the dependence of calcium-activated potassium channels (Ik(Ca)) on the inward flux of calcium ions, the patch-clamp pipettes were filled with a solution containing 100 microM CaCl2, and the Ca2+ in the bathing solution was replaced with EGTA.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. R., Burnstock G., Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Feb;182(3):541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Ho S. Adenosine modulation of potassium currents in preganglionic nerve terminals of avian ciliary ganglia. Neurosci Lett. 1992 Mar 16;137(1):41–44. doi: 10.1016/0304-3940(92)90293-g. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Ho S. Probabilistic secretion of quanta from nerve terminals in avian ciliary ganglia modulated by adenosine. J Physiol. 1991;440:513–527. doi: 10.1113/jphysiol.1991.sp018722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Kerr R., Khurana G. Adenosine modulation of calcium currents in postganglionic neurones of avian cultured ciliary ganglia. Br J Pharmacol. 1992 May;106(1):25–32. doi: 10.1111/j.1476-5381.1992.tb14287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Kerr R., Nichol K. Adenosine modulation of potassium currents in postganglionic neurones of cultured avian ciliary ganglia. Br J Pharmacol. 1991 Oct;104(2):459–465. doi: 10.1111/j.1476-5381.1991.tb12451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide, a novel neuronal messenger. Neuron. 1992 Jan;8(1):3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- Böhme G. A., Bon C., Stutzmann J. M., Doble A., Blanchard J. C. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol. 1991 Jul 9;199(3):379–381. doi: 10.1016/0014-2999(91)90505-k. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):462–470. doi: 10.1007/BF00497774. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Lincoln T. M. Regulation of intracellular Ca2+ levels in cultured vascular smooth muscle cells. Reduction of Ca2+ by atriopeptin and 8-bromo-cyclic GMP is mediated by cyclic GMP-dependent protein kinase. J Biol Chem. 1989 Jan 15;264(2):1146–1155. [PubMed] [Google Scholar]
- DE LORENZO A. J. The fine structure of synapses in the ciliary ganglion of the chick. J Biophys Biochem Cytol. 1960 Feb;7:31–36. doi: 10.1083/jcb.7.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F. Peptide toxins and potassium channels. Rev Physiol Biochem Pharmacol. 1990;115:93–136. [PubMed] [Google Scholar]
- Dryer S. E., Dourado M. M., Wisgirda M. E. Characteristics of multiple Ca(2+)-activated K+ channels in acutely dissociated chick ciliary-ganglion neurones. J Physiol. 1991 Nov;443:601–627. doi: 10.1113/jphysiol.1991.sp018854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher G. H., Chiappinelli V. A. An inward rectifier is present in presynaptic nerve terminals in the chick ciliary ganglion. Brain Res. 1992 Mar 13;575(1):103–112. doi: 10.1016/0006-8993(92)90429-d. [DOI] [PubMed] [Google Scholar]
- Fox A. P., Nowycky M. C., Tsien R. W. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987 Dec;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola M., Ducreux C., Chagneux H. Ca2(+)-activated K+ current involvement in neuronal function revealed by in situ single-channel analysis in Helix neurones. J Physiol. 1990 Jan;420:73–109. doi: 10.1113/jphysiol.1990.sp017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kaang B. K., Pfaffinger P. J., Grant S. G., Kandel E. R., Furukawa Y. Overexpression of an Aplysia shaker K+ channel gene modifies the electrical properties and synaptic efficacy of identified Aplysia neurons. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1133–1137. doi: 10.1073/pnas.89.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Blockade of Ca-activated K conductance by apamin in rat sympathetic neurones. Br J Pharmacol. 1986 Jan;87(1):225–232. doi: 10.1111/j.1476-5381.1986.tb10175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana G., Bennett M. R. Nitric oxide and arachidonic acid modulation of calcium currents in postganglionic neurones of avian cultured ciliary ganglia. Br J Pharmacol. 1993 Jun;109(2):480–485. doi: 10.1111/j.1476-5381.1993.tb13594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Kumamoto E. Long-term potentiations in vertebrate synapses: a variety of cascades with common subprocesses. Prog Neurobiol. 1990;34(3):197–269. doi: 10.1016/0301-0082(90)90012-6. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Adams P. R. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986 Jun;55(6):1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lancaster B., Pennefather P. Potassium currents evoked by brief depolarizations in bull-frog sympathetic ganglion cells. J Physiol. 1987 Jun;387:519–548. doi: 10.1113/jphysiol.1987.sp016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Oberhauser A., Labarca P., Alvarez O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989;51:385–399. doi: 10.1146/annurev.ph.51.030189.002125. [DOI] [PubMed] [Google Scholar]
- Magliola L., Jones A. W. Sodium nitroprusside alters Ca2+ flux components and Ca2(+)-dependent fluxes of K+ and Cl- in rat aorta. J Physiol. 1990 Feb;421:411–424. doi: 10.1113/jphysiol.1990.sp017952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. J., Brown D. A. Potassium currents contributing to action potential repolarization in dissociated cultured rat superior cervical sympathetic neurones. Neurosci Lett. 1991 Dec 9;133(2):298–302. doi: 10.1016/0304-3940(91)90593-i. [DOI] [PubMed] [Google Scholar]
- Nelson T. J., Collin C., Alkon D. L. Isolation of a G protein that is modified by learning and reduces potassium currents in Hermissenda. Science. 1990 Mar 23;247(4949 Pt 1):1479–1483. doi: 10.1126/science.247.4949.1479. [DOI] [PubMed] [Google Scholar]
- Roberts W. M., Jacobs R. A., Hudspeth A. J. Colocalization of ion channels involved in frequency selectivity and synaptic transmission at presynaptic active zones of hair cells. J Neurosci. 1990 Nov;10(11):3664–3684. doi: 10.1523/JNEUROSCI.10-11-03664.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels on a cholinergic presynaptic nerve terminal. Neuron. 1991 Oct;7(4):585–591. doi: 10.1016/0896-6273(91)90371-6. [DOI] [PubMed] [Google Scholar]
- Storm J. F. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987 Apr;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Minota S., Kuba K., Koyano K., Abe T. Differential effects of apamin on Ca2+-dependent K+ currents in bullfrog sympathetic ganglion cells. Neurosci Lett. 1986 Sep 12;69(3):233–238. doi: 10.1016/0304-3940(86)90485-4. [DOI] [PubMed] [Google Scholar]
- White R. E., Schonbrunn A., Armstrong D. L. Somatostatin stimulates Ca(2+)-activated K+ channels through protein dephosphorylation. Nature. 1991 Jun 13;351(6327):570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Oda M., Ikemoto Y. Kinetics of the Ca(2+)-activated K+ channel in rat hippocampal neurons. Jpn J Physiol. 1991;41(2):297–315. doi: 10.2170/jjphysiol.41.297. [DOI] [PubMed] [Google Scholar]



