Abstract
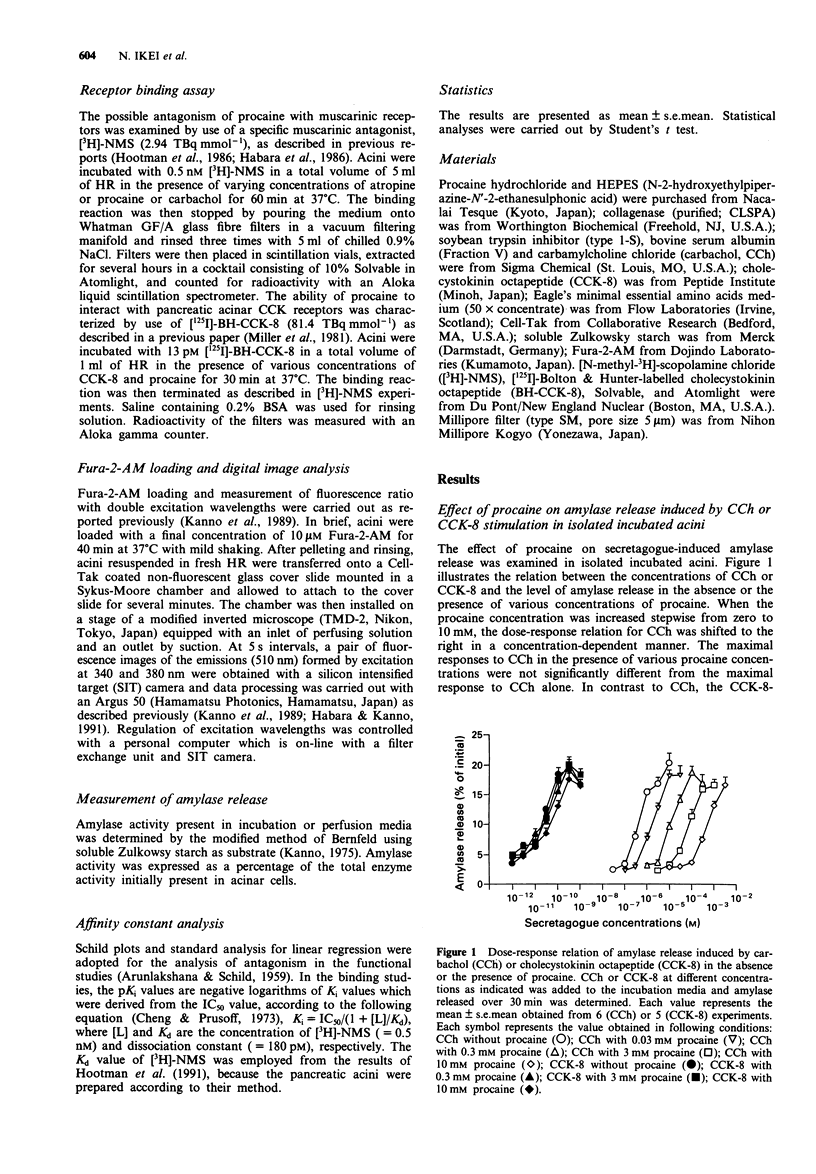
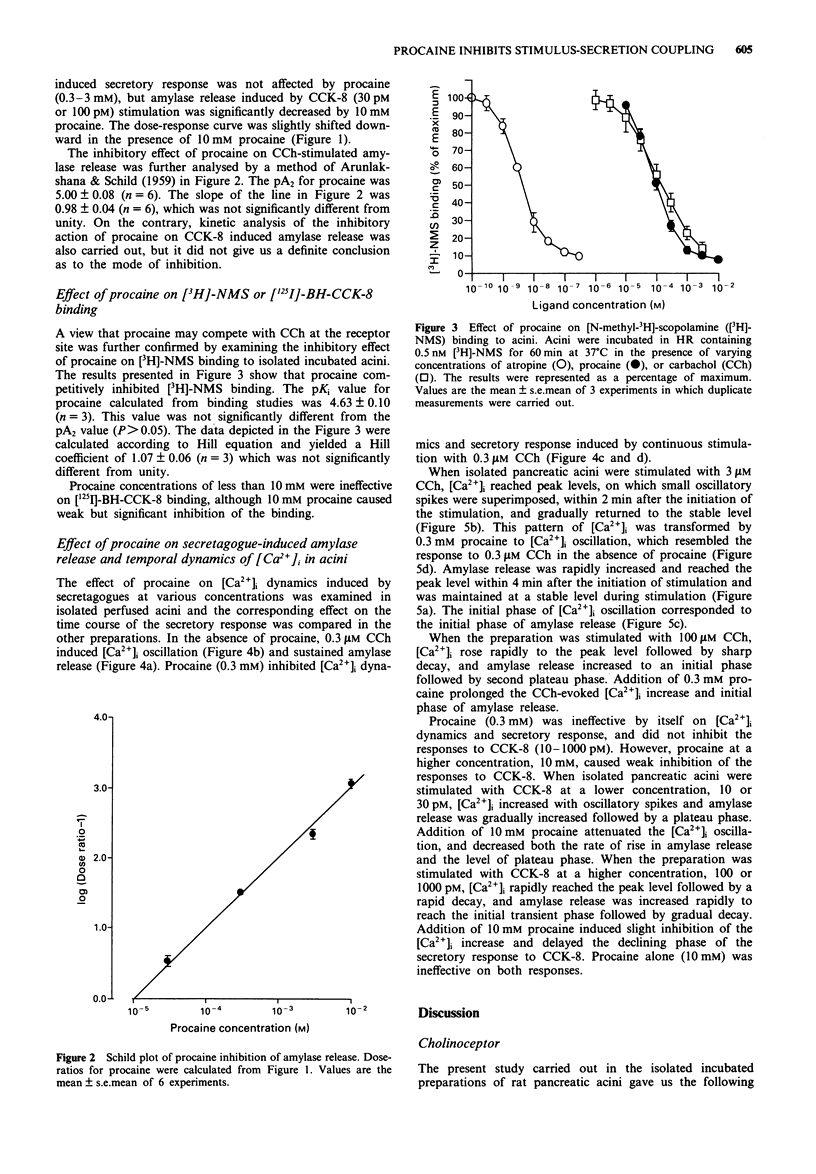
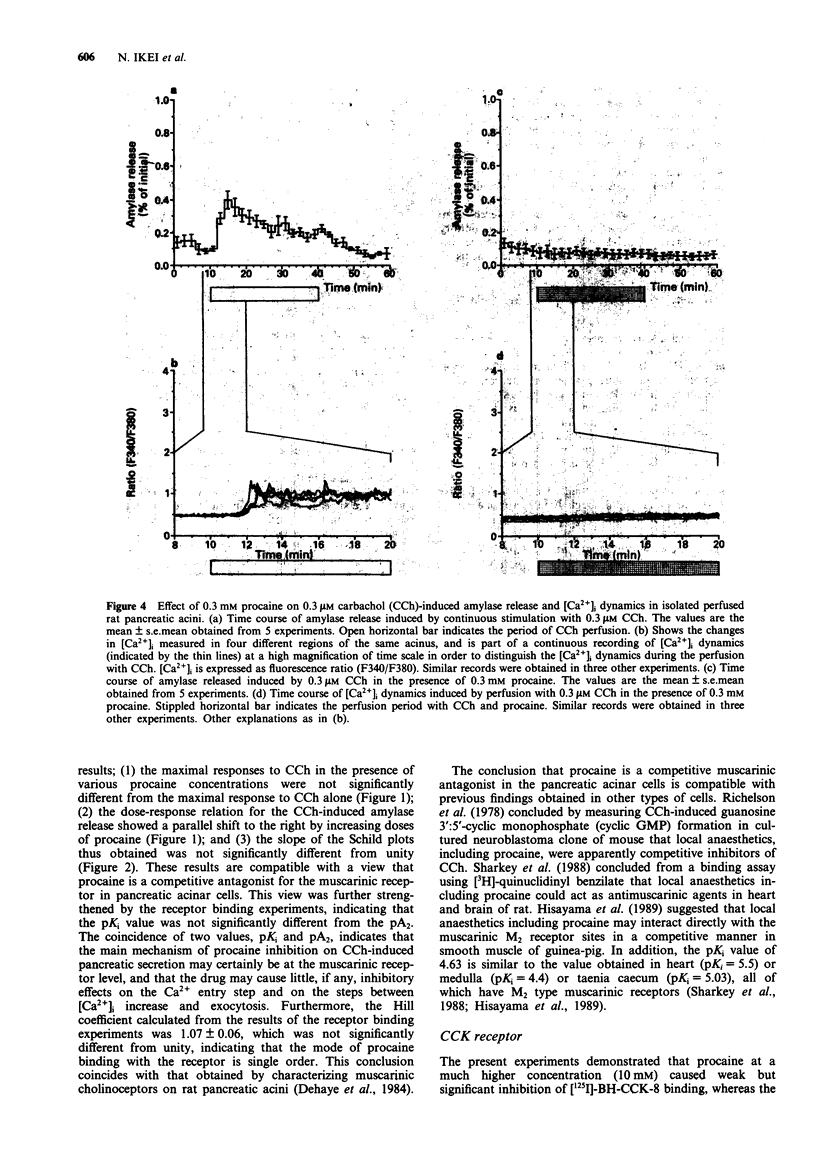
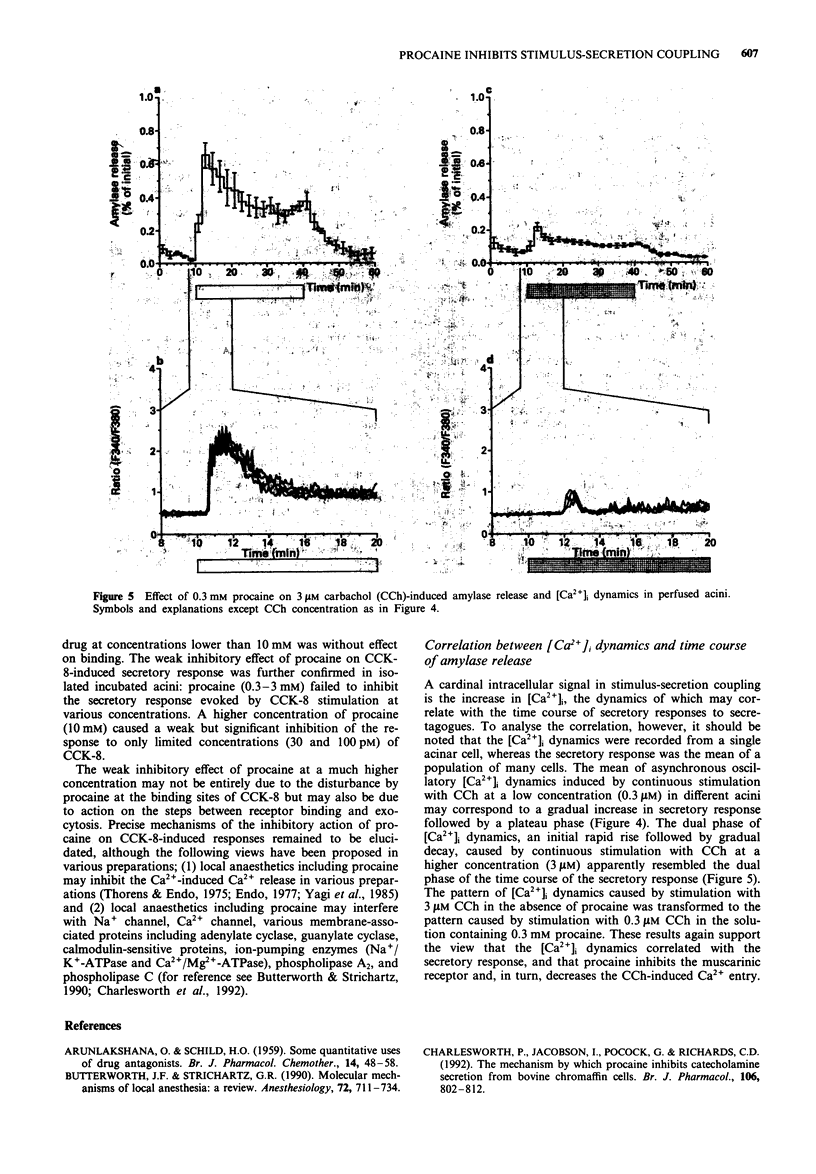
1. Procaine (0.03-10 mM) inhibited carbachol (CCh)-induced amylase release from rat isolated pancreatic acini in a competitive manner. Kinetic analysis of the relation between CCh concentrations and the amount of amylase released in the presence of various procaine concentrations indicated that procaine caused competitive inhibition with the affinity constant (pA2) value of 5.00 +/- 0.08. 2. Receptor binding assay confirmed that procaine (0.01-10 mM) competitively inhibited [N-methyl-3H]-scopolamine chloride ([3H]-NMS) binding to its receptor with binding affinity (pKi) of 4.63 +/- 0.10. 3. Procaine transformed CCh-evoked [Ca2+]i dynamics: the initial rise in [Ca2+]i followed by a gradual decay during continuous stimulation with 3 microM CCh was transformed by 0.3 mM procaine to the oscillatory [Ca2+]i dynamics, which resembled the response to 0.3 microM CCh in the absence of procaine. The initial phase of [Ca2+]i oscillation corresponded to the initial phase of CCh-induced amylase release in isolated perfused acini. 4. Procaine (0.3-3 mM) did not inhibit the secretory response to cholecystokinin octapeptide (CCK-8) in isolated incubated acini. A higher concentration of procaine (10 mM) caused weak but significant inhibition of the response to only limited concentrations of CCK-8, 30 and 100 pM. Procaine lower than 10 mM was ineffective on [125I]-BH-CCK-8 binding, although procaine (10 mM) caused weak but significant inhibition of the binding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth J. F., 4th, Strichartz G. R. Molecular mechanisms of local anesthesia: a review. Anesthesiology. 1990 Apr;72(4):711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- Charlesworth P., Jacobson I., Pocock G., Richards C. D. The mechanism by which procaine inhibits catecholamine secretion from bovine chromaffin cells. Br J Pharmacol. 1992 Aug;106(4):802–812. doi: 10.1111/j.1476-5381.1992.tb14416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dehaye J. P., Winand J., Poloczek P., Christophe J. Characterization of muscarinic cholinergic receptors on rat pancreatic acini by N-[3H]methylscopolamine binding. Their relationship with calcium 45 efflux and amylase secretion. J Biol Chem. 1984 Jan 10;259(1):294–300. [PubMed] [Google Scholar]
- Douglas W. W., Kanno T. The effect of amethocaine on acetylcholine-induced depolarization and catecholamine secretion in the adrenal chromaffin cell. Br J Pharmacol Chemother. 1967 Aug;30(3):612–619. doi: 10.1111/j.1476-5381.1967.tb02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Habara Y., Kanno T. Dose-dependency in spatial dynamics of [Ca2+]c in pancreatic acinar cells. Cell Calcium. 1991 Sep;12(8):533–542. doi: 10.1016/0143-4160(91)90073-n. [DOI] [PubMed] [Google Scholar]
- Habara Y., Williams J. A., Hootman S. R. Antimuscarinic effects of chloroquine in rat pancreatic acini. Biochem Biophys Res Commun. 1986 Jun 13;137(2):664–669. doi: 10.1016/0006-291x(86)91129-0. [DOI] [PubMed] [Google Scholar]
- Hisayama T., Takayanagi I., Kumagai N., Kubo H. Interaction of 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride, ryanodine and procaine with muscarinic cholinergic M2 receptor sites in smooth muscle. J Pharmacol Exp Ther. 1989 May;249(2):646–651. [PubMed] [Google Scholar]
- Hootman S. R., Brown M. E., Williams J. A., Logsdon C. D. Regulation of muscarinic acetylcholine receptors in cultured guinea pig pancreatic acini. Am J Physiol. 1986 Jul;251(1 Pt 1):G75–G83. doi: 10.1152/ajpgi.1986.251.1.G75. [DOI] [PubMed] [Google Scholar]
- Hootman S. R., Valles S. M., Kovalcik S. A. Mechanism of agonist-induced downregulation of muscarinic receptors in rat pancreatic acini. Am J Physiol. 1991 Jul;261(1 Pt 1):G128–G135. doi: 10.1152/ajpgi.1991.261.1.G128. [DOI] [PubMed] [Google Scholar]
- Imamura K., Wakasugi H., Shinozaki H., Ibayashi H. Dynamic analysis of secretagogue-induced amylase secretion from rat pancreatic acini studied by perifusion system. Jpn J Physiol. 1983;33(5):687–698. doi: 10.2170/jjphysiol.33.687. [DOI] [PubMed] [Google Scholar]
- Kanno T. Calcium-dependent amylase release and electrophysiological measurements in cells of the pancreas. J Physiol. 1972 Oct;226(2):353–371. doi: 10.1113/jphysiol.1972.sp009988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T. The electrogenic sodium pump in the hyperpolarizing and secretory effects of pancreozymin in the pancreatic acinar cell. J Physiol. 1975 Mar;245(3):599–616. doi: 10.1113/jphysiol.1975.sp010864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Rosenzweig S. A., Jamieson J. D. Preparation and characterization of a probe for the cholecystokinin octapeptide receptor, N alpha (125I-desaminotyrosyl)CCK-8, and its interactions with pancreatic acini. J Biol Chem. 1981 Dec 10;256(23):12417–12423. [PubMed] [Google Scholar]
- Richelson E., Prendergast F. G., Divinetz-Romero S. Muscarinic receptor-mediated cyclic GMP formation by cultured nerve cells--ionic dependence and effects of local anesthetics. Biochem Pharmacol. 1978;27(16):2039–2048. doi: 10.1016/0006-2952(78)90064-3. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Feinstein M. B., Jaanus S. D., Paimre M. Inhibition of catecholamine secretion and calcium exchange in perfused cat adrenal glands by tetracaine and magnesium. J Pharmacol Exp Ther. 1967 Mar;155(3):463–471. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- Sharkey J., Ritz M. C., Schenden J. A., Hanson R. C., Kuhar M. J. Cocaine inhibits muscarinic cholinergic receptors in heart and brain. J Pharmacol Exp Ther. 1988 Sep;246(3):1048–1052. [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J., Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium. 1985 Apr;6(1-2):145–157. doi: 10.1016/0143-4160(85)90041-7. [DOI] [PubMed] [Google Scholar]


