Abstract
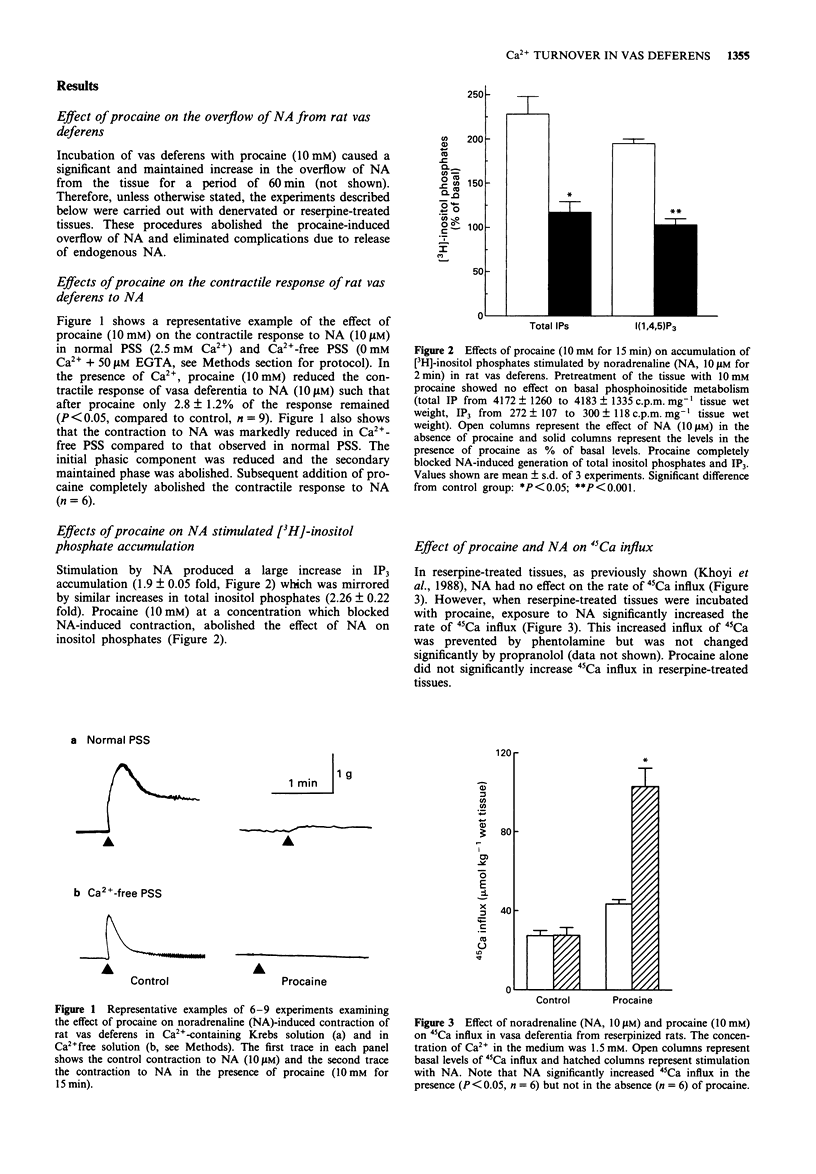
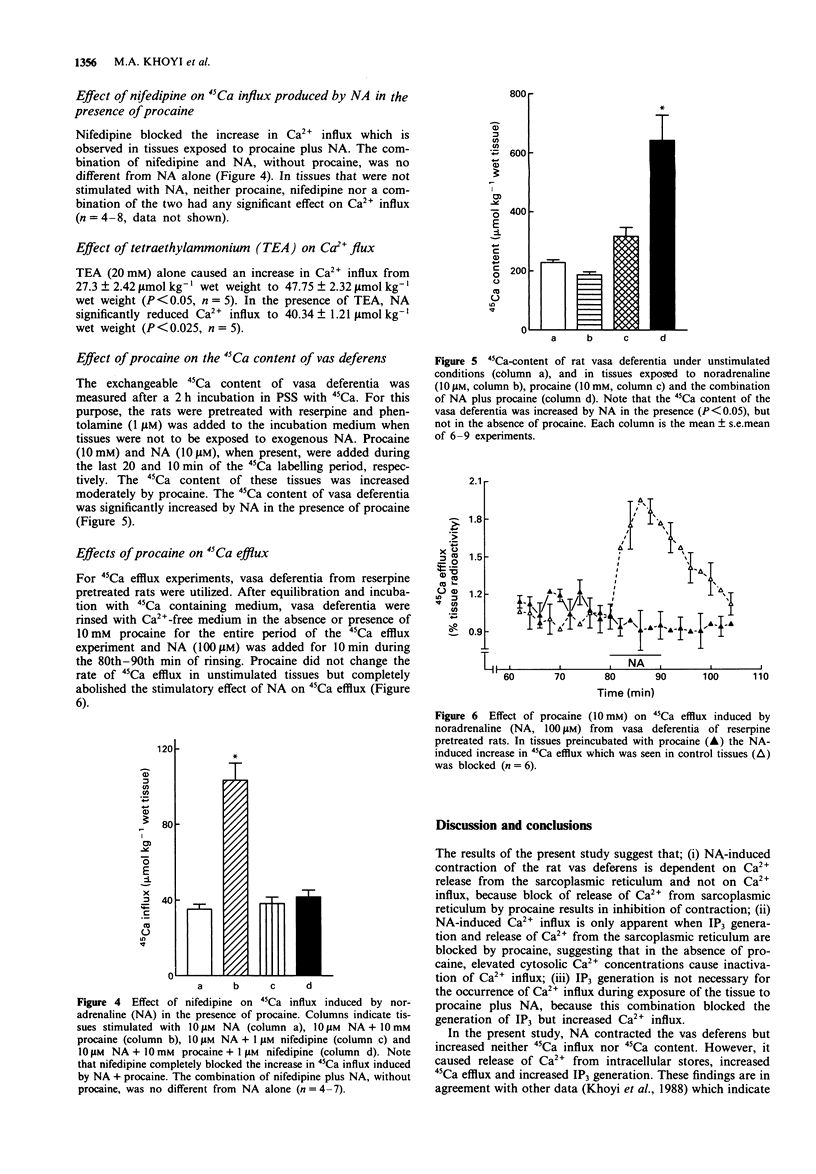
1. The actions of procaine (10 mM) on noradrenaline-induced effects on 45Ca-influx, 45Ca-efflux, 45Ca-content, total inositol phosphates, inositol-1,4,5-trisphosphate, and contractile status of the rat was deferens were examined. 2. Noradrenaline alone had no effect on 45Ca-influx or 45Ca-content, but released Ca2+ from intracellular stores as indicated by an increased 45Ca-efflux and increased total inositol phosphates, specifically inositol-1,4,5-trisphosphate, leading to contraction of the rat vas deferens. 3. Noradrenaline, in the presence of 10 mM procaine, increased 45Ca-influx and 45Ca-content. Procaine blocked the noradrenaline-induced 45Ca-efflux, the increase in total inositol phosphates, the increase in inositol-1,4,5-trisphosphate, and contraction. 4. The noradrenaline-induced increase in 45Ca influx which was observed in the presence of procaine was abolished by phentolamine and nifedipine but was not altered significantly by propranolol suggesting that, in the presence of procaine, noradrenaline activates dihydropyridine-sensitive calcium channels through alpha-adrenoceptors. 5. These findings indicate that, in the rat vas deferens, noradrenaline induces contraction by releasing intracellularly stored Ca2+. The effects of procaine appear to be due to its ability to block the release of Ca2+ from intracellular stores. Furthermore, the simultaneous increase in 45Ca influx and inhibition of inositol-1,4,5-trisphosphate formation in tissues treated with procaine plus noradrenaline indicates that Ca2+ influx is independent of inositol-1,4,5-trisphosphate formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P. I. Estimation of high K- and noradrenaline-induced 45Ca uptakes in isolated rat aorta: effects of washing in icecold solutions. Pflugers Arch. 1989 Sep;414(5):579–583. doi: 10.1007/BF00580994. [DOI] [PubMed] [Google Scholar]
- Ahn H. Y., Karaki H. Inhibitory effects of procaine on contraction and calcium movement in vascular and intestinal smooth muscles. Br J Pharmacol. 1988 Jul;94(3):789–796. doi: 10.1111/j.1476-5381.1988.tb11590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprigliano O., Hermsmeyer K. In vitro denervation of the portal vein and caudal artery of the rat. J Pharmacol Exp Ther. 1976 Sep;198(3):568–577. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl A., Frey B. W., Ward S. M., Sanders K. M., Kenyon J. L. Inhibition of slow-wave repolarization and Ca(2+)-activated K+ channels by quaternary ammonium ions. Am J Physiol. 1993 Mar;264(3 Pt 1):C625–C631. doi: 10.1152/ajpcell.1993.264.3.C625. [DOI] [PubMed] [Google Scholar]
- Chad J., Eckert R., Ewald D. Kinetics of calcium-dependent inactivation of calcium current in voltage-clamped neurones of Aplysia californica. J Physiol. 1984 Feb;347:279–300. doi: 10.1113/jphysiol.1984.sp015066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Feddersen M., Vesperinas G., Lewin J., Huidobro-Toro J. P. Different calcium sources mobilized by noradrenaline (NA) and adenosine 5'-triphosphate (ATP): a basis for co-transmission in the rat vas deferens. Eur J Pharmacol. 1988 Aug 24;153(2-3):313–314. doi: 10.1016/0014-2999(88)90622-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hay D. W., Wadsworth R. M. Effects of KCl on 45Ca uptake and efflux in the rat vas deferens. Br J Pharmacol. 1984 Mar;81(3):441–447. doi: 10.1111/j.1476-5381.1984.tb10096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S., Nakazawa M., Imai H., Nabata H. Effects of procaine on the isolated dog coronary artery. Arch Int Pharmacodyn Ther. 1984 Sep;271(1):98–105. [PubMed] [Google Scholar]
- Imaizumi Y., Takeda M., Muraki K., Watanabe M. Mechanisms of NE-induced reduction of Ca current in single smooth muscle cells from guinea pig vas deferens. Am J Physiol. 1991 Jan;260(1 Pt 1):C17–C25. doi: 10.1152/ajpcell.1991.260.1.C17. [DOI] [PubMed] [Google Scholar]
- Ito K., Takakura S., Sato K., Sutko J. L. Ryanodine inhibits the release of calcium from intracellular stores in guinea pig aortic smooth muscle. Circ Res. 1986 May;58(5):730–734. doi: 10.1161/01.res.58.5.730. [DOI] [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemond V., Rougier O. Nifedipine and BAY K inhibit contraction independently from their action on calcium channels. Biochem Biophys Res Commun. 1988 May 16;152(3):1002–1007. doi: 10.1016/s0006-291x(88)80383-8. [DOI] [PubMed] [Google Scholar]
- Khoyi M. A., Westfall D. P., Buxton I. L., Akhtar-Khavari F., Rezaei E., Salaices M., Sanchez-Garcia P. Norepinephrine and potassium induced calcium translocation in rat vas deferens. J Pharmacol Exp Ther. 1988 Sep;246(3):917–923. [PubMed] [Google Scholar]
- Khoyi M. A., Westfall D. P., Gerthoffer W. T. Effects of potassium and norepinephrine on calcium influx in guinea-pig vas deferens. Eur J Pharmacol. 1987 Aug 4;140(1):55–62. doi: 10.1016/0014-2999(87)90633-9. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Hwang O., van Breemen C. Evidence for two separated Ca2+ pathways in smooth muscle plasmalemma. J Membr Biol. 1981 Mar 15;59(1):19–25. doi: 10.1007/BF01870817. [DOI] [PubMed] [Google Scholar]
- Pacaud P., Loirand G., Mironneau C., Mironneau J. Opposing effects of noradrenaline on the two classes of voltage-dependent calcium channels of single vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1987 Nov;410(4-5):557–559. doi: 10.1007/BF00586539. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Inositol phosphates and calcium entry. Adv Second Messenger Phosphoprotein Res. 1992;26:143–160. [PubMed] [Google Scholar]
- Rinaldi G. J., Amado Cattaneo E., Mattiazzi A., Cingolani H. E. Dissociation between calcium influx blockage and smooth muscle relaxation by nifedipine in spontaneously hypertensive rats. Circ Res. 1987 Mar;60(3):367–374. doi: 10.1161/01.res.60.3.367. [DOI] [PubMed] [Google Scholar]
- Shinozuka K., Bjur R. A., Westfall D. P. Characterization of prejunctional purinoceptors on adrenergic nerves of the rat caudal artery. Naunyn Schmiedebergs Arch Pharmacol. 1988 Sep;338(3):221–227. doi: 10.1007/BF00173391. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Vesperinas G., Feddersen M., Lewin J., Huidobro-Toro J. P. The use of ryanodine and calcium channel blockers to characterize intra- and extracellular calcium pools mobilized by noradrenaline in the rat vas deferens. Eur J Pharmacol. 1989 Jun 20;165(2-3):309–313. doi: 10.1016/0014-2999(89)90727-9. [DOI] [PubMed] [Google Scholar]
- Xuan Y. T., Whorton A. R., Watkins W. D. Inhibition by nicardipine of endothelin-mediated inositol phosphate formation and Ca2+ mobilization in smooth muscle cell. Biochem Biophys Res Commun. 1989 Apr 28;160(2):758–764. doi: 10.1016/0006-291x(89)92498-4. [DOI] [PubMed] [Google Scholar]
- Zhang L. B., Buxton I. L. Muscarinic receptors in canine colonic circular smooth muscle. II. Signal transduction pathways. Mol Pharmacol. 1991 Dec;40(6):952–959. [PubMed] [Google Scholar]


