Abstract
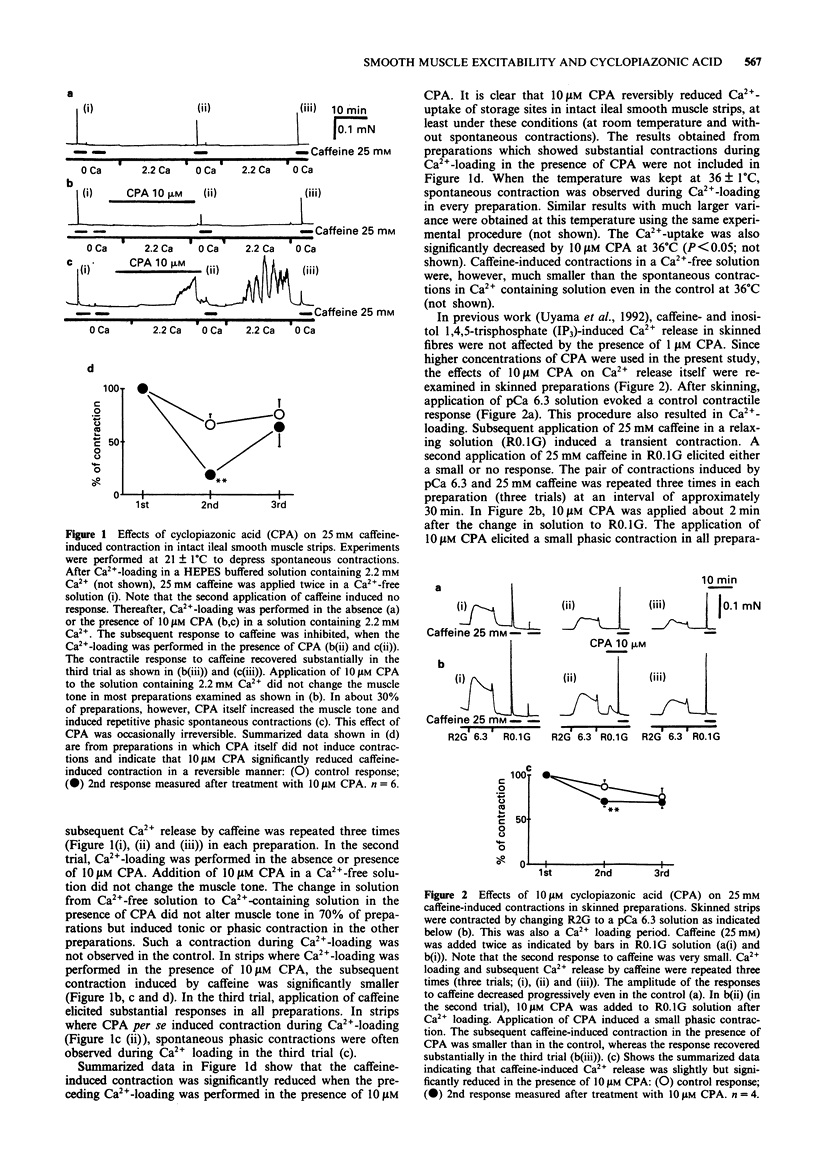
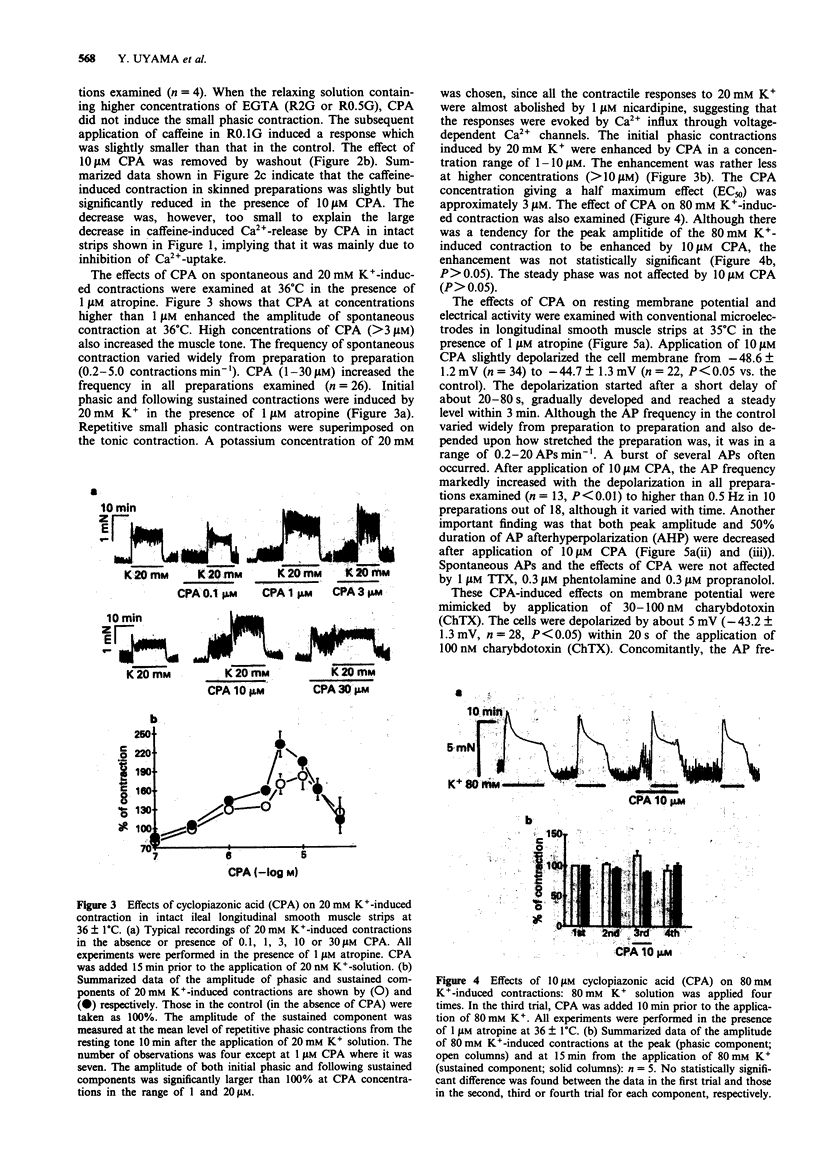
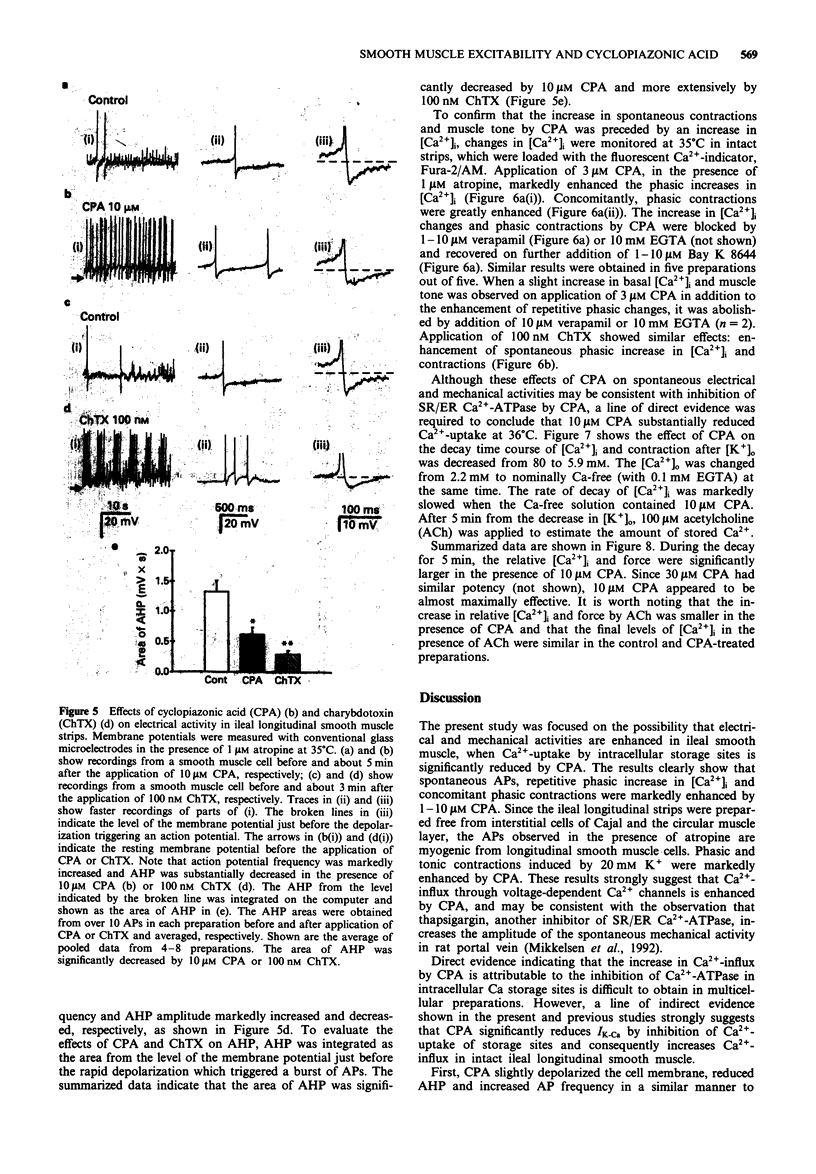
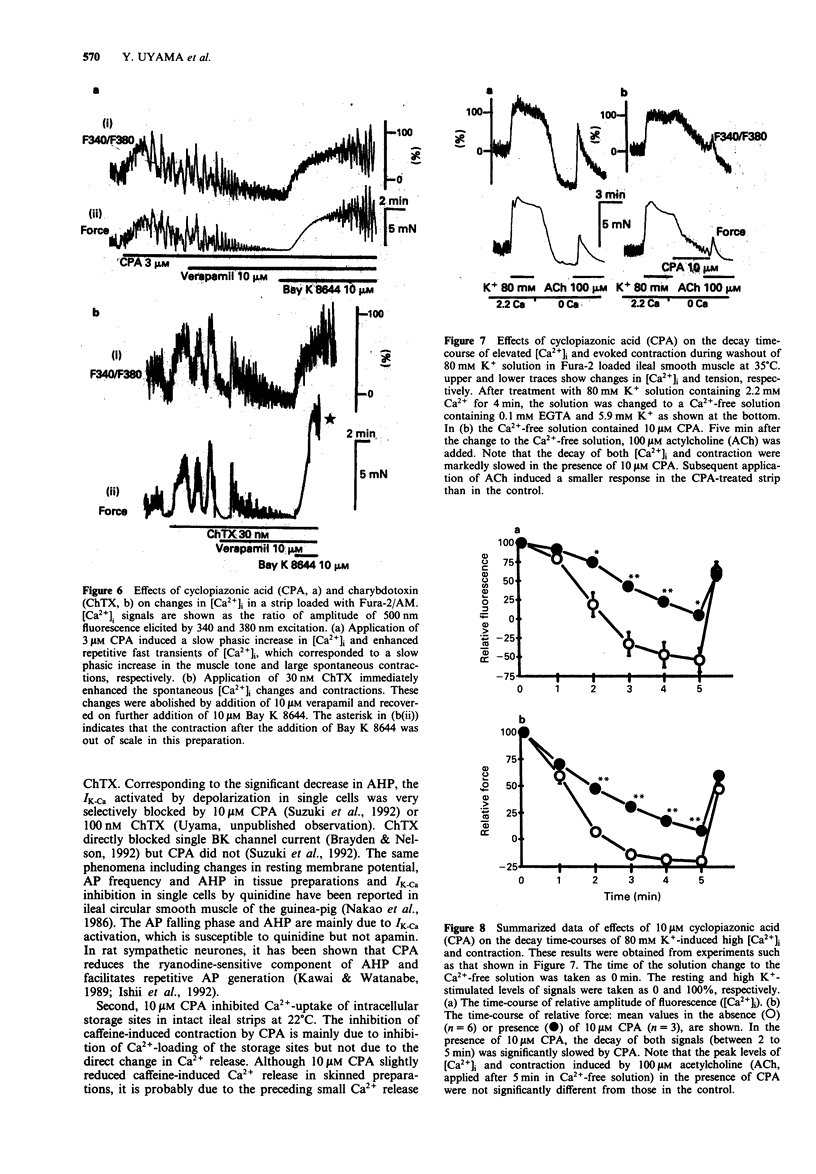
1. Effects of cyclopiazonic acid (CPA), a specific inhibitor of Ca(2+)-ATPase in endo- and sarcoplasmic reticulum (ER/SR), on contractile responses, cytosolic Ca2+ concentration and spontaneous electrical activity were examined in ileal longitudinal smooth muscle strips. 2. After intracellular stored Ca2+ in intact ileal strips was depleted by application of 25 mM caffeine in Ca(2+)-free solution, Ca(2+)-loading was performed in the absence or presence of 10 microns CPA in a standard solution containing 2.2 mM Ca2+. Subsequent application of caffeine in Ca(2+)-free solution induced a phasic contraction which was significantly smaller in the strip pretreated with CPA than that in the control. 3. Spontaneous and 20 mM K(+)-induced contractions in the presence of 1 microM atropine were markedly enhanced by 1-30 microM CPA, whereas that induced by 80 mM K+ was not. The magnitude of repetitive transient elevation of cytosolic Ca2+ concentration ([Ca2+])i) and concomitant phasic contractions were markedly enhanced by CPA. The effects were abolished by 10 microM verapamil and restored by 10 microM Bay K 8644. 4. Application of 10 microM CPA depolarized the cell by about 5 mV, decreased the action potential (AP) afterhyperpolarization and markedly increased the frequency of spontaneous AP. These effects were mimicked by 100 nM charybdotoxin. 5. The rate of decay of [Ca2+]i and tension after the bathing solution was changed from one containing 140 mM K+ and 2.2 mM Ca2+ to one containing 5.9 mM K+ and 0 mM Ca2+ was significantly slowed when 10 microM CPA was added to the latter solution.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amédée T., Benham C. D., Bolton T. B., Byrne N. G., Large W. A. Potassium, chloride and non-selective cation conductances opened by noradrenaline in rabbit ear artery cells. J Physiol. 1990 Apr;423:551–568. doi: 10.1113/jphysiol.1990.sp018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. L., Singer J. J., Walsh J. V., Jr, Fay F. S. Regulation of calcium concentration in voltage-clamped smooth muscle cells. Science. 1989 Apr 14;244(4901):211–214. doi: 10.1126/science.2704996. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourreau J. P., Abela A. P., Kwan C. Y., Daniel E. E. Acetylcholine Ca2+ stores refilling directly involves a dihydropyridine-sensitive channel in dog trachea. Am J Physiol. 1991 Sep;261(3 Pt 1):C497–C505. doi: 10.1152/ajpcell.1991.261.3.C497. [DOI] [PubMed] [Google Scholar]
- Brayden J. E., Nelson M. T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992 Apr 24;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Byron K. L., Babnigg G., Villereal M. L. Bradykinin-induced Ca2+ entry, release, and refilling of intracellular Ca2+ stores. Relationships revealed by image analysis of individual human fibroblasts. J Biol Chem. 1992 Jan 5;267(1):108–118. [PubMed] [Google Scholar]
- Chen Q., Cannell M., van Breemen C. The superficial buffer barrier in vascular smooth muscle. Can J Physiol Pharmacol. 1992 Apr;70(4):509–514. doi: 10.1139/y92-066. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Deng H. W., Kwan C. Y. Cyclopiazonic acid is a sarcoplasmic reticulum Ca(2+)-pump inhibitor of rat aortic muscle. Zhongguo Yao Li Xue Bao. 1991 Jan;12(1):53–58. [PubMed] [Google Scholar]
- Dolor R. J., Hurwitz L. M., Mirza Z., Strauss H. C., Whorton A. R. Regulation of extracellular calcium entry in endothelial cells: role of intracellular calcium pool. Am J Physiol. 1992 Jan;262(1 Pt 1):C171–C181. doi: 10.1152/ajpcell.1992.262.1.C171. [DOI] [PubMed] [Google Scholar]
- Ganitkevich V. Y., Isenberg G. Contribution of Ca(2+)-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. J Physiol. 1992 Dec;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T., Dorner J. W., Cole R. J. Cyclopiazonic acid inhibition of the Ca2+-transport ATPase in rat skeletal muscle sarcoplasmic reticulum vesicles. Biochem Pharmacol. 1988 Mar 1;37(5):978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- Goeger D. E., Riley R. T. Interaction of cyclopiazonic acid with rat skeletal muscle sarcoplasmic reticulum vesicles. Effect on Ca2+ binding and Ca2+ permeability. Biochem Pharmacol. 1989 Nov 15;38(22):3995–4003. doi: 10.1016/0006-2952(89)90679-5. [DOI] [PubMed] [Google Scholar]
- Hoth M., Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Yamamoto Y., Kao C. Y. The Ca2+-activated K+ channel and its functional roles in smooth muscle cells of guinea pig taenia coli. J Gen Physiol. 1989 Nov;94(5):833–847. doi: 10.1085/jgp.94.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Calcium-induced calcium release mechanism in guinea pig taenia caeci. J Gen Physiol. 1989 Aug;94(2):363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Watanabe M. Characteristics of transient outward currents in single smooth muscle cells from the ureter of the guinea-pig. J Physiol. 1990 Aug;427:301–324. doi: 10.1113/jphysiol.1990.sp018173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Kawai T., Watanabe M. Inhibitory effects of cyclopiazonic acid on the spike after-hyperpolarization in rat sympathetic neurons. Jpn J Pharmacol. 1992 Apr;58(4):451–456. doi: 10.1254/jjp.58.451. [DOI] [PubMed] [Google Scholar]
- Janssen L. J., Sims S. M. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol. 1992;453:197–218. doi: 10.1113/jphysiol.1992.sp019224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T., Watanabe M. Effects of ryanodine on the spike after-hyperpolarization in sympathetic neurones of the rat superior cervical ganglion. Pflugers Arch. 1989 Mar;413(5):470–475. doi: 10.1007/BF00594175. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Sakai T., Kajioka S., Kuriyama H. Activations of the Ca dependent K channel by Ca released from the sarcoplasmic reticulum of mammalian smooth muscles. Biomed Biochim Acta. 1989;48(5-6):S364–S369. [PubMed] [Google Scholar]
- Kitazawa T., Somlyo A. P. Desensitization and muscarinic re-sensitization of force and myosin light chain phosphorylation to cytoplasmic Ca2+ in smooth muscle. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1291–1297. doi: 10.1016/0006-291x(90)91589-k. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kitazawa T., Somlyo A. V., Somlyo A. P. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989 Oct 25;264(30):17997–18004. [PubMed] [Google Scholar]
- Kurebayashi N., Ogawa Y. Discrimination of Ca(2+)-ATPase activity of the sarcoplasmic reticulum from actomyosin-type ATPase activity of myofibrils in skinned mammalian skeletal muscle fibres: distinct effects of cyclopiazonic acid on the two ATPase activities. J Muscle Res Cell Motil. 1991 Aug;12(4):355–365. doi: 10.1007/BF01738590. [DOI] [PubMed] [Google Scholar]
- Low A. M., Kwan C. Y., Daniel E. E. Evidence for two types of internal Ca2+ stores in canine mesenteric artery with different refilling mechanisms. Am J Physiol. 1992 Jan;262(1 Pt 2):H31–H37. doi: 10.1152/ajpheart.1992.262.1.H31. [DOI] [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- Mikkelsen E. O., Poulsen S. H., Christensen S. B. Comparison of the effects of thapsigargin and BAY K 8644 on spontaneous mechanical activity in rat portal vein and contractile responses of rat cardiac muscle. Pharmacol Toxicol. 1992 Feb;70(2):152–156. doi: 10.1111/j.1600-0773.1992.tb00447.x. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Declerck I., Droogmans G., Plessers L., De Smedt H., Raeymaekers L., Casteels R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J Physiol. 1990 Aug;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Morad M. Ca2+ and Ca2+-activated K+ currents in mammalian gastric smooth muscle cells. Science. 1985 Jul 19;229(4710):269–272. doi: 10.1126/science.2409600. [DOI] [PubMed] [Google Scholar]
- Nakao K., Inoue R., Yamanaka K., Kitamura K. Actions of quinidine and apamin on after-hyperpolarization of the spike in circular smooth muscle cells of the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1986 Dec;334(4):508–513. doi: 10.1007/BF00569394. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Kitamura K., Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987 Apr;252(4 Pt 1):C401–C410. doi: 10.1152/ajpcell.1987.252.4.C401. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Sato K., Satoh T., Karaki H. Simultaneous recordings of calcium signals and mechanical activity using fluorescent dye fura 2 in isolated strips of vascular smooth muscle. Jpn J Pharmacol. 1987 Nov;45(3):429–433. doi: 10.1254/jjp.45.429. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Sakai T., Terada K., Kitamura K., Kuriyama H. Ryanodine inhibits the Ca-dependent K current after depletion of Ca stored in smooth muscle cells of the rabbit ileal longitudinal muscle. Br J Pharmacol. 1988 Dec;95(4):1089–1100. doi: 10.1111/j.1476-5381.1988.tb11743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Shima H., Blaustein M. P. Modulation of evoked contractions in rat arteries by ryanodine, thapsigargin, and cyclopiazonic acid. Circ Res. 1992 May;70(5):968–977. doi: 10.1161/01.res.70.5.968. [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L., Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehno-Bittel L., Sturek M. Spontaneous sarcoplasmic reticulum calcium release and extrusion from bovine, not porcine, coronary artery smooth muscle. J Physiol. 1992;451:49–78. doi: 10.1113/jphysiol.1992.sp019153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M., Muraki K., Imaizumi Y., Watanabe M. Cyclopiazonic acid, an inhibitor of the sarcoplasmic reticulum Ca(2+)-pump, reduces Ca(2+)-dependent K+ currents in guinea-pig smooth muscle cells. Br J Pharmacol. 1992 Sep;107(1):134–140. doi: 10.1111/j.1476-5381.1992.tb14475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Takemura H., Ohshika H., Yokosawa N., Oguma K., Thastrup O. The thapsigargin-sensitive intracellular Ca2+ pool is more important in plasma membrane Ca2+ entry than the IP3-sensitive intracellular Ca2+ pool in neuronal cell lines. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1518–1526. doi: 10.1016/s0006-291x(05)81368-3. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992 May;106(1):208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Imaizumi Y., Muraki K., Takeda M. A comparative study about voltage-dependent Ca currents in smooth muscle cells isolated from several tissues. Adv Exp Med Biol. 1989;255:119–128. doi: 10.1007/978-1-4684-5679-0_13. [DOI] [PubMed] [Google Scholar]
- Wong B. S. Ionic conductances in dissociated smooth muscle cells. Proc Soc Exp Biol Med. 1991 Jun;197(2):125–134. doi: 10.3181/00379727-197-43234. [DOI] [PubMed] [Google Scholar]
- Xiong Z. L., Kitamura K., Kuriyama H. Evidence for contribution of Ca2+ storage sites on unitary K+ channel currents in inside-out membrane of rabbit portal vein. Pflugers Arch. 1992 Jan;420(1):112–114. doi: 10.1007/BF00378651. [DOI] [PubMed] [Google Scholar]
- Xuan Y. T., Wang O. L., Whorton A. R. Thapsigargin stimulates Ca2+ entry in vascular smooth muscle cells: nicardipine-sensitive and -insensitive pathways. Am J Physiol. 1992 May;262(5 Pt 1):C1258–C1265. doi: 10.1152/ajpcell.1992.262.5.C1258. [DOI] [PubMed] [Google Scholar]


