Abstract
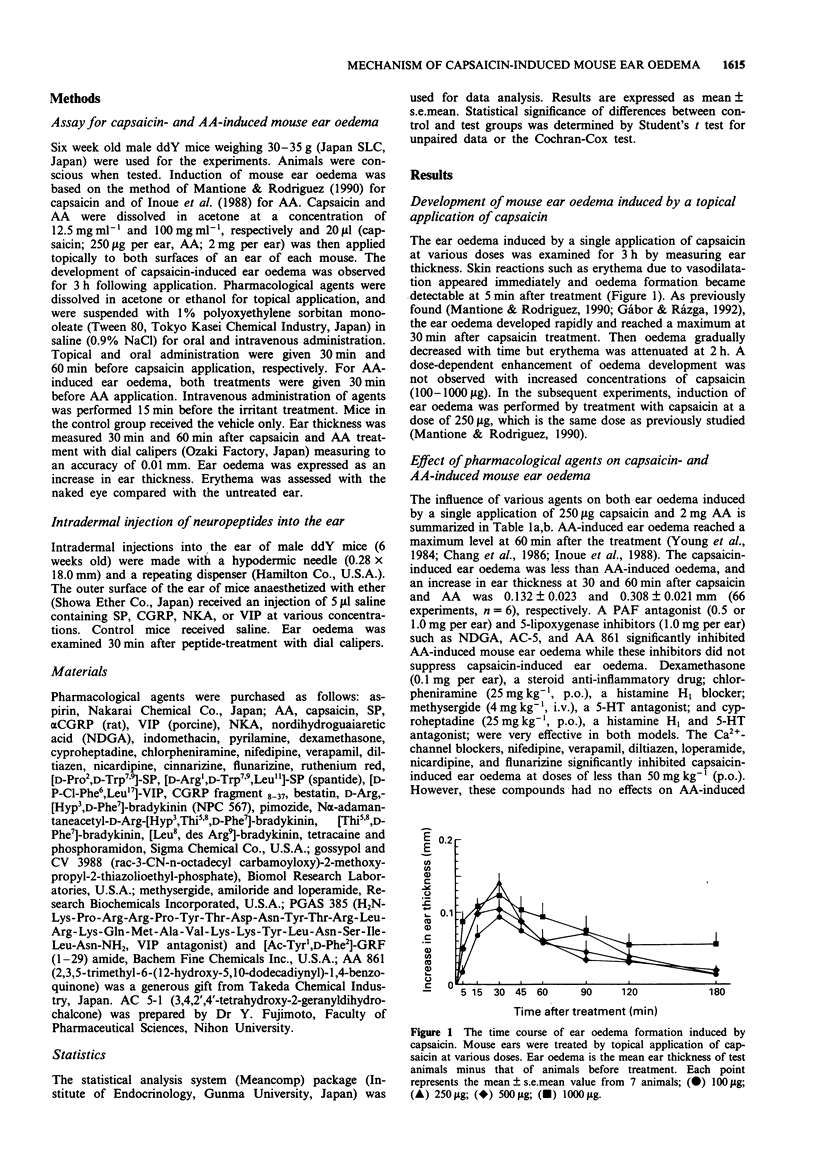
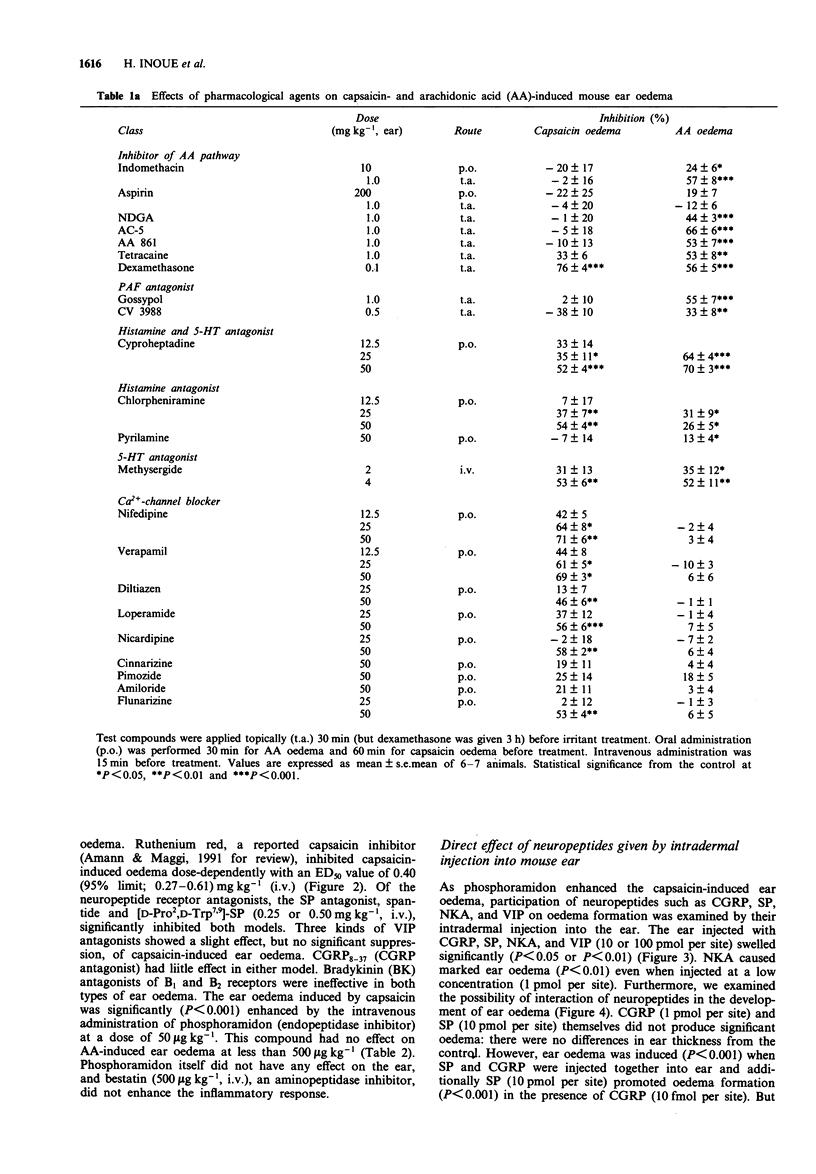
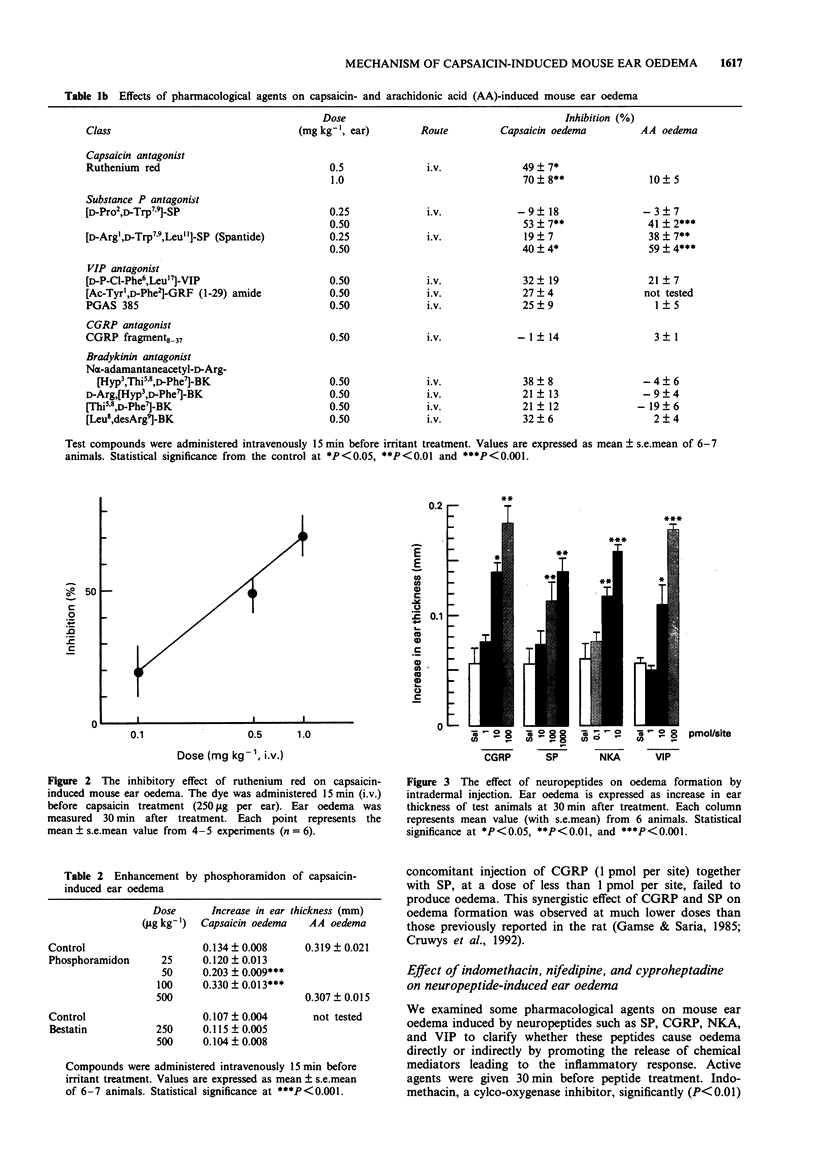
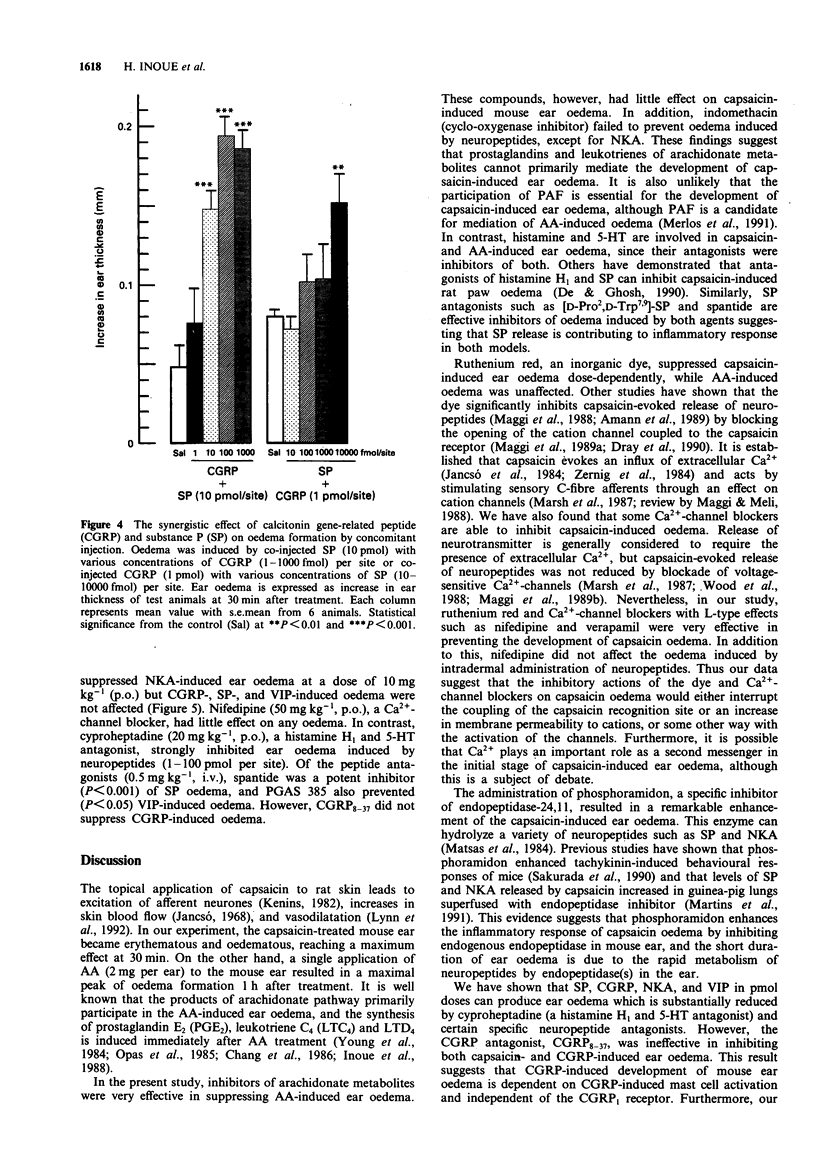
1. We have investigated the mechanism of capsaicin-induced mouse ear oedema compared with that of arachidonic acid (AA)-induced ear oedema, and evaluated the possible involvement of neuropeptides in the development of capsaicin-induced oedema. 2. Topical application of capsaicin (0.1-1.0 mg per ear) to the ear of mice produced immediate vasodilatation and erythema followed by the development of oedema which was maximal at 30 min after the treatment. This oedema was of shorter duration with less swelling than AA-induced oedema (2.0 mg per ear). 3. Capsaicin-induced ear oedema was unaffected when inhibitors of arachidonate metabolites including platelet activating factor (PAF) were administered before capsaicin (250 micrograms per ear) application, while these agents significantly prevented AA-induced oedema. Dexamethasone, histamine H1 and/or 5-hydroxytryptamine (5-HT) antagonists, and substance P (SP) antagonists were effective in inhibiting both models. Furthermore, a Ca(2+)-channel blocker and the capsaicin inhibitor, ruthenium red, were effective inhibitors of capsaicin oedema but had no effect on AA-induced oedema. 4. Phosphoramidon (50 micrograms kg-1, i.v.), an endopeptidase inhibitor, markedly (P < 0.001) enhanced only capsaicin-induced ear oedema, but bestatin (0.5 mg kg-1, i.v.), an aminopeptidase, failed to enhance oedema formation. 5. Neuropeptides (1-100 pmol per site) such as rat calcitonin gene-related peptide (CGRP), SP, neurokinin A (NKA), and vasoactive intestinal peptide (VIP), which are released from capsaicin-sensitive neurones, caused ear oedema by intradermal injection. Furthermore, a synergistic effect of CGRP (10 fmol per site) and SP (10pmol per site) on oedema formation was observed.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R., Donnerer J., Lembeck F. Ruthenium red selectively inhibits capsaicin-induced release of calcitonin gene-related peptide from the isolated perfused guinea pig lung. Neurosci Lett. 1989 Jul 3;101(3):311–315. doi: 10.1016/0304-3940(89)90551-x. [DOI] [PubMed] [Google Scholar]
- Amann R., Maggi C. A. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49(12):849–856. doi: 10.1016/0024-3205(91)90169-c. [DOI] [PubMed] [Google Scholar]
- Brain S. D., Williams T. J. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature. 1988 Sep 1;335(6185):73–75. doi: 10.1038/335073a0. [DOI] [PubMed] [Google Scholar]
- Carlson R. P., O'Neill-Davis L., Chang J., Lewis A. J. Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents. Agents Actions. 1985 Dec;17(2):197–204. doi: 10.1007/BF01966592. [DOI] [PubMed] [Google Scholar]
- Chang J., Carlson R. P., O'Neill-Davis L., Lamb B., Sharma R. N., Lewis A. J. Correlation between mouse skin inflammation induced by arachidonic acid and eicosanoid synthesis. Inflammation. 1986 Sep;10(3):205–214. doi: 10.1007/BF00916116. [DOI] [PubMed] [Google Scholar]
- Cruwys S. C., Kidd B. L., Mapp P. I., Walsh D. A., Blake D. R. The effects of calcitonin gene-related peptide on formation of intra-articular oedema by inflammatory mediators. Br J Pharmacol. 1992 Sep;107(1):116–119. doi: 10.1111/j.1476-5381.1992.tb14472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillier P., Regoli D., Asseraf A., Descours B., Marsac J., Renoux M. Histamine release and local responses of rat and human skin to substance P and other mammalian tachykinins. Pharmacology. 1986;32(6):340–347. doi: 10.1159/000138190. [DOI] [PubMed] [Google Scholar]
- Dray A., Forbes C. A., Burgess G. M. Ruthenium red blocks the capsaicin-induced increase in intracellular calcium and activation of membrane currents in sensory neurones as well as the activation of peripheral nociceptors in vitro. Neurosci Lett. 1990 Mar 2;110(1-2):52–59. doi: 10.1016/0304-3940(90)90786-9. [DOI] [PubMed] [Google Scholar]
- Farber E. M., Nickoloff B. J., Recht B., Fraki J. E. Stress, symmetry, and psoriasis: possible role of neuropeptides. J Am Acad Dermatol. 1986 Feb;14(2 Pt 1):305–311. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- Gamse R., Saria A. Potentiation of tachykinin-induced plasma protein extravasation by calcitonin gene-related peptide. Eur J Pharmacol. 1985 Aug 7;114(1):61–66. doi: 10.1016/0014-2999(85)90520-5. [DOI] [PubMed] [Google Scholar]
- Gábor M., Rázga Z. Development and inhibition of mouse ear oedema induced with capsaicin. Agents Actions. 1992 May;36(1-2):83–86. doi: 10.1007/BF01991233. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143–201. [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Inoue H., Mori T., Koshihara Y. Sulfidopeptide-leukotrienes are major mediators of arachidonic acid-induced mouse ear edema. Prostaglandins. 1988 Nov;36(5):731–739. doi: 10.1016/0090-6980(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Iwamoto I., Nadel J. A. Tachykinin receptor subtype that mediates the increase in vascular permeability in guinea pig skin. Life Sci. 1989;44(16):1089–1095. doi: 10.1016/0024-3205(89)90336-6. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Karcsú S., Király E., Szebeni A., Tóth L., Bácsy E., Joó F., Párducz A. Neurotoxin induced nerve cell degeneration: possible involvement of calcium. Brain Res. 1984 Mar 19;295(2):211–216. doi: 10.1016/0006-8993(84)90969-7. [DOI] [PubMed] [Google Scholar]
- Kenins P. Responses of single nerve fibres to capsaicin applied to the skin. Neurosci Lett. 1982 Mar 17;29(1):83–88. doi: 10.1016/0304-3940(82)90369-x. [DOI] [PubMed] [Google Scholar]
- Larsson J., Ekblom A., Henriksson K., Lundeberg T., Theodorsson E. Immunoreactive tachykinins, calcitonin gene-related peptide and neuropeptide Y in human synovial fluid from inflamed knee joints. Neurosci Lett. 1989 May 22;100(1-3):326–330. doi: 10.1016/0304-3940(89)90707-6. [DOI] [PubMed] [Google Scholar]
- Lynn B., Ye W., Cotsell B. The actions of capsaicin applied topically to the skin of the rat on C-fibre afferents, antidromic vasodilatation and substance P levels. Br J Pharmacol. 1992 Oct;107(2):400–406. doi: 10.1111/j.1476-5381.1992.tb12758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi C. A., Meli A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen Pharmacol. 1988;19(1):1–43. doi: 10.1016/0306-3623(88)90002-x. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Patacchini R., Santicioli P., Giuliani S., Del Bianco E., Geppetti P., Meli A. The 'efferent' function of capsaicin-sensitive nerves: ruthenium red discriminates between different mechanisms of activation. Eur J Pharmacol. 1989 Nov 7;170(3):167–177. doi: 10.1016/0014-2999(89)90537-2. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Geppetti P., Parlani M., Astolfi M., Del Bianco E., Patacchini R., Giuliani S., Meli A. The effect of calcium free medium and nifedipine on the release of substance P-like immunoreactivity and contractions induced by capsaicin in the isolated guinea-pig and rat bladder. Gen Pharmacol. 1989;20(4):445–456. doi: 10.1016/0306-3623(89)90194-8. [DOI] [PubMed] [Google Scholar]
- Maggi C. A., Santicioli P., Geppetti P., Parlani M., Astolfi M., Pradelles P., Patacchini R., Meli A. The antagonism induced by ruthenium red of the actions of capsaicin on the peripheral terminals of sensory neurons: further studies. Eur J Pharmacol. 1988 Sep 1;154(1):1–10. doi: 10.1016/0014-2999(88)90356-1. [DOI] [PubMed] [Google Scholar]
- Mantione C. R., Rodriguez R. A bradykinin (BK)1 receptor antagonist blocks capsaicin-induced ear inflammation in mice. Br J Pharmacol. 1990 Mar;99(3):516–518. doi: 10.1111/j.1476-5381.1990.tb12960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S. J., Stansfeld C. E., Brown D. A., Davey R., McCarthy D. The mechanism of action of capsaicin on sensory C-type neurons and their axons in vitro. Neuroscience. 1987 Oct;23(1):275–289. doi: 10.1016/0306-4522(87)90289-2. [DOI] [PubMed] [Google Scholar]
- Marshall K. W., Chiu B., Inman R. D. Substance P and arthritis: analysis of plasma and synovial fluid levels. Arthritis Rheum. 1990 Jan;33(1):87–90. doi: 10.1002/art.1780330111. [DOI] [PubMed] [Google Scholar]
- Martins M. A., Shore S. A., Drazen J. M. Capsaicin-induced release of tachykinins: effects of enzyme inhibitors. J Appl Physiol (1985) 1991 May;70(5):1950–1956. doi: 10.1152/jappl.1991.70.5.1950. [DOI] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlos M., Gómez L. A., Giral M., Vericat M. L., García-Rafanell J., Forn J. Effects of PAF-antagonists in mouse ear oedema induced by several inflammatory agents. Br J Pharmacol. 1991 Dec;104(4):990–994. doi: 10.1111/j.1476-5381.1991.tb12538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Alving K., Ahlstedt S., Hökfelt T., Lundberg J. M. Peptidergic innervation of rat lymphoid tissue and lung: relation to mast cells and sensitivity to capsaicin and immunization. Cell Tissue Res. 1990 Oct;262(1):125–133. doi: 10.1007/BF00327753. [DOI] [PubMed] [Google Scholar]
- Opas E. E., Bonney R. J., Humes J. L. Prostaglandin and leukotriene synthesis in mouse ears inflamed by arachidonic acid. J Invest Dermatol. 1985 Apr;84(4):253–256. doi: 10.1111/1523-1747.ep12265320. [DOI] [PubMed] [Google Scholar]
- Piotrowski W., Foreman J. C. On the actions of substance P, somatostatin, and vasoactive intestinal polypeptide on rat peritoneal mast cells and in human skin. Naunyn Schmiedebergs Arch Pharmacol. 1985 Dec;331(4):364–368. doi: 10.1007/BF00500821. [DOI] [PubMed] [Google Scholar]
- Piotrowski W., Foreman J. C. Some effects of calcitonin gene-related peptide in human skin and on histamine release. Br J Dermatol. 1986 Jan;114(1):37–46. doi: 10.1111/j.1365-2133.1986.tb02777.x. [DOI] [PubMed] [Google Scholar]
- Sakurada T., Tan-No K., Yamada T., Sakurada S., Kisara K. Phosphoramidon potentiates mammalian tachykinin-induced biting, licking and scratching behaviour in mice. Pharmacol Biochem Behav. 1990 Dec;37(4):779–783. doi: 10.1016/0091-3057(90)90563-w. [DOI] [PubMed] [Google Scholar]
- Saria A., Martling C. R., Yan Z., Theodorsson-Norheim E., Gamse R., Lundberg J. M. Release of multiple tachykinins from capsaicin-sensitive sensory nerves in the lung by bradykinin, histamine, dimethylphenyl piperazinium, and vagal nerve stimulation. Am Rev Respir Dis. 1988 Jun;137(6):1330–1335. doi: 10.1164/ajrccm/137.6.1330. [DOI] [PubMed] [Google Scholar]
- Wallengren J., Ekman R., Möller H. Substance P and vasoactive intestinal peptide in bullous and inflammatory skin disease. Acta Derm Venereol. 1986;66(1):23–28. [PubMed] [Google Scholar]
- Wallengren J., Möller H., Ekman R. Occurrence of substance P, vasoactive intestinal peptide, and calcitonin gene-related peptide in dermographism and cold urticaria. Arch Dermatol Res. 1987;279(8):512–515. doi: 10.1007/BF00413281. [DOI] [PubMed] [Google Scholar]
- Williams T. J. Vasoactive intestinal polypeptide is more potent than prostaglandin E2 as a vasodilator and oedema potentiator in rabbit skin. Br J Pharmacol. 1982 Nov;77(3):505–509. doi: 10.1111/j.1476-5381.1982.tb09324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. N., Winter J., James I. F., Rang H. P., Yeats J., Bevan S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J Neurosci. 1988 Sep;8(9):3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. M., Spires D. A., Bedord C. J., Wagner B., Ballaron S. J., De Young L. M. The mouse ear inflammatory response to topical arachidonic acid. J Invest Dermatol. 1984 Apr;82(4):367–371. doi: 10.1111/1523-1747.ep12260709. [DOI] [PubMed] [Google Scholar]
- Zernig G., Holzer P., Lembeck F. A study of the mode and site of action of capsaicin in guinea-pig heart and rat uterus. Naunyn Schmiedebergs Arch Pharmacol. 1984 May;326(1):58–63. doi: 10.1007/BF00518779. [DOI] [PubMed] [Google Scholar]


