Abstract
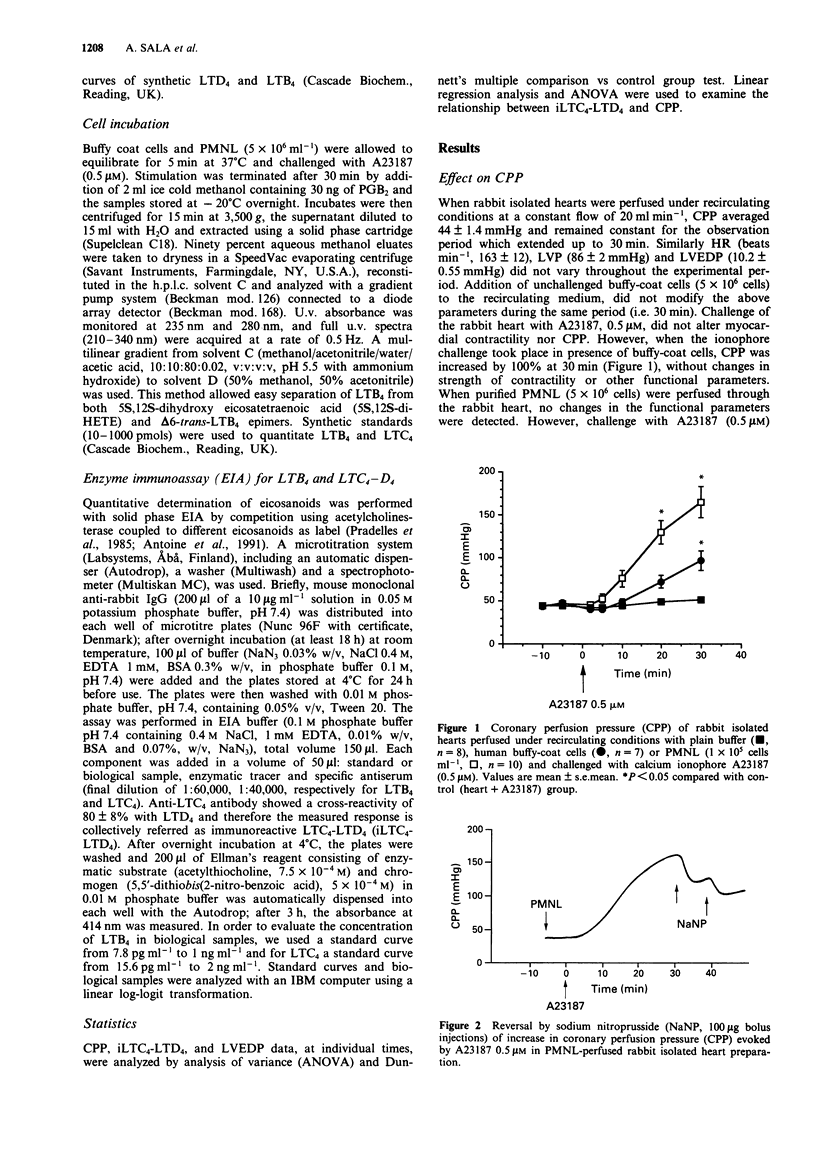
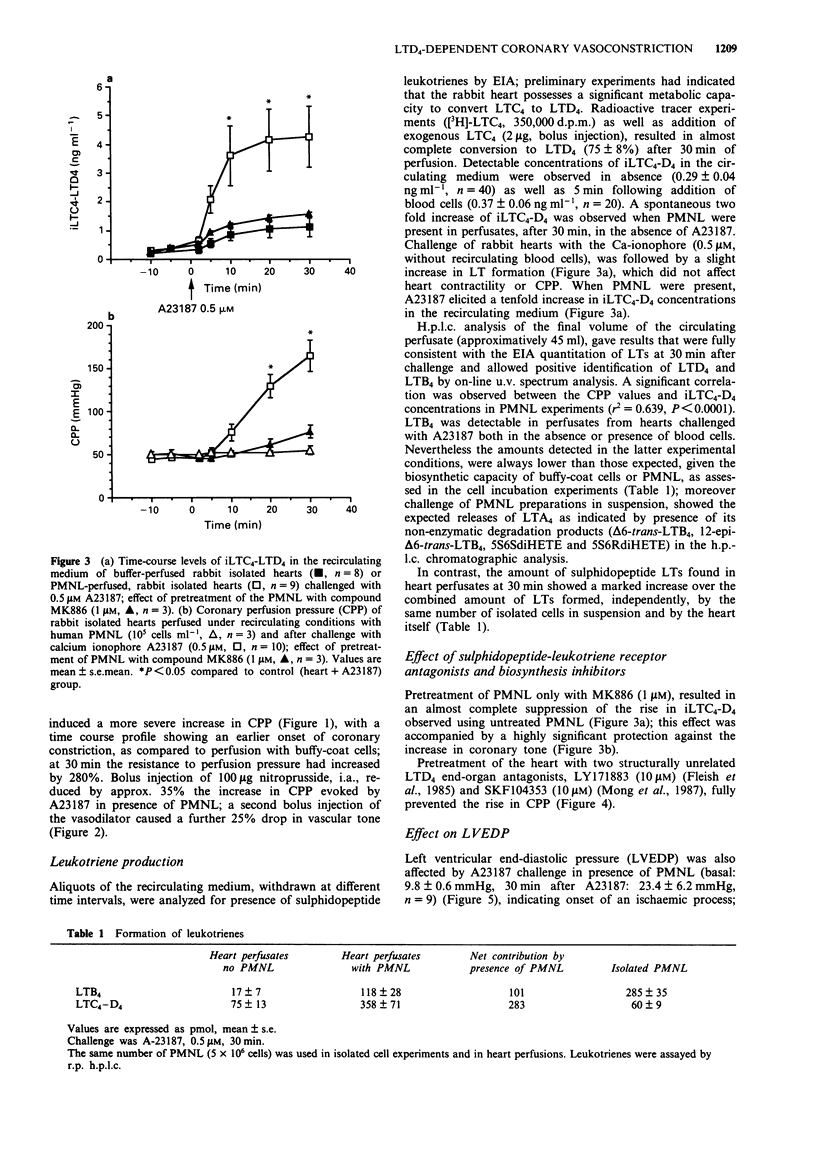
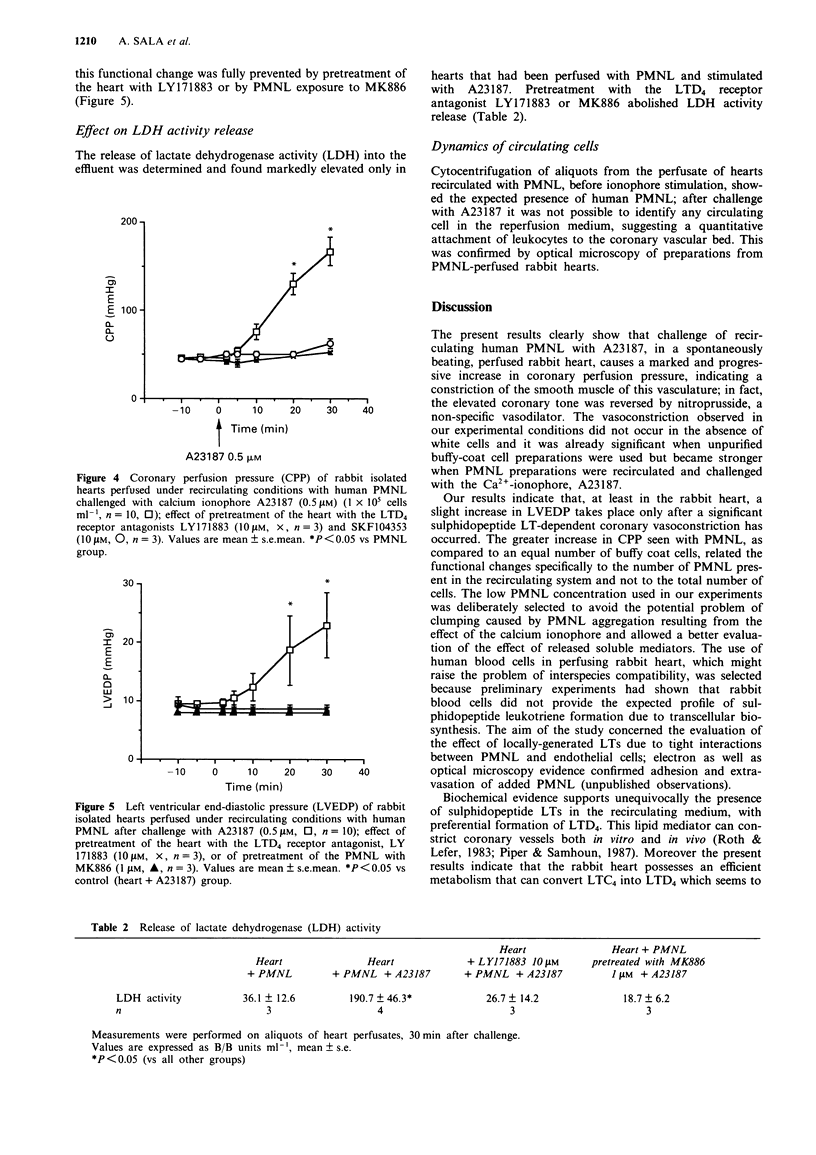
1. We have studied the transcellular biosynthesis of bioactive leukotrienes (LTs), generated upon blood cell-vascular wall interactions and their functional consequences, in the spontaneously beating, cell-perfused, heart of the rabbit. Rabbit isolated hearts were perfused under recirculating conditions (50 ml) with 5 x 10(6) cells of unpurified (buffy coat) or purified human neutrophils (PMNL), and challenged with 0.5 microM A23187 for 30 min. Coronary perfusion pressure (CPP), heart rate (HR), left ventricular end-diastolic pressure (LVEDP) and left ventricular pressure (LVP) were monitored continuously. Leukotriene formation was measured by specific enzyme-immunoassay and confirmed by reversed phase h.p.l.c. and u.v. spectral analysis. 2. Basal CPP values averaged 44 +/- 1.4 mmHg; A23187 triggered a marked increase in CPP both in the presence of buffy coat cells (+100% above basal) and PMNL (+270% above basal); the latter change in CPP was accompanied by a rise in LVEDP (+138% above basal). 3. The increase in CPP was preceded by a statistically significant rise in iLTC4-D4 concentration in the circulating buffer. Pretreatment with two structurally unrelated LTD4 receptor antagonists, LY171883 and SKF104353 (10 microM), fully prevented the increase in CPP and LVEDP. A similar protection was also observed when the rabbit heart was perfused with PMNL that had been pretreated with MK886 (1 microM), a potent inhibitor of leukotriene biosynthesis. 4. The increased coronary tone was accompanied by a marked release of lactate dehydrogenase (LDH), a marker of ischaemic damage; pretreatment of the heart with the LTD4 receptor antagonists as well as of the PMNL with MK886 resulted in a complete suppression of LDH activity release.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoine C., Lellouche J. P., Maclouf J., Pradelles P. Development of enzyme immunoassays for leukotrienes using acetylcholinesterase. Biochim Biophys Acta. 1991 Oct 10;1075(2):162–168. doi: 10.1016/0304-4165(91)90247-e. [DOI] [PubMed] [Google Scholar]
- Berti F., Rossoni G., Magni F., Caruso D., Omini C., Puglisi L., Galli G. Nonsteroidal antiinflammatory drugs aggravate acute myocardial ischemia in the perfused rabbit heart: a role for prostacyclin. J Cardiovasc Pharmacol. 1988 Oct;12(4):438–444. doi: 10.1097/00005344-198810000-00009. [DOI] [PubMed] [Google Scholar]
- Borgeat P., Samuelsson B. Arachidonic acid metabolism in polymorphonuclear leukocytes: effects of ionophore A23187. Proc Natl Acad Sci U S A. 1979 May;76(5):2148–2152. doi: 10.1073/pnas.76.5.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carry M., Korley V., Willerson J. T., Weigelt L., Ford-Hutchinson A. W., Tagari P. Increased urinary leukotriene excretion in patients with cardiac ischemia. In vivo evidence for 5-lipoxygenase activation. Circulation. 1992 Jan;85(1):230–236. doi: 10.1161/01.cir.85.1.230. [DOI] [PubMed] [Google Scholar]
- Evers A. S., Murphree S., Saffitz J. E., Jakschik B. A., Needleman P. Effects of endogenously produced leukotrienes, thromboxane, and prostaglandins on coronary vascular resistance in rabbit myocardial infarction. J Clin Invest. 1985 Mar;75(3):992–999. doi: 10.1172/JCI111801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinmark S. J., Cannon P. J. Endothelial cell leukotriene C4 synthesis results from intercellular transfer of leukotriene A4 synthesized by polymorphonuclear leukocytes. J Biol Chem. 1986 Dec 15;261(35):16466–16472. [PubMed] [Google Scholar]
- Feinmark S. J., Cannon P. J. Vascular smooth muscle cell leukotriene C4 synthesis: requirement for transcellular leukotriene A4 metabolism. Biochim Biophys Acta. 1987 Nov 21;922(2):125–135. doi: 10.1016/0005-2760(87)90146-9. [DOI] [PubMed] [Google Scholar]
- Feuerstein G. Cardiac and vascular effects of leukotrienes. Adv Prostaglandin Thromboxane Leukot Res. 1986;16:299–308. [PubMed] [Google Scholar]
- Fleisch J. H., Rinkema L. E., Haisch K. D., Swanson-Bean D., Goodson T., Ho P. P., Marshall W. S. LY171883, 1-less than 2-hydroxy-3-propyl-4-less than 4-(1H-tetrazol-5-yl) butoxy greater than phenyl greater than ethanone, an orally active leukotriene D4 antagonist. J Pharmacol Exp Ther. 1985 Apr;233(1):148–157. [PubMed] [Google Scholar]
- Gillard J., Ford-Hutchinson A. W., Chan C., Charleson S., Denis D., Foster A., Fortin R., Leger S., McFarlane C. S., Morton H. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- Grimminger F., Kreusler B., Schneider U., Becker G., Seeger W. Influence of microvascular adherence on neutrophil leukotriene generation. Evidence for cooperative eicosanoid synthesis. J Immunol. 1990 Mar 1;144(5):1866–1872. [PubMed] [Google Scholar]
- Haslett C., Guthrie L. A., Kopaniak M. M., Johnston R. B., Jr, Henson P. M. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985 Apr;119(1):101–110. [PMC free article] [PubMed] [Google Scholar]
- Hock C. E., Beck L. D., Papa L. A. Peptide leukotriene receptor antagonism in myocardial ischaemia and reperfusion. Cardiovasc Res. 1992 Dec;26(12):1206–1211. doi: 10.1093/cvr/26.12.1206. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Johnston G. I., Bliss G. A., Newman P. J., McEver R. P. Structure of the human gene encoding granule membrane protein-140, a member of the selectin family of adhesion receptors for leukocytes. J Biol Chem. 1990 Dec 5;265(34):21381–21385. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi B. R., Mullane K. M. Leukocytes and ischemia-induced myocardial injury. Annu Rev Pharmacol Toxicol. 1986;26:201–224. doi: 10.1146/annurev.pa.26.040186.001221. [DOI] [PubMed] [Google Scholar]
- Maclouf J. A., Murphy R. C. Transcellular metabolism of neutrophil-derived leukotriene A4 by human platelets. A potential cellular source of leukotriene C4. J Biol Chem. 1988 Jan 5;263(1):174–181. [PubMed] [Google Scholar]
- Maclouf J., Murphy R. C., Henson P. M. Transcellular sulfidopeptide leukotriene biosynthetic capacity of vascular cells. Blood. 1989 Aug 1;74(2):703–707. [PubMed] [Google Scholar]
- Marcus A. J., Broekman M. J., Safier L. B., Ullman H. L., Islam N., Sherhan C. N., Rutherford L. E., Korchak H. M., Weissmann G. Formation of leukotrienes and other hydroxy acids during platelet-neutrophil interactions in vitro. Biochem Biophys Res Commun. 1982 Nov 16;109(1):130–137. doi: 10.1016/0006-291x(82)91575-3. [DOI] [PubMed] [Google Scholar]
- McGee J. E., Fitzpatrick F. A. Erythrocyte-neutrophil interactions: formation of leukotriene B4 by transcellular biosynthesis. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1349–1353. doi: 10.1073/pnas.83.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelassi F., Landa L., Hill R. D., Lowenstein E., Watkins W. D., Petkau A. J., Zapol W. M. Leukotriene D4: a potent coronary artery vasoconstrictor associated with impaired ventricular contraction. Science. 1982 Aug 27;217(4562):841–843. doi: 10.1126/science.6808665. [DOI] [PubMed] [Google Scholar]
- Mong S., Wu H. L., Miller J., Hall R. F., Gleason J. G., Crooke S. T. SKF 104353, a high affinity antagonist for human and guinea pig lung leukotriene D4 receptor, blocked phosphatidylinositol metabolism and thromboxane synthesis induced by leukotriene D4. Mol Pharmacol. 1987 Aug;32(1):223–229. [PubMed] [Google Scholar]
- Piper P. J., Samhoun M. N. Leukotrienes. Br Med Bull. 1987 Apr;43(2):297–311. doi: 10.1093/oxfordjournals.bmb.a072184. [DOI] [PubMed] [Google Scholar]
- Pradelles P., Grassi J., Maclouf J. Enzyme immunoassays of eicosanoids using acetylcholine esterase as label: an alternative to radioimmunoassay. Anal Chem. 1985 Jun;57(7):1170–1173. doi: 10.1021/ac00284a003. [DOI] [PubMed] [Google Scholar]
- Roth D. M., Lefer A. M. Studies on the mechanism of leukotriene induced coronary artery constriction. Prostaglandins. 1983 Oct;26(4):573–581. doi: 10.1016/0090-6980(83)90195-8. [DOI] [PubMed] [Google Scholar]
- Voelkel N. F., Czartolomna J., Simpson J., Murphy R. C. FMLP causes eicosanoid-dependent vasoconstriction and edema in lungs from endotoxin-primed rats. Am Rev Respir Dis. 1992 Mar;145(3):701–711. doi: 10.1164/ajrccm/145.3.701. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Lee C. W., Foster D. W., Corey E. J., Austen K. F., Lewis R. A. Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C4. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7626–7630. doi: 10.1073/pnas.80.24.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]


