Abstract
1. The effect of endotoxin (E. coli lipopolysaccharide) on the induction of nitric oxide synthase (NOS) and the changes in vascular permeability in the colon and jejunum over a 5 h period have been investigated in the rat. 2. Under resting conditions, a calcium-dependent constitutive NOS, determined by the conversion of radiolabelled L-arginine to citrulline, was detected in homogenates of both colonic and jejunal tissue. 3. Administration of endotoxin (3 mg kg-1, i.v.) led, after a 2 h lag period, to the appearance of calcium-independent NOS activity in the colon and jejunum ex vivo, characteristic of the inducible NOS enzyme. 4. Administration of endotoxin led to an increase in colonic and jejunal vascular permeability after a lag period of 3 h, determined by the leakage of radiolabelled albumin. 5. Pretreatment with dexamethasone (1 mg kg-1 s.c., 2 h prior to challenge) inhibited both the induction of NOS and the vascular leakage induced by endotoxin. 6. Administration of the NO synthase inhibitor NG-monomethyl-L-arginine (12.5-50 mg kg-1, s.c.) 3 h after endotoxin injection, dose-dependently reduced the subsequent increase in vascular permeability in jejunum and colon, an effect reversed by L-arginine (300 mg kg-1, s.c.). 7. These findings suggest that induction of NOS is associated with the vascular injury induced by endotoxin in the rat colon and jejunum.
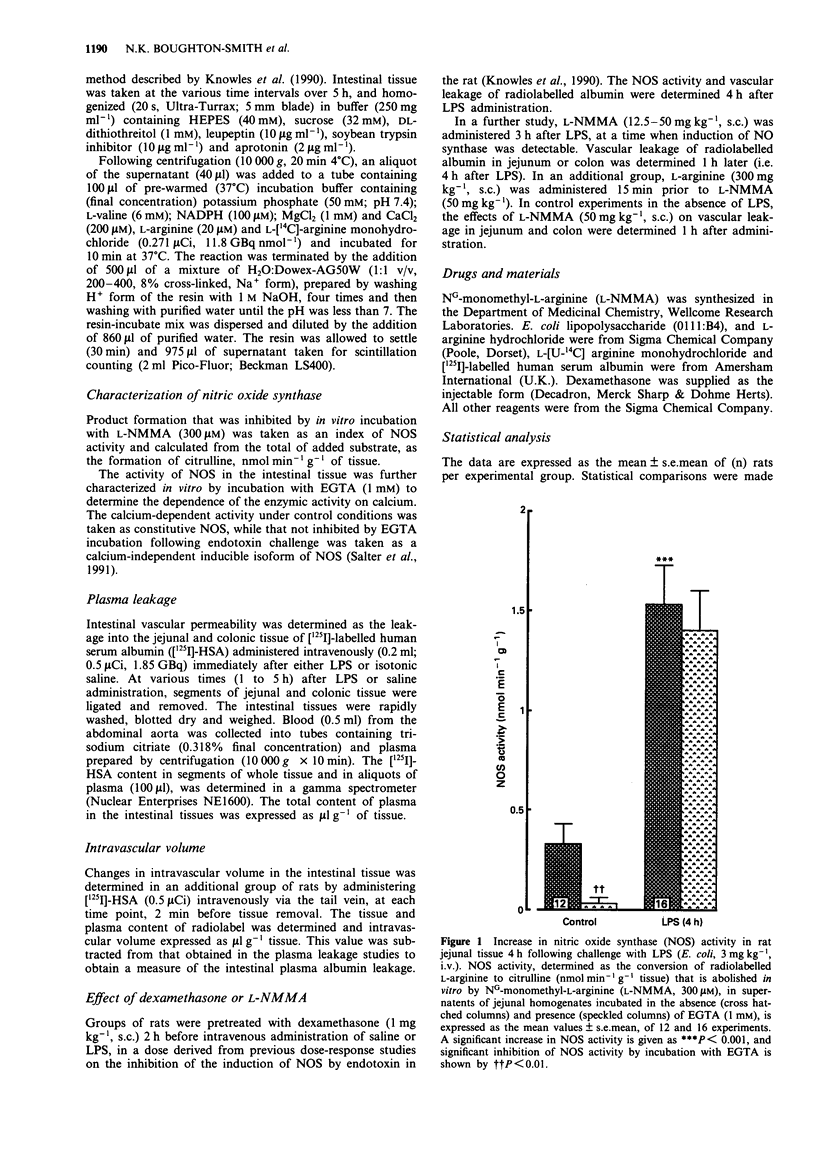
Full text
PDF
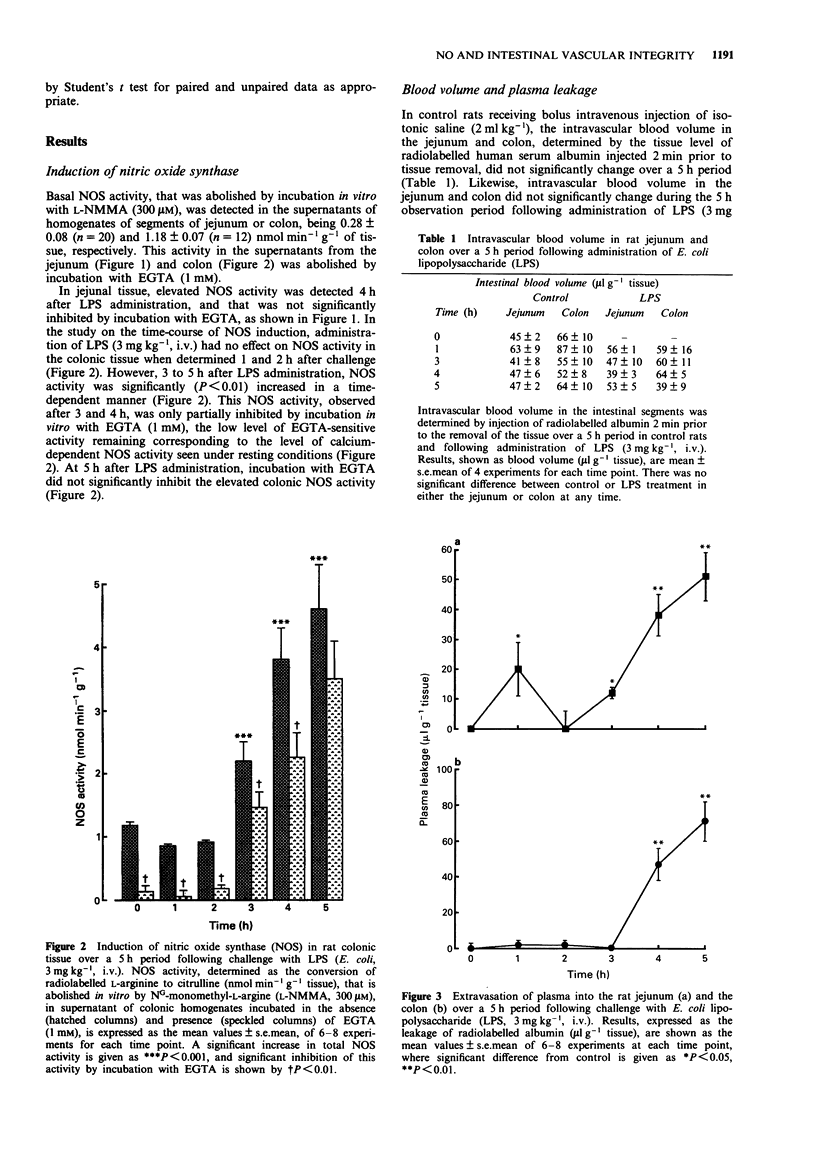



Selected References
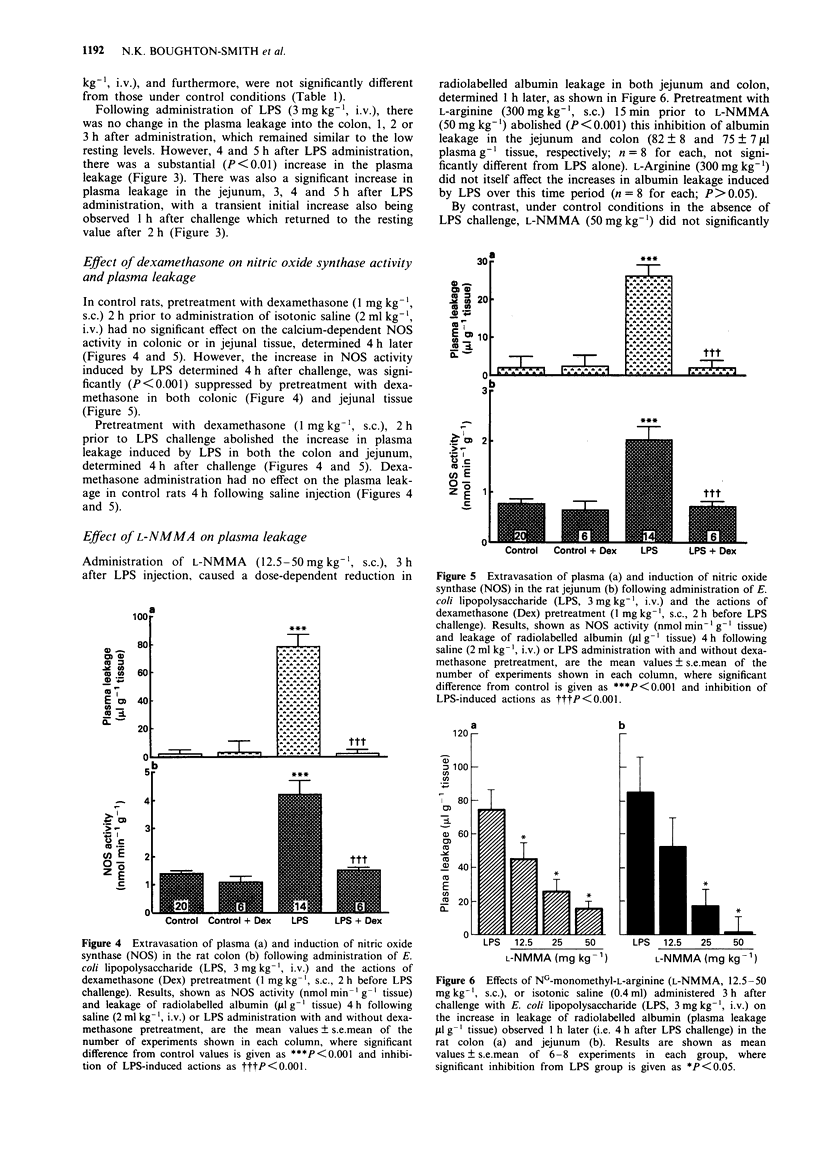
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Parente L., Persico P. Macrocortin: a polypeptide causing the anti-phospholipase effect of glucocorticoids. Nature. 1980 Sep 11;287(5778):147–149. doi: 10.1038/287147a0. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Hutcheson I. R., Deakin A. M., Whittle B. J., Moncada S. Protective effect of S-nitroso-N-acetyl-penicillamine in endotoxin-induced acute intestinal damage in the rat. Eur J Pharmacol. 1990 Dec 4;191(3):485–488. doi: 10.1016/0014-2999(90)94185-z. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith N. K., Hutcheson I., Whittle B. J. Relationship between PAF-acether and thromboxane A2 biosynthesis in endotoxin-induced intestinal damage in the rat. Prostaglandins. 1989 Sep;38(3):319–333. doi: 10.1016/0090-6980(89)90136-6. [DOI] [PubMed] [Google Scholar]
- Busse R., Mülsch A. Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett. 1990 Nov 26;275(1-2):87–90. doi: 10.1016/0014-5793(90)81445-t. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Radomski M., Carnuccio R., Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Fleming I., Julou-Schaeffer G., Gray G. A., Parratt J. R., Stoclet J. C. Evidence that an L-arginine/nitric oxide dependent elevation of tissue cyclic GMP content is involved in depression of vascular reactivity by endotoxin. Br J Pharmacol. 1991 May;103(1):1047–1052. doi: 10.1111/j.1476-5381.1991.tb12298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förstermann U., Pollock J. S., Schmidt H. H., Heller M., Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. R., Williams T. J., Brain S. D. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990 Dec 4;191(3):481–484. doi: 10.1016/0014-2999(90)94184-y. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Whittle B. J., Boughton-Smith N. K. Role of nitric oxide in maintaining vascular integrity in endotoxin-induced acute intestinal damage in the rat. Br J Pharmacol. 1990 Dec;101(4):815–820. doi: 10.1111/j.1476-5381.1990.tb14163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ialenti A., Ianaro A., Moncada S., Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992 Feb 11;211(2):177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- Kern J. A., Lamb R. J., Reed J. C., Daniele R. P., Nowell P. C. Dexamethasone inhibition of interleukin 1 beta production by human monocytes. Posttranscriptional mechanisms. J Clin Invest. 1988 Jan;81(1):237–244. doi: 10.1172/JCI113301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn R. G., Jubran A., Gross S. S., Griffith O. W., Levi R., Adams J., Lodato R. F. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Middleton S. J., Shorthouse M., Hunter J. O. Increased nitric oxide synthesis in ulcerative colitis. Lancet. 1993 Feb 20;341(8843):465–466. doi: 10.1016/0140-6736(93)90211-x. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Sadowska-Krowicka H., Chotinaruemol S., Kakkis J. L., Clark D. A. Amelioration of chronic ileitis by nitric oxide synthase inhibition. J Pharmacol Exp Ther. 1993 Jan;264(1):11–16. [PubMed] [Google Scholar]
- Mitchell J. A., Sheng H., Förstermann U., Murad F. Characterization of nitric oxide synthases in non-adrenergic non-cholinergic nerve containing tissue from the rat anococcygeus muscle. Br J Pharmacol. 1991 Oct;104(2):289–291. doi: 10.1111/j.1476-5381.1991.tb12422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka K., Funakoshi A., Shikado F., Kitani K. Stimulatory and inhibitory effects of bile salts on rat pancreatic secretion. Gastroenterology. 1992 Feb;102(2):598–604. doi: 10.1016/0016-5085(92)90108-b. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nava E., Palmer R. M., Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet. 1991 Dec 21;338(8782-8783):1555–1557. doi: 10.1016/0140-6736(91)92375-c. [DOI] [PubMed] [Google Scholar]
- Nishida K., Harrison D. G., Navas J. P., Fisher A. A., Dockery S. P., Uematsu M., Nerem R. M., Alexander R. W., Murphy T. J. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. J Clin Invest. 1992 Nov;90(5):2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor K. J., Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase and the related cell damage in adenocarcinoma cells. Biochim Biophys Acta. 1991 Oct 21;1097(3):227–231. doi: 10.1016/0925-4439(91)90040-g. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Bridge L., Foxwell N. A., Moncada S. The role of nitric oxide in endothelial cell damage and its inhibition by glucocorticoids. Br J Pharmacol. 1992 Jan;105(1):11–12. doi: 10.1111/j.1476-5381.1992.tb14202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983 Aug;245(2):G285–G289. doi: 10.1152/ajpgi.1983.245.2.G285. [DOI] [PubMed] [Google Scholar]
- Petros A., Bennett D., Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991 Dec 21;338(8782-8783):1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- Pique J. M., Esplugues J. V., Whittle B. J. Endogenous nitric oxide as a mediator of gastric mucosal vasodilatation during acid secretion. Gastroenterology. 1992 Jan;102(1):168–174. doi: 10.1016/0016-5085(92)91797-8. [DOI] [PubMed] [Google Scholar]
- Pique J. M., Whittle B. J., Esplugues J. V. The vasodilator role of endogenous nitric oxide in the rat gastric microcirculation. Eur J Pharmacol. 1989 Dec 19;174(2-3):293–296. doi: 10.1016/0014-2999(89)90324-5. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Waage A., Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide-stimulated human monocytes. Immunology. 1988 Feb;63(2):299–302. [PMC free article] [PubMed] [Google Scholar]
- Walder C. E., Thiemermann C., Vane J. R. Endothelium-derived relaxing factor participates in the increased blood flow in response to pentagastrin in the rat stomach mucosa. Proc Biol Sci. 1990 Sep 22;241(1302):195–200. doi: 10.1098/rspb.1990.0085. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Steel G., Whittle B. J., Lagente V., Vargaftig B. Evidence for platelet-activating factor as a mediator of endotoxin-induced gastrointestinal damage in the rat. Effects of three platelet-activating factor antagonists. Gastroenterology. 1987 Oct;93(4):765–773. doi: 10.1016/0016-5085(87)90438-0. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Coughlan M. L., Williams T. J. Endotoxin-induced vasodilatation in anaesthetized rat skin involves nitric oxide and prostaglandin synthesis. Br J Pharmacol. 1992 Aug;106(4):953–957. doi: 10.1111/j.1476-5381.1992.tb14441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B. J., Boughton-Smith N. K., Hutcheson I. R., Esplugues J. V., Wallace J. L. Increased intestinal formation of Paf in endotoxin-induced damage in the rat. Br J Pharmacol. 1987 Sep;92(1):3–4. doi: 10.1111/j.1476-5381.1987.tb11287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Moncada S. Regulation of gastric mucosal integrity by endogenous nitric oxide: interactions with prostanoids and sensory neuropeptides in the rat. Br J Pharmacol. 1990 Mar;99(3):607–611. doi: 10.1111/j.1476-5381.1990.tb12977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- de Belder A. J., Radomski M. W., Why H. J., Richardson P. J., Bucknall C. A., Salas E., Martin J. F., Moncada S. Nitric oxide synthase activities in human myocardium. Lancet. 1993 Jan 9;341(8837):84–85. doi: 10.1016/0140-6736(93)92559-c. [DOI] [PubMed] [Google Scholar]


