Abstract
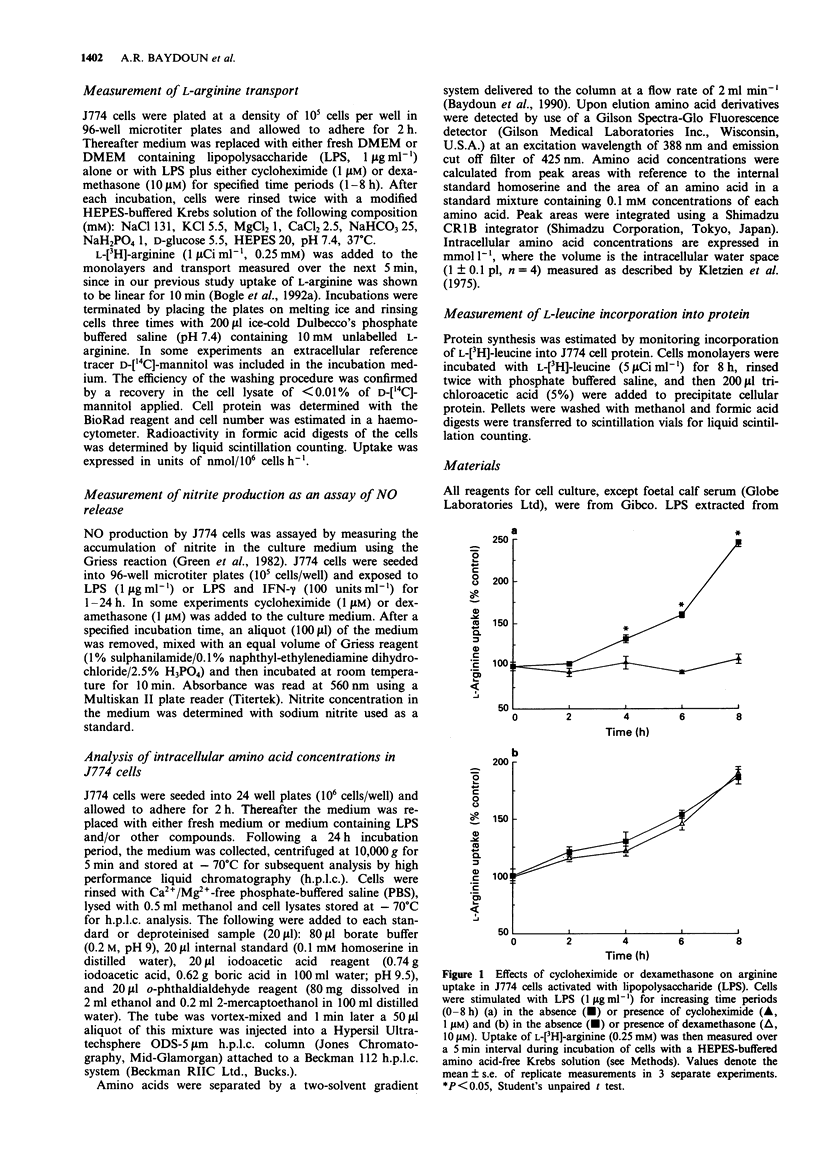
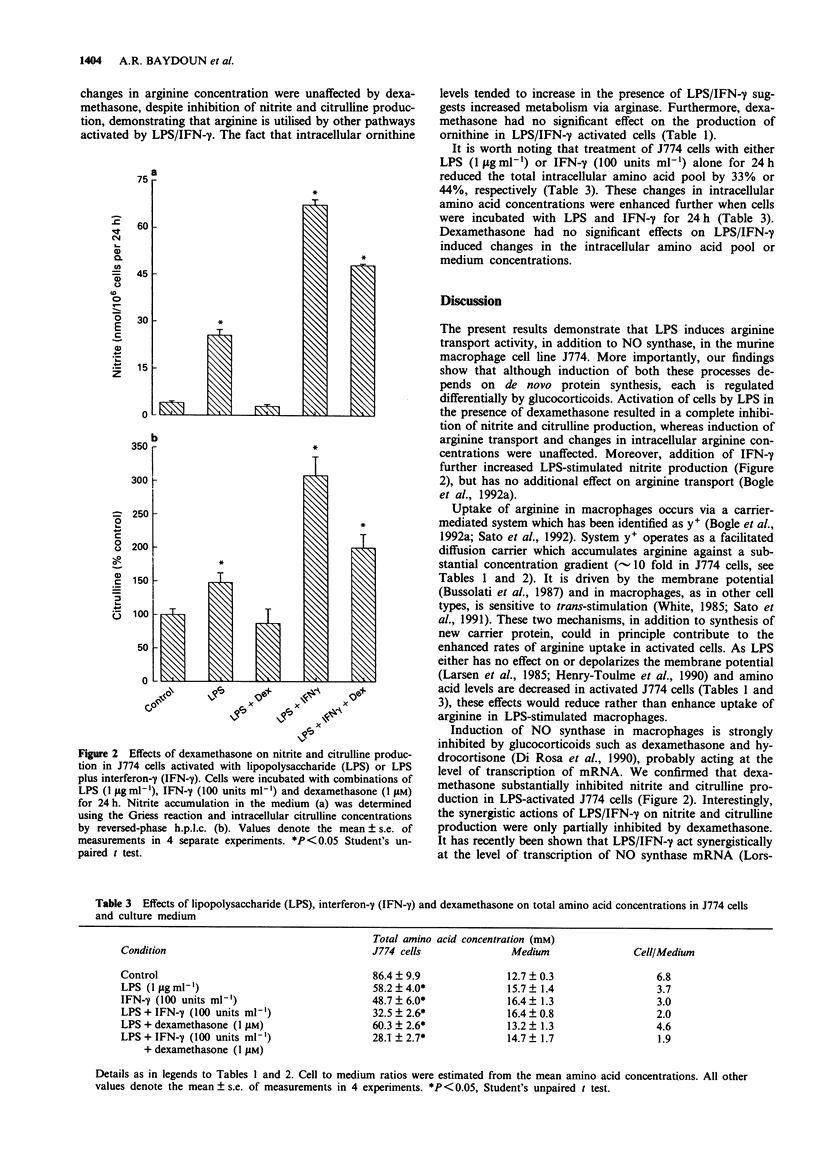
1. Effects of dexamethasone on induction of nitric oxide (NO) synthase and L-arginine transport by lipopolysaccharide (LPS) were examined in a murine cultured macrophage cell line J774. Metabolism of L-arginine to L-citrulline and subsequent changes in intracellular amino acids pools were correlated with changes in nitrite production. 2. Despite a high intracellular concentration of arginine in activated J774 cells, LPS (1 microgram ml-1, 8 h) induced a 2.4 fold increase in arginine transport. Treatment of cells with cycloheximide (1 microgram ml-1) inhibited the time-dependent (1-8 h) induction of NO synthase and arginine transport mediated by LPS. 3. Induction of NO synthase by LPS (1 microgram ml-1, 24 h) alone was accompanied by a marked increase in arginine utilisation leading to decreased intracellular arginine levels and elevated intracellular and extracellular L-citrulline levels. These changes were further enhanced in the presence of interferon-gamma (IFN-gamma, 100 units ml-1, 24 h). 4. Dexamethasone (1 microM) abolished the increases in both nitrite and citrulline production induced by LPS alone but only partially reversed the combined effects of LPS and IFN-gamma. In contrast, treatment of cells with dexamethasone (10 microM) had no effect on the LPS-mediated induction of arginine transport or the decrease in intracellular arginine concentration. 5. We conclude that induction of arginine transporter activity in LPS-stimulated J774 cells involves de novo synthesis of carrier proteins, which increases transport of exogenous arginine during enhanced NO production.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assreuy J., Moncada S. A perfusion system for the long term study of macrophage activation. Br J Pharmacol. 1992 Oct;107(2):317–321. doi: 10.1111/j.1476-5381.1992.tb12744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baydoun A. R., Emery P. W., Pearson J. D., Mann G. E. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1990 Dec 31;173(3):940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- Benninghoff B., Lehmann V., Eck H. P., Dröge W. Production of citrulline and ornithine by interferon-gamma treated macrophages. Int Immunol. 1991 May;3(5):413–417. doi: 10.1093/intimm/3.5.413. [DOI] [PubMed] [Google Scholar]
- Bogle R. G., Baydoun A. R., Pearson J. D., Moncada S., Mann G. E. L-arginine transport is increased in macrophages generating nitric oxide. Biochem J. 1992 May 15;284(Pt 1):15–18. doi: 10.1042/bj2840015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogle R. G., Coade S. B., Moncada S., Pearson J. D., Mann G. E. Bradykinin and ATP stimulate L-arginine uptake and nitric oxide release in vascular endothelial cells. Biochem Biophys Res Commun. 1991 Oct 31;180(2):926–932. doi: 10.1016/s0006-291x(05)81154-4. [DOI] [PubMed] [Google Scholar]
- Bussolati O., Laris P. C., Nucci F. A., Dall'Asta V., Longo N., Guidotti G. G., Gazzola G. C. Dependence of L-arginine accumulation on membrane potential in cultured human fibroblasts. Am J Physiol. 1987 Sep;253(3 Pt 1):C391–C397. doi: 10.1152/ajpcell.1987.253.3.C391. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Radomski M., Carnuccio R., Moncada S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1246–1252. doi: 10.1016/0006-291x(90)91583-e. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Metabolic fate of L-arginine in relation to microbiostatic capability of murine macrophages. J Clin Invest. 1990 Jan;85(1):264–273. doi: 10.1172/JCI114422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Hecker M., Sessa W. C., Harris H. J., Anggård E. E., Vane J. R. The metabolism of L-arginine and its significance for the biosynthesis of endothelium-derived relaxing factor: cultured endothelial cells recycle L-citrulline to L-arginine. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8612–8616. doi: 10.1073/pnas.87.21.8612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry-Toulmé N., Sarthou P., Bolard J. Early membrane potential and cytoplasmic calcium changes during mitogenic stimulation of WEHI 231 cell line by polyene antibiotics, lipopolysaccharide and anti-immunoglobulin. Biochim Biophys Acta. 1990 Mar 9;1051(3):285–292. doi: 10.1016/0167-4889(90)90136-2. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaysen G. A., Strecker H. J. Purification and properties of arginase of rat kidney. Biochem J. 1973 Aug;133(4):779–788. doi: 10.1042/bj1330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Pariza M. W., Becker J. E., Potter V. R. A method using 3-O-methyl-D-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Anal Biochem. 1975 Oct;68(2):537–544. doi: 10.1016/0003-2697(75)90649-1. [DOI] [PubMed] [Google Scholar]
- Larsen N. E., Enelow R. I., Simons E. R., Sullivan R. Effect of bacterial endotoxin on the transmembrane electrical potential and plasma membrane fluidity of human monocytes. Biochim Biophys Acta. 1985 Apr 26;815(1):1–8. doi: 10.1016/0005-2736(85)90466-3. [DOI] [PubMed] [Google Scholar]
- Lorsbach R. B., Murphy W. J., Lowenstein C. J., Snyder S. H., Russell S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993 Jan 25;268(3):1908–1913. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Sato H., Fujiwara M., Bannai S. Effect of lipopolysaccharide on transport and metabolism of arginine in mouse peritoneal macrophages. J Leukoc Biol. 1992 Aug;52(2):161–164. doi: 10.1002/jlb.52.2.161. [DOI] [PubMed] [Google Scholar]
- Sato H., Ishii T., Sugita Y., Bannai S. Induction of cationic amino acid transport activity in mouse peritoneal macrophages by lipopolysaccharide. Biochim Biophys Acta. 1991 Oct 14;1069(1):46–52. doi: 10.1016/0005-2736(91)90102-e. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Takema M., Inaba K., Uno K., Kakihara K., Tawara K., Muramatsu S. Effect of L-arginine on the retention of macrophage tumoricidal activity. J Immunol. 1991 Mar 15;146(6):1928–1933. [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Wu G. Y., Brosnan J. T. Macrophages can convert citrulline into arginine. Biochem J. 1992 Jan 1;281(Pt 1):45–48. doi: 10.1042/bj2810045. [DOI] [PMC free article] [PubMed] [Google Scholar]


