Abstract
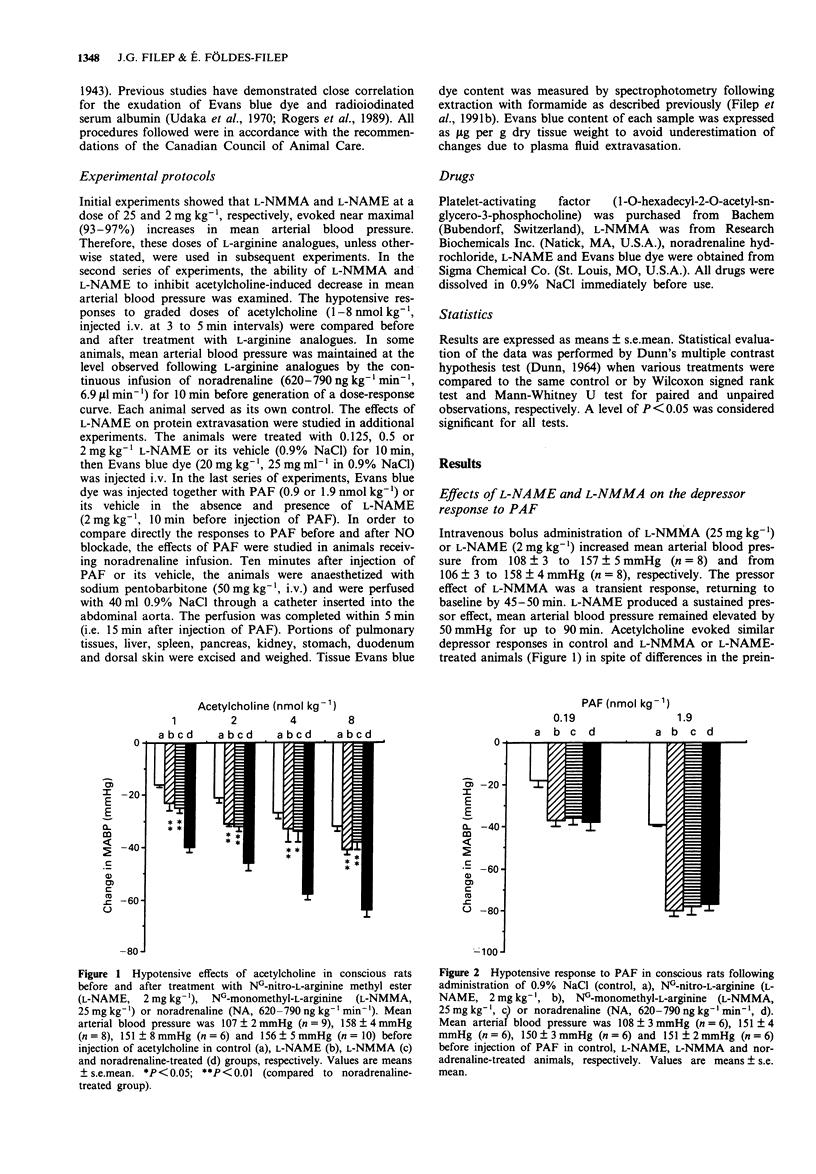
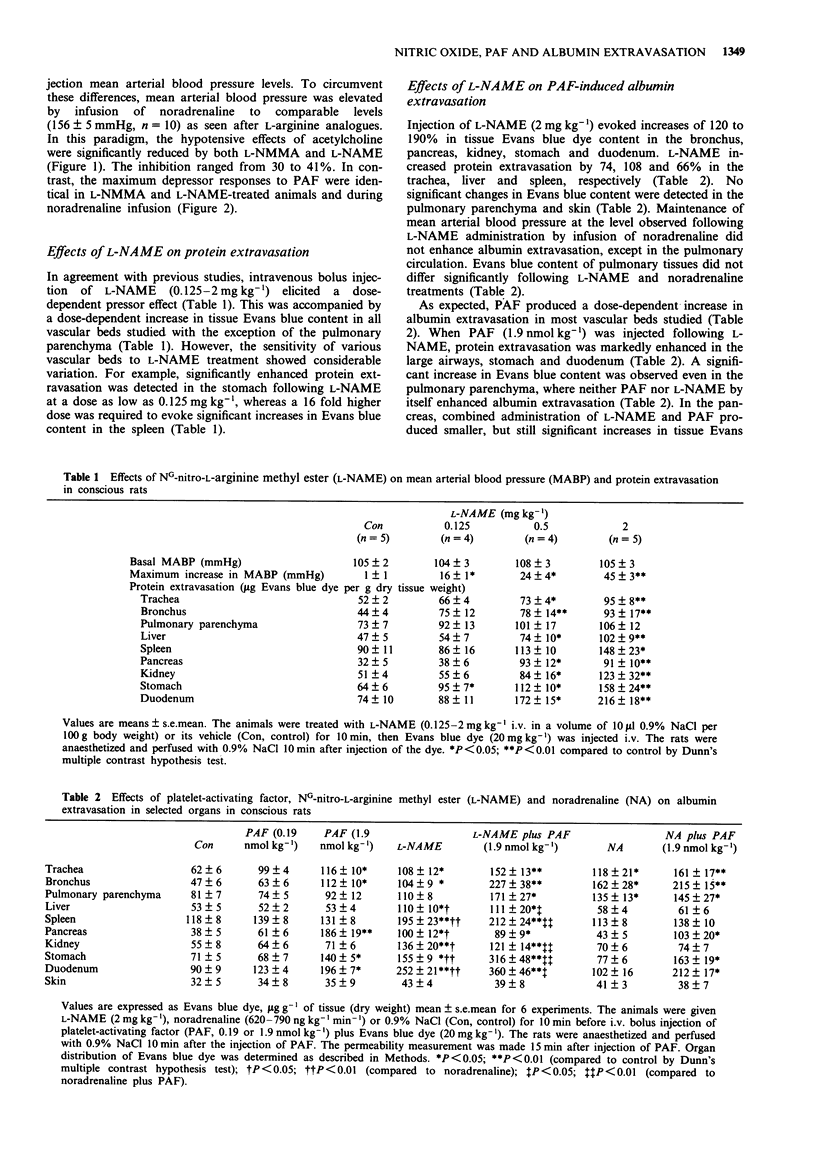
1. The objective of this study was to assess whether or not endogenous nitric oxide (NO) could mediate the hypotensive response to platelet-activating factor (PAF) and modulate PAF-induced microvascular albumin leakage in the conscious rat. 2. PAF (0.19 and 1.9 nmol kg-1, i.v.) evoked dose-dependent hypotension and significantly enhanced albumin extravasation in the large airways, pancreas, stomach and duodenum 15 min after its administration. Inhibition of NO synthesis by NG-nitro-L-arginine methyl ester (L-NAME, 0.125-2 mg kg-1, i.v.) produced marked dose-dependent increases in albumin accumulation (up to 290%) in large airways, liver, spleen, pancreas, kidney, stomach and duodenum as measured by the extravasation of Evans blue dye. L-NAME (2 mg kg-1) treatment markedly potentiated PAF (1.9 nmol kg-1)-induced albumin extravasation in these tissues, whereas it did not modify the hypotensive response to PAF. 3. Maintenance of mean arterial blood pressure at the level observed following 2 mg kg-1 L-NAME by infusion of noradrenaline (620-790 ng kg-1 min-1) neither affected significantly albumin extravasation nor potentiated the permeability effect of PAF in the vascular beds studied with the exception of large airways, where noradrenaline mimicked the effects of L-NAME. 4. These results indicate that inhibition of endogenous NO formation leads to an increase in albumin extravasation and to potentiation of the vascular permeability effect of PAF, whereas the hypotensive action of PAF seems to be independent of NO formation in the conscious rat. These data suggest an important role for NO in the regulation of albumin extravasation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang S. W., Feddersen C. O., Henson P. M., Voelkel N. F. Platelet-activating factor mediates hemodynamic changes and lung injury in endotoxin-treated rats. J Clin Invest. 1987 May;79(5):1498–1509. doi: 10.1172/JCI112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maestro R. F., Björk J., Arfors K. E. Increase in microvascular permeability induced by enzymatically generated free radicals. I. In vivo study. Microvasc Res. 1981 Nov;22(3):239–254. doi: 10.1016/0026-2862(81)90095-9. [DOI] [PubMed] [Google Scholar]
- Doebber T. W., Wu M. S., Robbins J. C., Choy B. M., Chang M. N., Shen T. Y. Platelet activating factor (PAF) involvement in endotoxin-induced hypotension in rats. Studies with PAF-receptor antagonist kadsurenone. Biochem Biophys Res Commun. 1985 Mar 29;127(3):799–808. doi: 10.1016/s0006-291x(85)80014-0. [DOI] [PubMed] [Google Scholar]
- Filep J. G., Sirois M. G., Rousseau A., Fournier A., Sirois P. Effects of endothelin-1 on vascular permeability in the conscious rat: interactions with platelet-activating factor. Br J Pharmacol. 1991 Dec;104(4):797–804. doi: 10.1111/j.1476-5381.1991.tb12509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filep J., Braquet P., Mózes T. Significance of platelet-activating factor in mesenteric ischemia-reperfusion. Lipids. 1991 Dec;26(12):1336–1339. doi: 10.1007/BF02536561. [DOI] [PubMed] [Google Scholar]
- Filep J., Földes-Filep E., Frölich J. C. Vascular responses to leukotriene B4, C4 and D4 following FPL 55712, indomethacin, saralasin, phentolamine and verapamil in the conscious rat. Br J Pharmacol. 1987 Feb;90(2):431–439. doi: 10.1111/j.1476-5381.1987.tb08973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filep J., Hermán F., Braquet P., Mózes T. Increased levels of platelet-activating factor in blood following intestinal ischemia in the dog. Biochem Biophys Res Commun. 1989 Jan 31;158(2):353–359. doi: 10.1016/s0006-291x(89)80055-5. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Gardiner S. M., Compton A. M., Kemp P. A., Bennett T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of NG-nitro-L-arginine methyl ester. Br J Pharmacol. 1990 Nov;101(3):632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grega G. J., Adamski S. W., Dobbins D. E. Physiological and pharmacological evidence for the regulation of permeability. Fed Proc. 1986 Feb;45(2):96–100. [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Hecker M., Mitchell J. A., Harris H. J., Katsura M., Thiemermann C., Vane J. R. Endothelial cells metabolize NG-monomethyl-L-arginine to L-citrulline and subsequently to L-arginine. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1037–1043. doi: 10.1016/0006-291x(90)90627-y. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P., Granger D. N. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992 Feb;262(2 Pt 2):H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H. P., Liu S., Barnes P. J. The effect of endogenous nitric oxide on neurogenic plasma exudation in guinea-pig airways. Eur J Pharmacol. 1992 Oct 20;221(2-3):385–388. doi: 10.1016/0014-2999(92)90728-m. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P. S., Lefer D. J., Ma X. L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991 Apr;5(7):2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- Marcel van Gelderen E., Heiligers J. P., Saxena P. R. Haemodynamic changes and acetylcholine-induced hypotensive responses after NG-nitro-L-arginine methyl ester in rats and cats. Br J Pharmacol. 1991 Aug;103(4):1899–1904. doi: 10.1111/j.1476-5381.1991.tb12349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montrucchio G., Alloatti G., Tetta C., De Luca R., Saunders R. N., Emanuelli G., Camussi G. Release of platelet-activating factor from ischemic-reperfused rabbit heart. Am J Physiol. 1989 Apr;256(4 Pt 2):H1236–H1246. doi: 10.1152/ajpheart.1989.256.4.H1236. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Guerra R., Jr, Harrison D. G. Release of NO and EDRF from cultured bovine aortic endothelial cells. Am J Physiol. 1989 Apr;256(4 Pt 2):H1030–H1037. doi: 10.1152/ajpheart.1989.256.4.H1030. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Miller C. H., Lewis J. C., Wykle R. L., Bass D. A., McCall C. E., Waite M., DeChatelet L. R. Neutrophil responses to platelet-activating factor. Inflammation. 1981 Sep;5(3):193–201. doi: 10.1007/BF00914443. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet. 1987 Nov 7;2(8567):1057–1058. doi: 10.1016/s0140-6736(87)91481-4. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. F., Boschetto P., Barnes P. J. Plasma exudation. Correlation between Evans blue dye and radiolabeled albumin in guinea pig airways in vivo. J Pharmacol Methods. 1989 Jul;21(4):309–315. doi: 10.1016/0160-5402(89)90068-5. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Scherf H., Nies A. S., Schwertschlag U., Hughes M., Gerber J. G. Hemodynamic effects of platelet activating factor in the dog kidney in vivo. Hypertension. 1986 Sep;8(9):737–741. doi: 10.1161/01.hyp.8.9.737. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Pinckard R. N., Ferrigni K. S., McManus L. M., Hanahan D. J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [PubMed] [Google Scholar]
- Siegfried M. R., Erhardt J., Rider T., Ma X. L., Lefer A. M. Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischemia-reperfusion. J Pharmacol Exp Ther. 1992 Feb;260(2):668–675. [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Vargas H. M., Cuevas J. M., Ignarro L. J., Chaudhuri G. Comparison of the inhibitory potencies of N(G)-methyl-, N(G)-nitro- and N(G)-amino-L-arginine on EDRF function in the rat: evidence for continuous basal EDRF release. J Pharmacol Exp Ther. 1991 Jun;257(3):1208–1215. [PubMed] [Google Scholar]
- Whittle B. J., Lopez-Belmonte J., Rees D. D. Modulation of the vasodepressor actions of acetylcholine, bradykinin, substance P and endothelin in the rat by a specific inhibitor of nitric oxide formation. Br J Pharmacol. 1989 Oct;98(2):646–652. doi: 10.1111/j.1476-5381.1989.tb12639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]


