Abstract
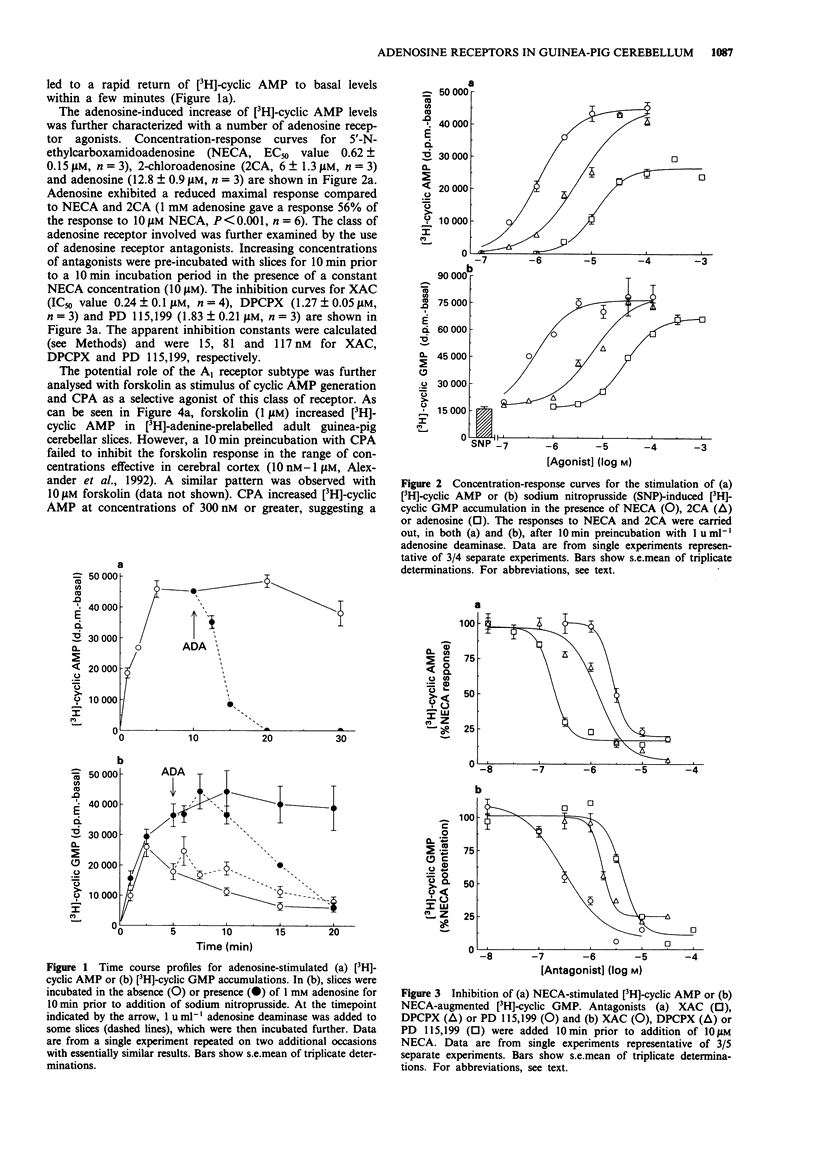
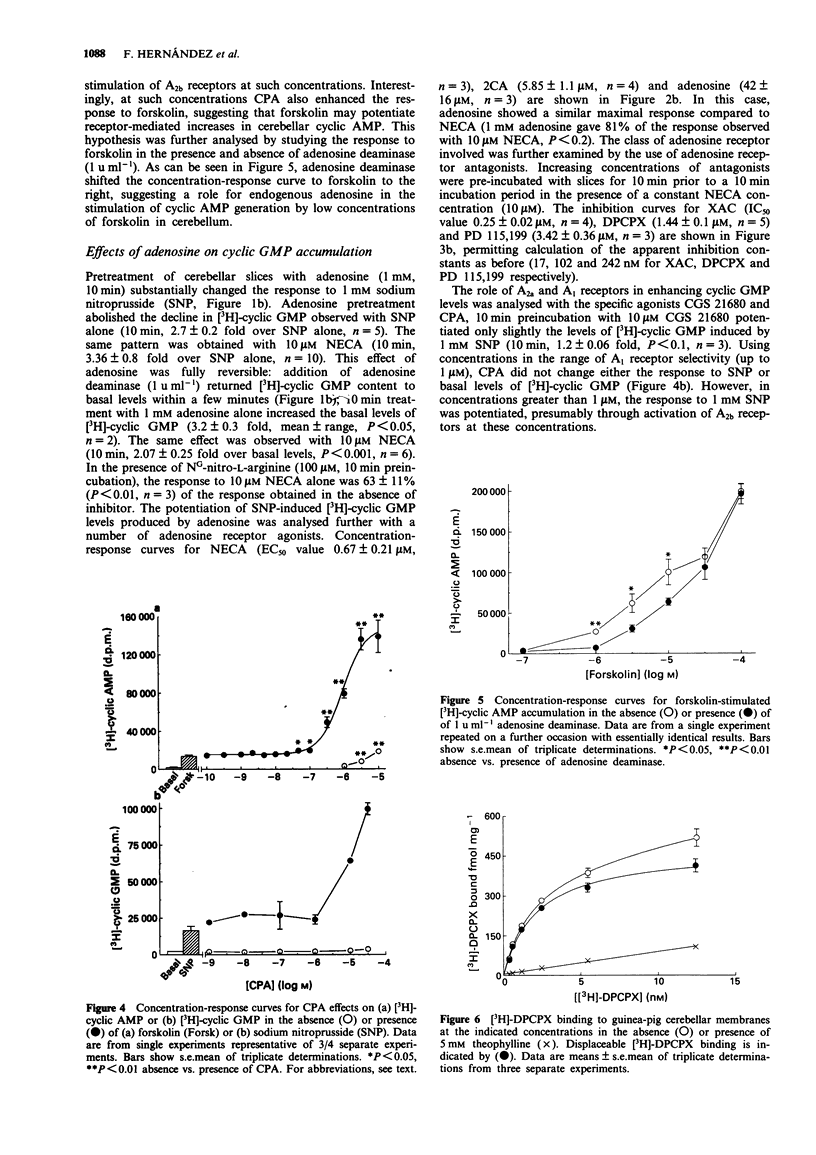
1. The effects of adenosine receptor agonists on cyclic nucleotides accumulation were investigated in adult guinea-pig cerebellar slices by use of radioactive precursors. 2. Adenosine elicited a rapid and maintained increase in cyclic AMP, that was fully reversed upon addition of adenosine deaminase. Adenosine analogues stimulated cyclic AMP generation up to 40 fold with the rank order of potency: 5'-N-ethylcarboxamidoadenosine (0.6 microM) > 2-chloroadenosine (6 microM) > adenosine (13 microM). CGS 21680 (10 microM) elicited only a small stimulation (1.2 fold). 3. The cyclic AMP response to NECA was reversed by the 1,3-dipropylxanthine-based adenosine receptor antagonists 8-[4-[[[[(2-aminoethyl)amino]amino]carbonyl]methyl]oxy]- phenyl]-1,3-dipropylxanthine (XAC), 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) and N-[2-(dimethylamino)ethyl]N-methyl-4-(1,3-dipropylxanthine)benzene sulphonamide (PD 115,199) with estimated apparent inhibition constants of 15, 81 and 117 nM, respectively. 4. Pretreatment with adenosine also potentiated the cyclic GMP response to sodium nitroprusside, abolishing the decline in [3H]-cyclic GMP observed with sodium nitroprusside alone, and allowing [3H]-cyclic GMP levels to be maintained for at least an additional 10 min. This potentiation was fully reversed by adenosine deaminase. 5. Adenosine analogues potentiated the sodium nitroprusside-elicited cyclic GMP generation with the rank order of potency: 5'-N-ethylcarboxamidoadenosine (0.7 microM) > 2-chloroadenosine (6 microM) > adenosine (42 microM). 6. NECA potentiation of cyclic GMP formation was reversed by the antagonists XAC, DPCPX and PD 115,199 with apparent inhibition constants of 17, 102 and 242 nM, respectively.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. P., Kendall D. A., Hill S. J. Differences in the adenosine receptors modulating inositol phosphates and cyclic AMP accumulation in mammalian cerebral cortex. Br J Pharmacol. 1989 Dec;98(4):1241–1248. doi: 10.1111/j.1476-5381.1989.tb12670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazil C. W., Minneman K. P. An investigation of the low intrinsic activity of adenosine and its analogs at low affinity (A2) adenosine receptors in rat cerebral cortex. J Neurochem. 1986 Aug;47(2):547–553. doi: 10.1111/j.1471-4159.1986.tb04534.x. [DOI] [PubMed] [Google Scholar]
- Braas K. M., Newby A. C., Wilson V. S., Snyder S. H. Adenosine-containing neurons in the brain localized by immunocytochemistry. J Neurosci. 1986 Jul;6(7):1952–1961. doi: 10.1523/JNEUROSCI.06-07-01952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hartman J. D., Hays S. J., Huang C. C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hays S. J. PD 115,199: an antagonist ligand for adenosine A2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):64–69. doi: 10.1007/BF00165038. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Lu G. H., Pugsley T. A. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986 Apr;29(4):331–346. [PubMed] [Google Scholar]
- Daly J. W., Butts-Lamb P., Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983 Mar;3(1):69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLapp N. W., Eckols K. Forskolin stimulation of cyclic AMP accumulation in rat brain cortex slices is markedly enhanced by endogenous adenosine. J Neurochem. 1992 Jan;58(1):237–242. doi: 10.1111/j.1471-4159.1992.tb09301.x. [DOI] [PubMed] [Google Scholar]
- Donaldson J., Kendall D. A., Hill S. J. Discriminatory effects of forskolin and EGTA on the indirect cyclic AMP responses to histamine, noradrenaline, 5-hydroxytryptamine, and glutamate in guinea-pig cerebral cortical slices. J Neurochem. 1990 May;54(5):1484–1491. doi: 10.1111/j.1471-4159.1990.tb01195.x. [DOI] [PubMed] [Google Scholar]
- Ferrendelli J. A., Kinscherf D. A., Chang M. M. Regulation of levels of guanosine cyclic 3',5'-monophosphate in the central nervous system: effects of depolarizing agents. Mol Pharmacol. 1973 Jul;9(4):445–454. [PubMed] [Google Scholar]
- Fredholm B. B. Activation of adenylate cyclase from rat striatum and tuberculum olfactorium by adenosine. Med Biol. 1977 Oct;55(5):262–267. [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988 Apr;9(4):130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Kuhar M. J., Hester L., Snyder S. H. Adenosine receptors: autoradiographic evidence for their location on axon terminals of excitatory neurons. Science. 1983 May 27;220(4600):967–969. doi: 10.1126/science.6302841. [DOI] [PubMed] [Google Scholar]
- Hill S. J., Kendall D. A. Studies on the adenosine-receptor mediating the augmentation of histamine-induced inositol phospholipid hydrolysis in guinea-pig cerebral cortex. Br J Pharmacol. 1987 Jul;91(3):661–669. doi: 10.1111/j.1476-5381.1987.tb11260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., Kirk K. L., Padgett W. L., Daly J. W. Functionalized congeners of 1,3-dialkylxanthines: preparation of analogues with high affinity for adenosine receptors. J Med Chem. 1985 Sep;28(9):1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., Ukena D., Padgett W., Daly J. W., Kirk K. L. Xanthine functionalized congeners as potent ligands at A2-adenosine receptors. J Med Chem. 1987 Jan;30(1):211–214. doi: 10.1021/jm00384a037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. A., van Galen P. J., Williams M. Adenosine receptors: pharmacology, structure-activity relationships, and therapeutic potential. J Med Chem. 1992 Feb 7;35(3):407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M. F., Schulz R., Hutchison A. J., Do U. H., Sills M. A., Williams M. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther. 1989 Dec;251(3):888–893. [PubMed] [Google Scholar]
- Lee K. S., Reddington M. 1,3-Dipropyl-8-cyclopentylxanthine (DPCPX) inhibition of [3H]N-ethylcarboxamidoadenosine (NECA) binding allows the visualization of putative non-A1 adenosine receptors. Brain Res. 1986 Mar 19;368(2):394–398. doi: 10.1016/0006-8993(86)90589-5. [DOI] [PubMed] [Google Scholar]
- Lewis M. E., Patel J., Edley S. M., Marangos P. J. Autoradiographic visualization of rat brain adenosine receptors using N6-cyclohexyl [3H]adenosine. Eur J Pharmacol. 1981 Jul 17;73(1):109–110. doi: 10.1016/0014-2999(81)90155-2. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Lupica C. R., Cass W. A., Zahniser N. R., Dunwiddie T. V. Effects of the selective adenosine A2 receptor agonist CGS 21680 on in vitro electrophysiology, cAMP formation and dopamine release in rat hippocampus and striatum. J Pharmacol Exp Ther. 1990 Mar;252(3):1134–1141. [PubMed] [Google Scholar]
- Murphy K. M., Snyder S. H. Heterogeneity of adenosine A1 receptor binding in brain tissue. Mol Pharmacol. 1982 Sep;22(2):250–257. [PubMed] [Google Scholar]
- Ohga Y., Daly J. W. The accumulation of cyclic AMP and cyclic GMP in guinea pig brain slices. Effect of calcium ions, norepinephrine and adenosine. Biochim Biophys Acta. 1977 Jun 23;498(1):46–60. doi: 10.1016/0304-4165(77)90086-1. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Bredt D., Snyder S. H. Messenger molecules in the cerebellum. Trends Neurosci. 1990 Jun;13(6):216–222. doi: 10.1016/0166-2236(90)90163-5. [DOI] [PubMed] [Google Scholar]
- Saito M. Elevation of guanosine 3',5'-monophosphate level by adenosine in cerebellar slices of guinea pig. Biochim Biophys Acta. 1977 Jul 21;498(1):316–324. doi: 10.1016/0304-4165(77)90269-0. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Wojcik W. J., Neff N. H. Adenosine A1 receptors are associated with cerebellar granule cells. J Neurochem. 1983 Sep;41(3):759–763. doi: 10.1111/j.1471-4159.1983.tb04805.x. [DOI] [PubMed] [Google Scholar]
- de Mendonça A., Ribeiro J. A. 2-Chloroadenosine decreases long-term potentiation in the hippocampal CA1 area of the rat. Neurosci Lett. 1990 Oct 2;118(1):107–111. doi: 10.1016/0304-3940(90)90260-g. [DOI] [PubMed] [Google Scholar]
- van Galen P. J., Stiles G. L., Michaels G., Jacobson K. A. Adenosine A1 and A2 receptors: structure--function relationships. Med Res Rev. 1992 Sep;12(5):423–471. doi: 10.1002/med.2610120502. [DOI] [PMC free article] [PubMed] [Google Scholar]


