Abstract
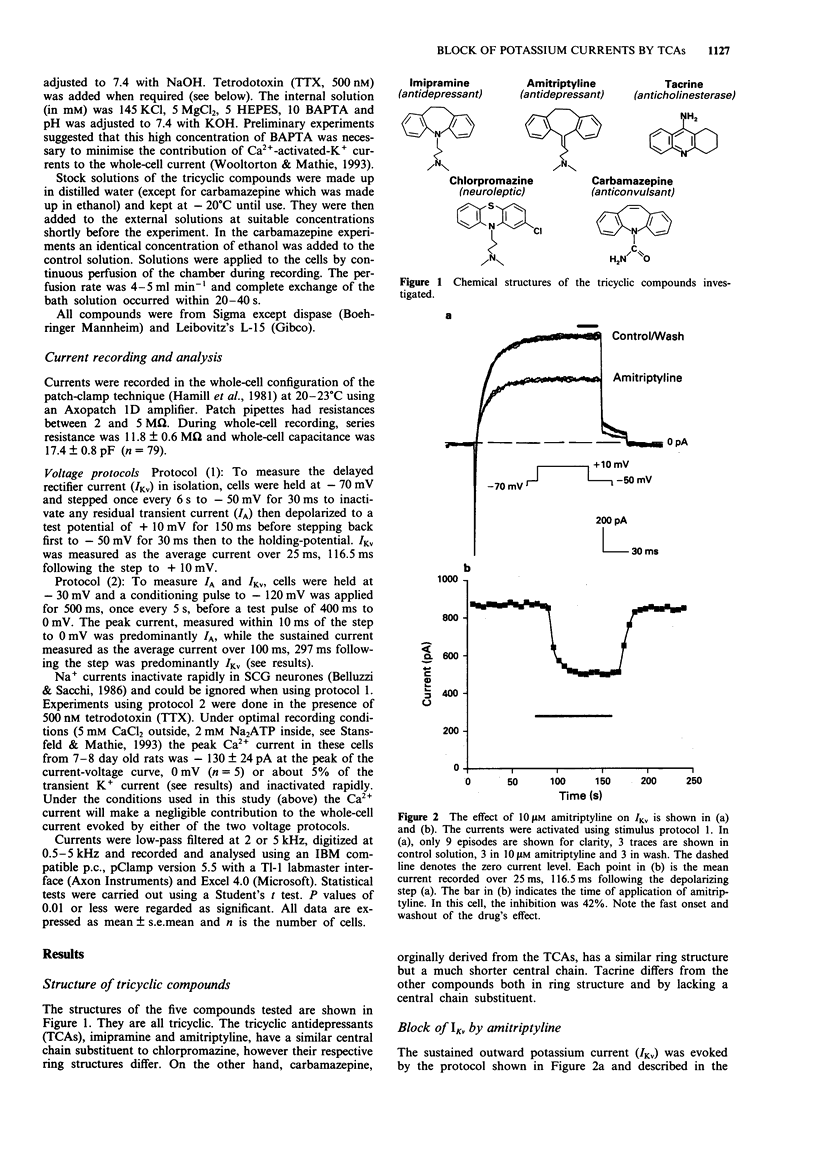
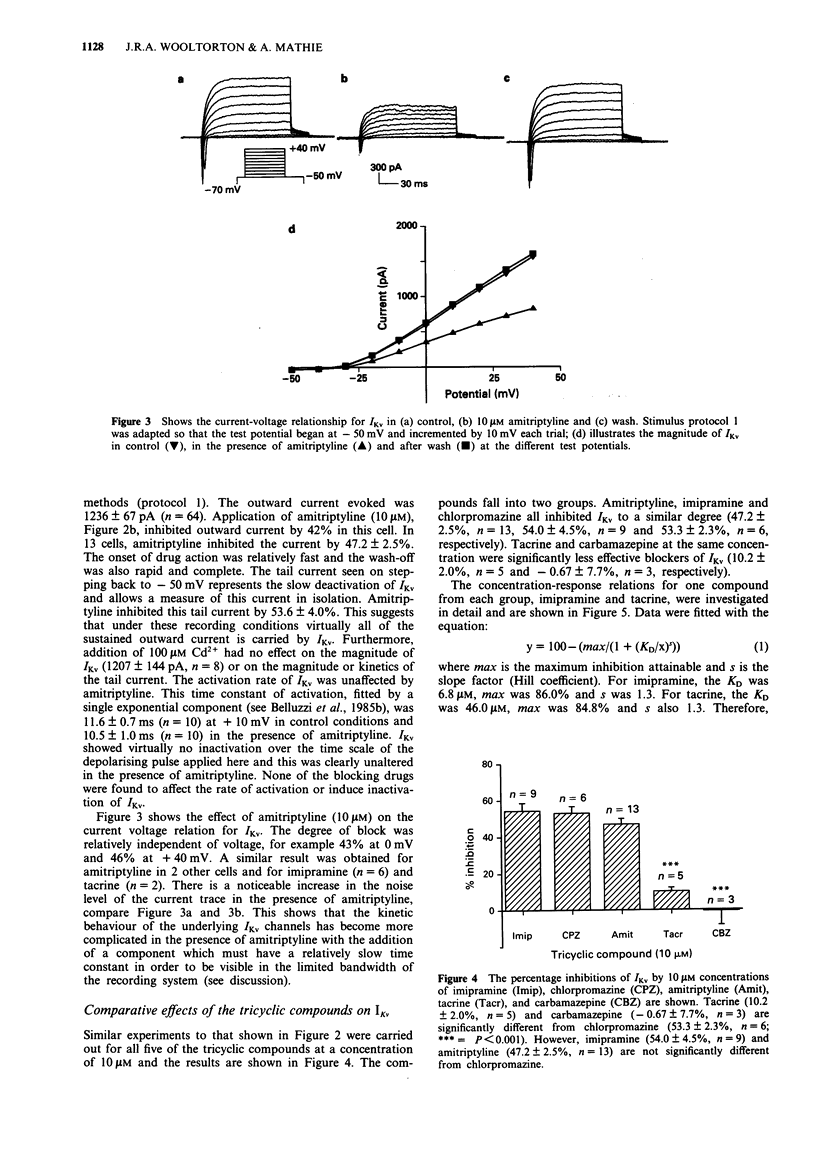
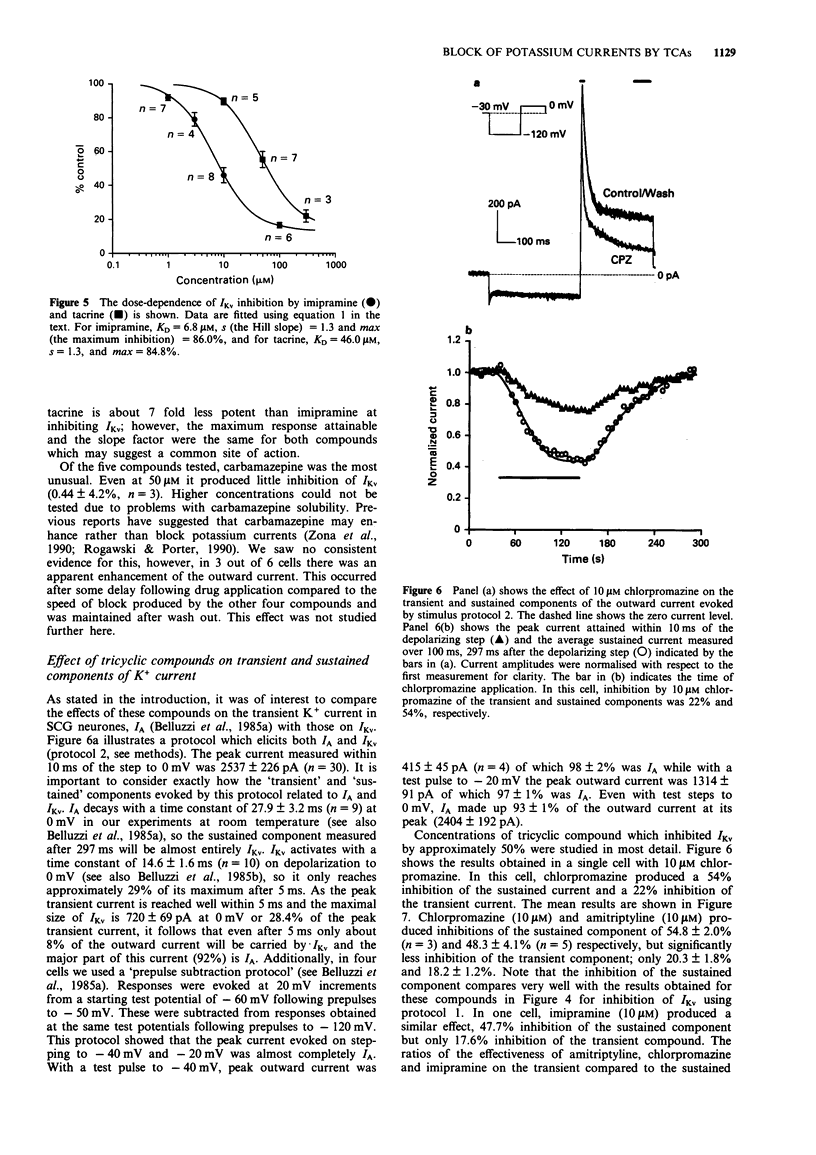
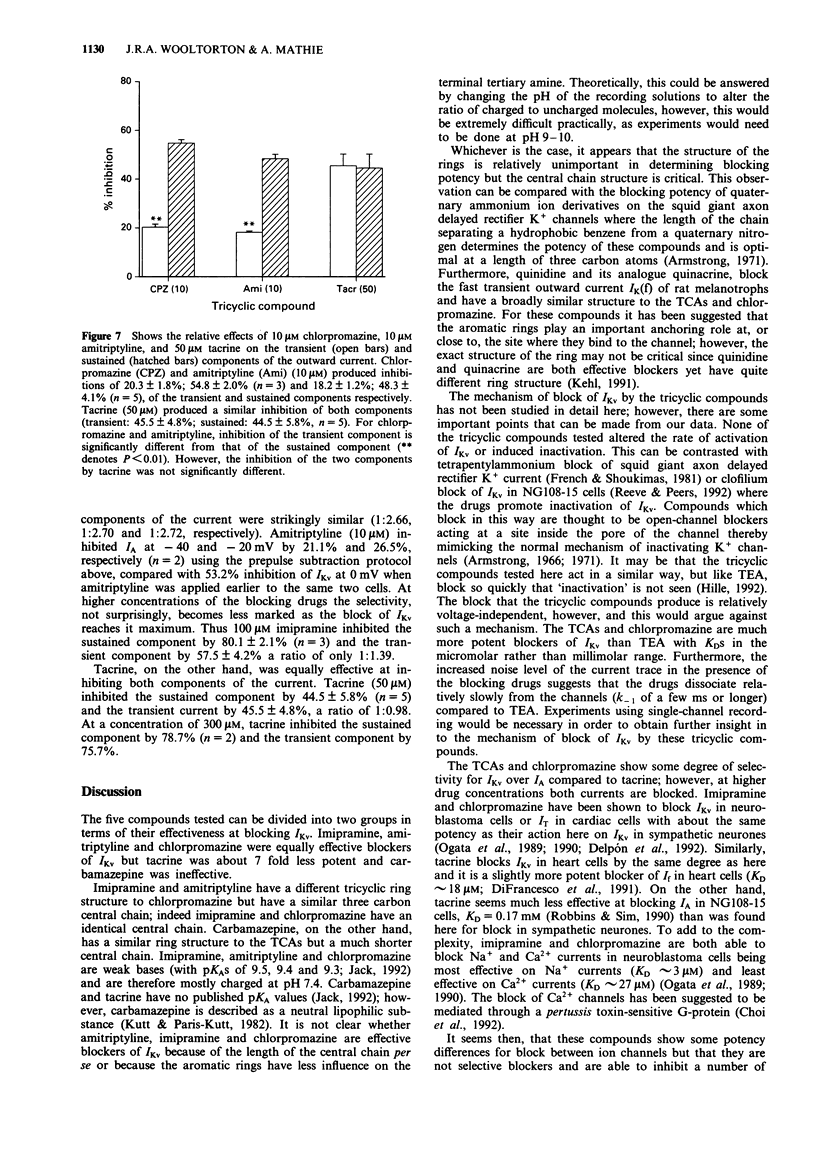
1. The block of K+ currents by the tricyclic antidepressants (TCAs), imipramine and amitriptyline and three structurally related compounds, chlorpromazine, tacrine and carbamazepine was investigated in rat isolated sympathetic neurones by whole-cell voltage-clamp recording. 2. At a concentration of 10 microM, imipramine, amitriptyline and chlorpromazine all blocked the delayed rectifier K+ current (IKv) by about the same extent, 54%, 47% and 53%. Tacrine was less effective (10%) while carbamazepine was ineffective at all concentrations tested. 3. The degree of block by the four effective compounds was relatively independent of the size of the voltage-step. Neither the activation nor the inactivation rates of IKv were altered by the blocking drugs. 4. Concentration-response relationships for imipramine and tacrine showed that imipramine was about 7 fold more potent than tacrine but that the maximum inhibition and the Hill slope were the same for both compounds. 5. Amitriptyline, chlorpromazine and imipramine (at 10 microM) were 2-3 fold more potent at inhibiting the sustained K+ current (mostly IKv) than the transient K+ current (mostly IA). Tacrine, however, was equally effective in blocking both components.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Time course of TEA(+)-induced anomalous rectification in squid giant axons. J Gen Physiol. 1966 Nov;50(2):491–503. doi: 10.1085/jgp.50.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bernheim L., Mathie A., Hille B. Intracellular Ca2+ buffers disrupt muscarinic suppression of Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O. A quantitative description of the sodium current in the rat sympathetic neurone. J Physiol. 1986 Nov;380:275–291. doi: 10.1113/jphysiol.1986.sp016285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi O., Sacchi O., Wanke E. Identification of delayed potassium and calcium currents in the rat sympathetic neurone under voltage clamp. J Physiol. 1985 Jan;358:109–129. doi: 10.1113/jphysiol.1985.sp015543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Middleton J. An electrophysiological analysis of the effects of amine-uptake blockers and alpha-adrenoceptor blockers on adrenergic neuromuscular transmission. Br J Pharmacol. 1975 Sep;55(1):87–95. doi: 10.1111/j.1476-5381.1975.tb07615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheim L., Beech D. J., Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991 Jun;6(6):859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- Bolotina V., Courtney K. R., Khodorov B. Gate-dependent blockade of sodium channels by phenothiazine derivatives: structure-activity relationships. Mol Pharmacol. 1992 Sep;42(3):423–431. [PubMed] [Google Scholar]
- Choi J. J., Huang G. J., Shafik E., Wu W. H., McArdle J. J. Imipramine's selective suppression of an L-type calcium channel in neurons of murine dorsal root ganglia involves G proteins. J Pharmacol Exp Ther. 1992 Oct;263(1):49–53. [PubMed] [Google Scholar]
- Delpón E., Tamargo J., Sánchez-Chapula J. Effects of imipramine on the transient outward current in rabbit atrial single cells. Br J Pharmacol. 1992 Jun;106(2):464–469. doi: 10.1111/j.1476-5381.1992.tb14357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Porciatti F., Janigro D., Maccaferri G., Mangoni M., Tritella T., Chang F., Cohen I. S. Block of the cardiac pacemaker current (If) in the rabbit sino-atrial node and in canine Purkinje fibres by 9-amino-1,2,3,4-tetrahydroacridine. Pflugers Arch. 1991 Feb;417(6):611–615. doi: 10.1007/BF00372959. [DOI] [PubMed] [Google Scholar]
- French R. J., Shoukimas J. J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys J. 1981 May;34(2):271–291. doi: 10.1016/S0006-3495(81)84849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi J. E. Membrane currents of cultured rat sympathetic neurons under voltage clamp. J Neurophysiol. 1983 Dec;50(6):1460–1478. doi: 10.1152/jn.1983.50.6.1460. [DOI] [PubMed] [Google Scholar]
- Galvan M., Sedlmeir C. Outward currents in voltage-clamped rat sympathetic neurones. J Physiol. 1984 Nov;356:115–133. doi: 10.1113/jphysiol.1984.sp015456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Tamargo J. Effect of imipramine on calcium and potassium currents in isolated bovine ventricular myocytes. Eur J Pharmacol. 1985 Jan 22;108(2):121–131. doi: 10.1016/0014-2999(85)90716-2. [DOI] [PubMed] [Google Scholar]
- Kehl S. J. Quinidine-induced inhibition of the fast transient outward K+ current in rat melanotrophs. Br J Pharmacol. 1991 Jul;103(3):1807–1813. doi: 10.1111/j.1476-5381.1991.tb09867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Narahashi T. Block of sodium channels by psychotropic drugs in single guinea-pig cardiac myocytes. Br J Pharmacol. 1989 Jul;97(3):905–913. doi: 10.1111/j.1476-5381.1989.tb12031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Narahashi T. Potent blocking action of chlorpromazine on two types of calcium channels in cultured neuroblastoma cells. J Pharmacol Exp Ther. 1990 Mar;252(3):1142–1149. [PubMed] [Google Scholar]
- Ogata N., Tatebayashi H. Modulation of sodium current kinetics by chlorpromazine in freshly-isolated striatal neurones of the adult guinea-pig. Br J Pharmacol. 1989 Dec;98(4):1173–1184. doi: 10.1111/j.1476-5381.1989.tb12662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Yoshii M., Narahashi T. Differential block of sodium and calcium channels by chlorpromazine in mouse neuroblastoma cells. J Physiol. 1990 Jan;420:165–183. doi: 10.1113/jphysiol.1990.sp017906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N., Yoshii M., Narahashi T. Psychotropic drugs block voltage-gated ion channels in neuroblastoma cells. Brain Res. 1989 Jan 2;476(1):140–144. doi: 10.1016/0006-8993(89)91546-1. [DOI] [PubMed] [Google Scholar]
- Pongs O. Structural basis of voltage-gated K+ channel pharmacology. Trends Pharmacol Sci. 1992 Sep;13(9):359–365. doi: 10.1016/0165-6147(92)90109-j. [DOI] [PubMed] [Google Scholar]
- Reeve H. L., Peers C. Blockade of delayed rectifier K+ currents in neuroblastoma x glioma hybrid (NG 108-15) cells by clofilium, a class III antidysrhythmic agent. Br J Pharmacol. 1992 Feb;105(2):458–462. doi: 10.1111/j.1476-5381.1992.tb14275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F., Galzi J. L., Giraudat J., Haumont P. Y., Lederer F., Changeux J. P. The noncompetitive blocker [3H]chlorpromazine labels three amino acids of the acetylcholine receptor gamma subunit: implications for the alpha-helical organization of regions MII and for the structure of the ion channel. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4675–4679. doi: 10.1073/pnas.87.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J., Sim J. A. A transient outward current in NG108-15 neuroblastoma x glioma hybrid cells. Pflugers Arch. 1990 Apr;416(1-2):130–137. doi: 10.1007/BF00370234. [DOI] [PubMed] [Google Scholar]
- Rogawski M. A., Porter R. J. Antiepileptic drugs: pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacol Rev. 1990 Sep;42(3):223–286. [PubMed] [Google Scholar]
- Strong P. N. Potassium channel toxins. Pharmacol Ther. 1990;46(1):137–162. doi: 10.1016/0163-7258(90)90040-9. [DOI] [PubMed] [Google Scholar]
- Van Erp M. G., Van Dongen A. M., Van den Berg R. J. Voltage-dependent action of valproate on potassium channels in frog node of Ranvier. Eur J Pharmacol. 1990 Aug 2;184(1):151–161. doi: 10.1016/0014-2999(90)90676-w. [DOI] [PubMed] [Google Scholar]
- Zhang Z. H., Follmer C. H., Sarma J. S., Chen F., Singh B. N. Effect of ambasilide, a new class III agent, on plateau currents in isolated guinea pig ventricular myocytes: block of delayed outward potassium current. J Pharmacol Exp Ther. 1992 Oct;263(1):40–48. [PubMed] [Google Scholar]
- Zhu Y., Im H. K., Im W. B. Block of voltage-gated potassium currents by anticonvulsant U-54494A in mouse neuroblastoma cells. J Pharmacol Exp Ther. 1992 Oct;263(1):207–213. [PubMed] [Google Scholar]
- Zona C., Tancredi V., Palma E., Pirrone G. C., Avoli M. Potassium currents in rat cortical neurons in culture are enhanced by the antiepileptic drug carbamazepine. Can J Physiol Pharmacol. 1990 Apr;68(4):545–547. doi: 10.1139/y90-079. [DOI] [PubMed] [Google Scholar]


