Abstract
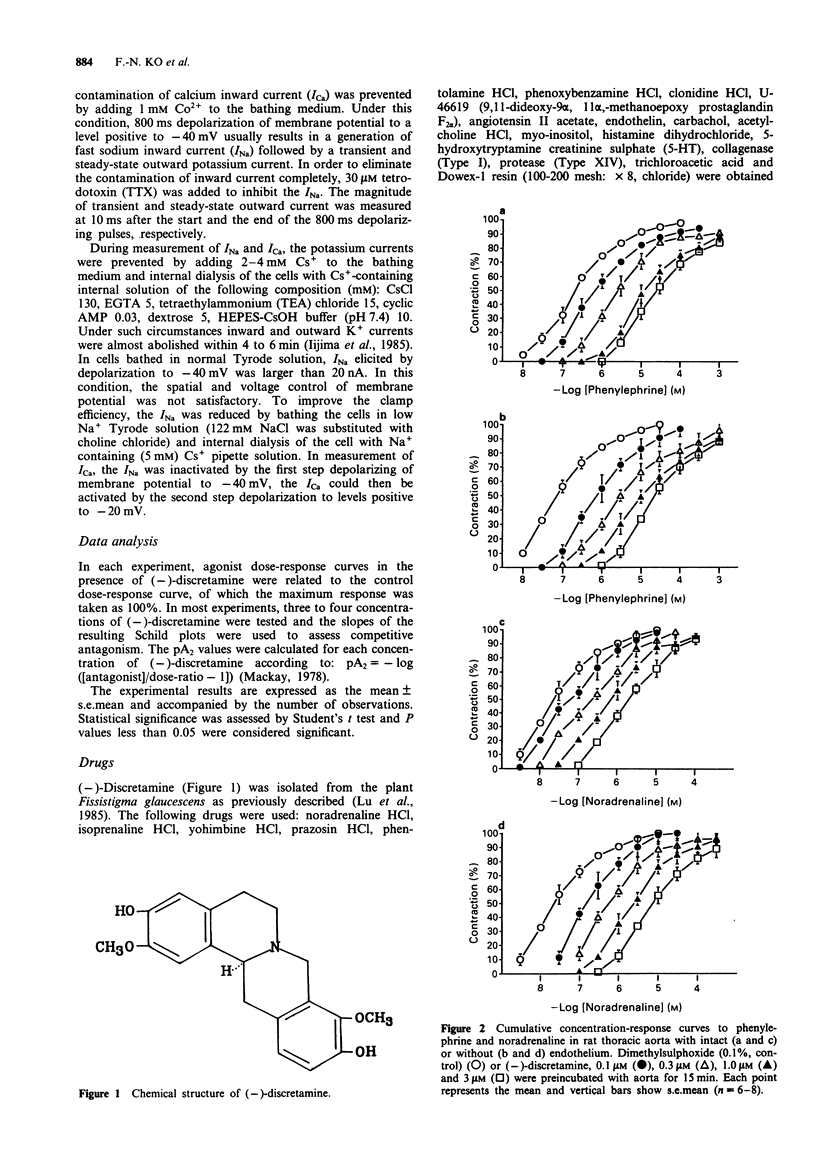
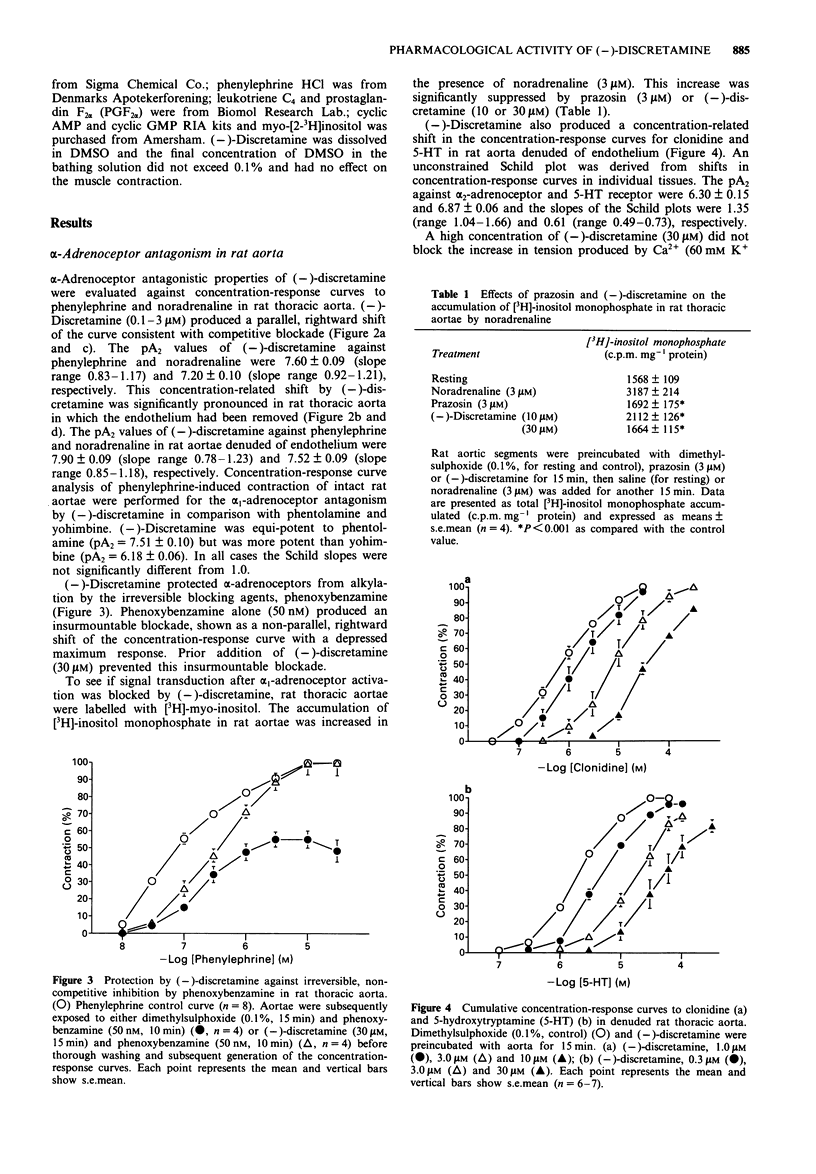
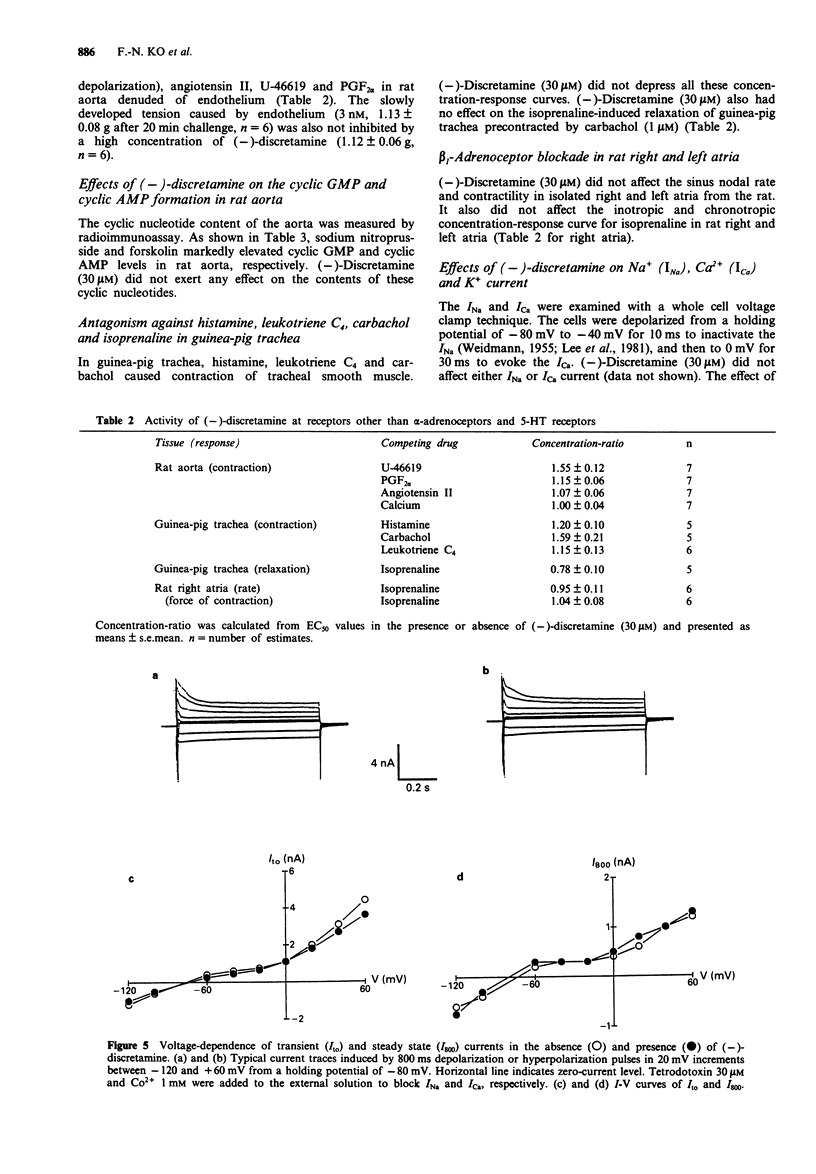
1. The pharmacological activity of (-)-discretamine, isolated from Fissistigma glaucescens, was determined in rat isolated thoracic aorta, cardiac tissues and ventricular myocytes and guinea-pig isolated trachea. 2. (-)-Discretamine was found to be an alpha 1-adrenoceptor blocking agent in rat thoracic aorta as revealed by its competitive antagonism of noradrenaline (pA2 = 7.20 +/- 0.10)- or phenylephrine (pA2 = 7.60 +/- 0.09)-induced vasoconstriction. It was as potent as phentolamine (pA2 = 7.51 +/- 0.10), but was more potent than yohimbine (pA2 = 6.18 +/- 0.06). Removal of endothelium significantly increased the antagonistic potency of (-)-discretamine on noradrenaline (pA2 = 7.52 +/- 0.09)- or phenylephrine (pA2 = 7.90 +/- 0.09)-induced vasoconstriction. 3. (-)-Discretamine was also an alpha 2-adrenoceptor blocking agent (pA2 = 6.30 +/- 0.15) and a 5-hydroxytryptamine antagonist (pA2 = 6.87 +/- 0.06), both in rat aorta denuded of endothelium. 4. (-)-Discretamine protected alpha-adrenoceptors from alkylation by the irreversible blocking agent, phenoxybenzamine. 5. [3H]-inositol monophosphate formation caused by noradrenaline (3 microM) in rat thoracic aorta was suppressed by (-)-discretamine (10 and 30 microM) and prazosin (3 microM). 6. A high concentration of (-)-discretamine (30 microM) did not affect the contraction induced by the thromboxane receptor agonist U-46619, prostaglandin F2 alpha (PGF2 alpha), angiotensin II, high K+ or endothelin in rat aorta denuded of endothelium. Neither cyclic AMP nor cyclic GMP content of rat thoracic aorta was changed by (-)-discretamine.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alosachie I., Godfraind T. Role of cyclic GMP in the modulation by endothelium of the adrenolytic action of prazosin in the rat isolated aorta. Br J Pharmacol. 1986 Nov;89(3):525–532. doi: 10.1111/j.1476-5381.1986.tb11152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apperley E., Humphrey P. P., Levy G. P. Receptors for 5-hydroxytryptamine and noradrenaline in rabbit isolated ear artery and aorta. Br J Pharmacol. 1976 Oct;58(2):211–221. doi: 10.1111/j.1476-5381.1976.tb10398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. L., French R. J., Mylecharane E. J. Receptor mechanisms for 5-hydroxytryptamine in rabbit arteries. Br J Pharmacol. 1981 Nov;74(3):619–626. doi: 10.1111/j.1476-5381.1981.tb10472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavero I., Roach A. G. The pharmacology of prazosin, a novel antihypertensive agent. Life Sci. 1980 Oct 27;27(17):1525–1540. doi: 10.1016/0024-3205(80)90561-5. [DOI] [PubMed] [Google Scholar]
- Eglème C., Godfraind T., Miller R. C. Enhanced responsiveness of rat isolated aorta to clonidine after removal of the endothelial cells. Br J Pharmacol. 1984 Jan;81(1):16–18. doi: 10.1111/j.1476-5381.1984.tb10736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruetter C. A., Barry B. K., McNamara D. B., Gruetter D. Y., Kadowitz P. J., Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5(3):211–224. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hirata M., Kohse K. P., Chang C. H., Ikebe T., Murad F. Mechanism of cyclic GMP inhibition of inositol phosphate formation in rat aorta segments and cultured bovine aortic smooth muscle cells. J Biol Chem. 1990 Jan 25;265(3):1268–1273. [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Izumi H., Kuriyama H. Mechanisms of relaxation induced by activation of beta-adrenoceptors in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1982 May;326:475–493. doi: 10.1113/jphysiol.1982.sp014207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A. Physiologic functions of normal endothelial cells. Ann N Y Acad Sci. 1985;454:279–291. doi: 10.1111/j.1749-6632.1985.tb11868.x. [DOI] [PubMed] [Google Scholar]
- Kalkman H. O., Timmermans P. B., Van Zwieten P. A. Characterization of the antihypertensive properties of ketanserin (R 41 468) in rats. J Pharmacol Exp Ther. 1982 Jul;222(1):227–231. [PubMed] [Google Scholar]
- Kauffman R. F., Schenck K. W., Utterback B. G., Crowe V. G., Cohen M. L. In vitro vascular relaxation by new inotropic agents: relationship to phosphodiesterase inhibition and cyclic nucleotides. J Pharmacol Exp Ther. 1987 Sep;242(3):864–872. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K. S., Hume J. R., Giles W., Brown A. M. Sodium current depression by lidocaine and quinidine in isolated ventricular cells. Nature. 1981 May 28;291(5813):325–327. doi: 10.1038/291325a0. [DOI] [PubMed] [Google Scholar]
- MacKay D. How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated? J Pharm Pharmacol. 1978 May;30(5):312–313. doi: 10.1111/j.2042-7158.1978.tb13237.x. [DOI] [PubMed] [Google Scholar]
- Minneman K. P. Alpha 1-adrenergic receptor subtypes, inositol phosphates, and sources of cell Ca2+. Pharmacol Rev. 1988 Jun;40(2):87–119. [PubMed] [Google Scholar]
- Mitra R., Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985 Nov;249(5 Pt 2):H1056–H1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986 Jul;78(1):1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neylon C. B., Summers R. J. Stimulation of alpha 1-adrenoceptors in rat kidney mediates increased inositol phospholipid hydrolysis. Br J Pharmacol. 1987 Jun;91(2):367–376. doi: 10.1111/j.1476-5381.1987.tb10291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousterhout J. M., Sperelakis N. Cyclic nucleotides depress action potentials in cultured aortic smooth muscle cells. Eur J Pharmacol. 1987 Nov 24;144(1):7–14. doi: 10.1016/0014-2999(87)90003-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Purdy R. E., Murray D. L., Stupecky G. L. Receptors for 5-hydroxytryptamine in rabbit blood vessels: activation of alpha adrenoceptors in rabbit thoracic aorta. J Pharmacol Exp Ther. 1987 Feb;240(2):535–541. [PubMed] [Google Scholar]
- Stanaszek W. F., Kellerman D., Brogden R. N., Romankiewicz J. A. Prazosin update. A review of its pharmacological properties and therapeutic use in hypertension and congestive heart failure. Drugs. 1983 Apr;25(4):339–384. doi: 10.2165/00003495-198325040-00002. [DOI] [PubMed] [Google Scholar]
- Titmarsh S., Monk J. P. Terazosin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in essential hypertension. Drugs. 1987 May;33(5):461–477. doi: 10.2165/00003495-198733050-00003. [DOI] [PubMed] [Google Scholar]
- Van Nueten J. M., Janssen P. A., Van Beek J., Xhonneux R., Verbeuren T. J., Vanhoutte P. M. Vascular effects of ketanserin (R 41 468), a novel antagonist of 5-HT2 serotonergic receptors. J Pharmacol Exp Ther. 1981 Jul;218(1):217–230. [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- WEIDMANN S. Effects of calcium ions and local anesthetics on electrical properties of Purkinje fibres. J Physiol. 1955 Sep 28;129(3):568–582. doi: 10.1113/jphysiol.1955.sp005379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zifa E., Fillion G. 5-Hydroxytryptamine receptors. Pharmacol Rev. 1992 Sep;44(3):401–458. [PubMed] [Google Scholar]


