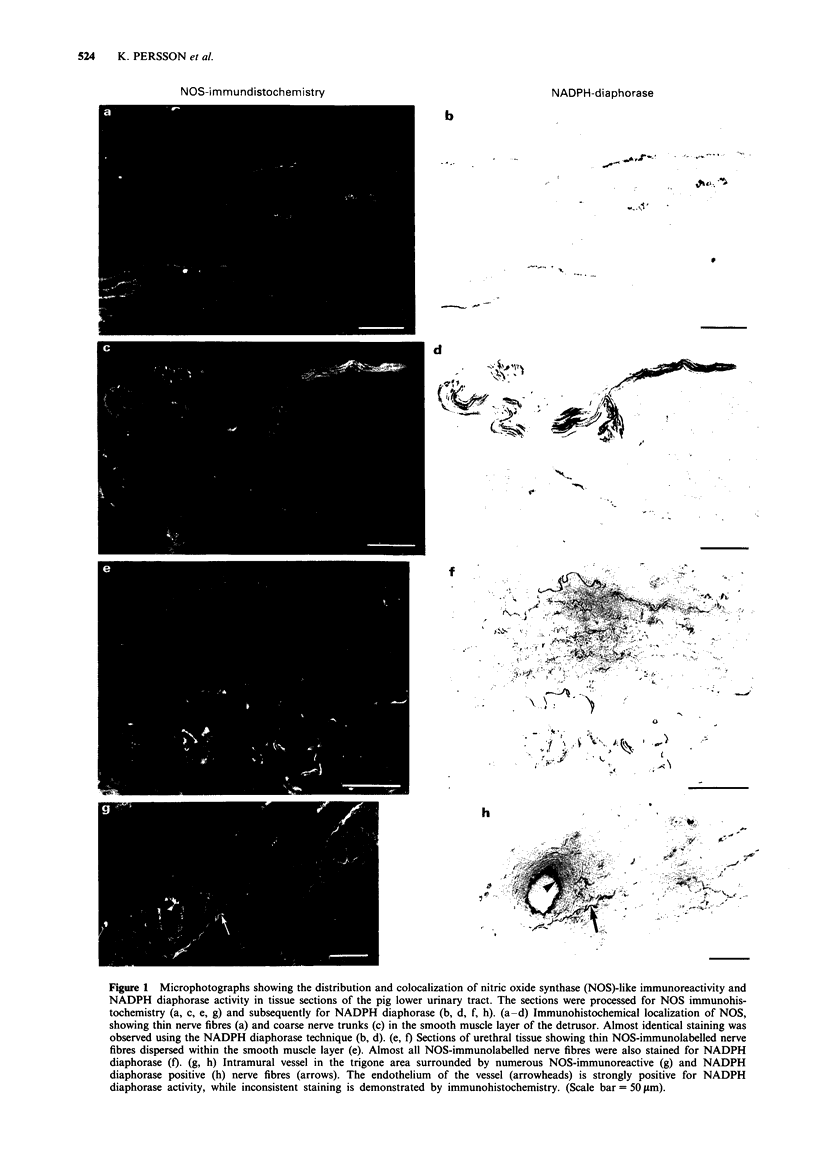
Abstract
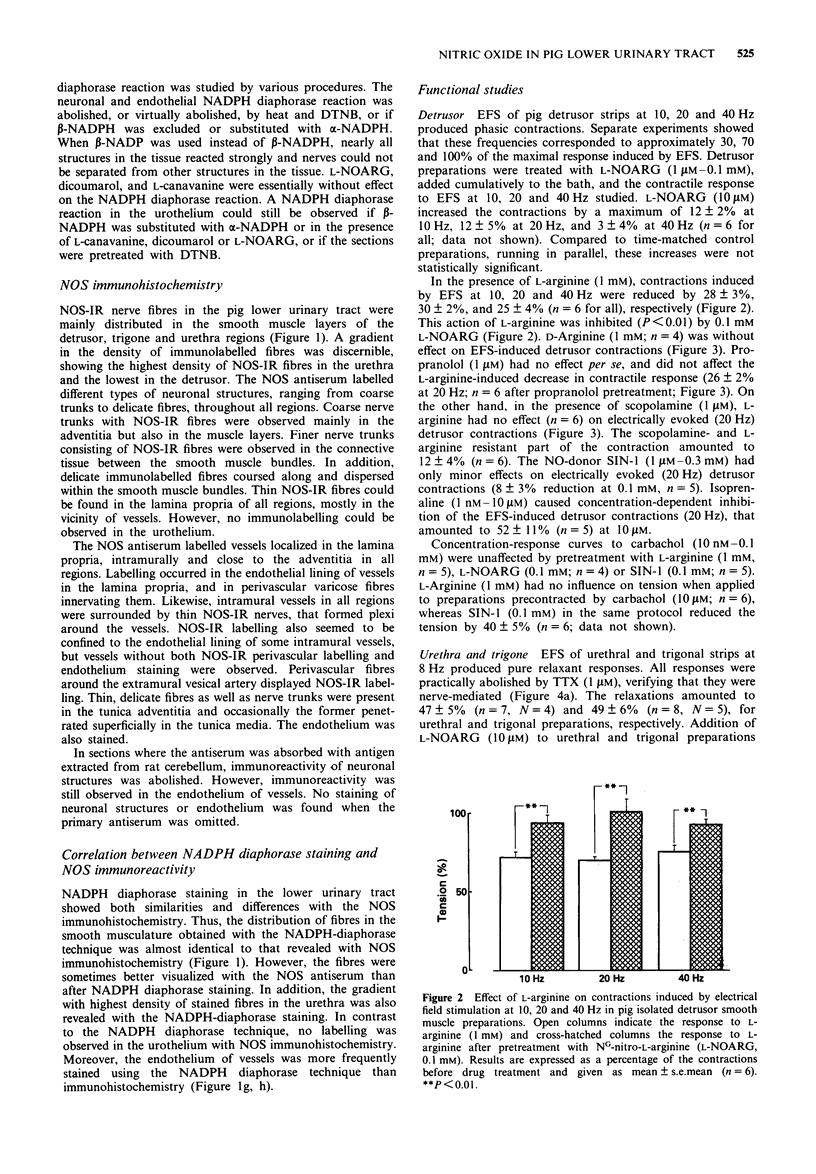
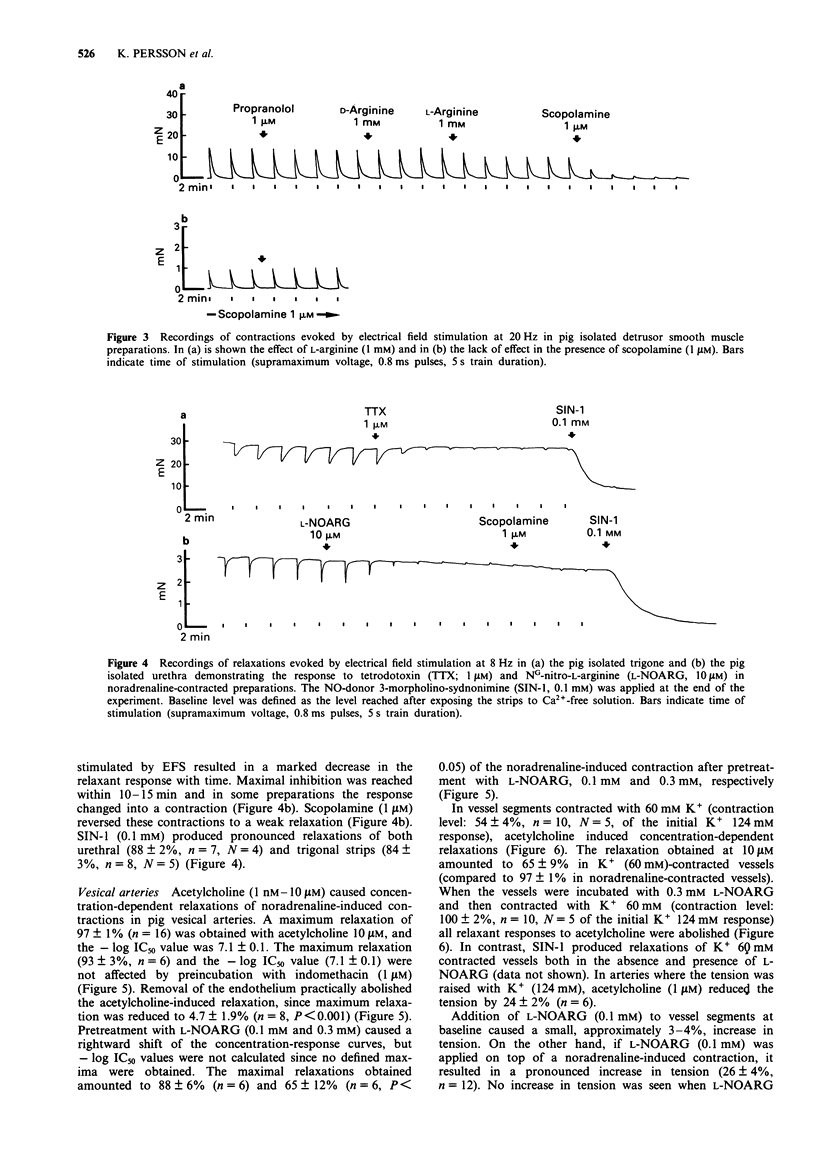
1. The distribution and colocalization of nitric oxide synthase (NOS)-like immunoreactivity and NADPH diaphorase activity in the pig lower urinary tract were investigated by immunohistochemical and histochemical staining techniques. Functional in vitro studies were performed to correlate the presence of NOS-immunoreactivity/NADPH diaphorase staining with smooth muscle responses involving the L-arginine/nitric oxide (NO) pathway. 2. NOS-immunoreactivity and NADPH diaphorase activity were expressed in nerve trunks and fine nerve fibres in and/or around muscular bundles in the detrusor, trigone and urethra. Thin nerve fibres that dispersed within the muscle bundles were mainly found in the urethral/trigonal area, whereas such fibres were less common in the detrusor. 3. Almost all neuronal structures that were NOS-immunolabeled were also stained for NADPH diaphorase. In contrast, the urothelium, which was intensively stained by the NADPH diaphorase technique, remained unstained by immunohistochemistry. 4. Electrical field stimulation of pig isolated trigonal and urethral preparations induced relaxations, which were inhibited by tetrodotoxin (1 microM) and NG-nitro-L-arginine (L-NOARG, 10 microM). 5. L-Arginine (1 mM), but not D-arginine, inhibited (25-30%) electrically evoked detrusor contractions. This inhibition was reversed by L-NOARG (0.1 mM). L-Arginine did not inhibit detrusor contractions in the presence of scopolamine (1 microM) and had no direct smooth muscle effects per se. 6. Acetylcholine (1 nM-10 microM) caused concentration-dependent relaxations of noradrenaline-induced contractions in pig vesical arteries. Removal of the endothelium practically abolished the acetylcholine-induced relaxation. Pretreatment with L-NOARG (0.1 mM and 0.3 mM) caused a rightward shift of the concentration-response curves to acetylcholine, but the maximal relaxation obtained was significantly reduced (to 65 +/- 12%; n = 6; P < 0.05) only at 0.3 mM L-NOARG. 7. In vessel segments contracted with K+ (60 mM), acetylcholine induced concentration-dependent relaxations. When the vessels were incubated with 0.3 mM L-NOARG and then contracted with K+ (60 mM) all relaxant responses to acetylcholine were abolished. 8. The presence of NO synthesizing enzyme in nerve fibres and the pharmacological evidence for NO-mediated relaxation of the trigone and urethra suggest that NO or a NO-related substance may have a role in inhibitory neurotransmission in these regions. In the detrusor, the presence of NO-synthesizing enzyme in nerves can be demonstrated, but its functional importance is unclear. NO, as well as other endothelium-derived factors seem to be involved in the endothelium-dependent acetylcholine-induced relaxation of pig vesical arteries.
Full text
PDF





Images in this article
Selected References
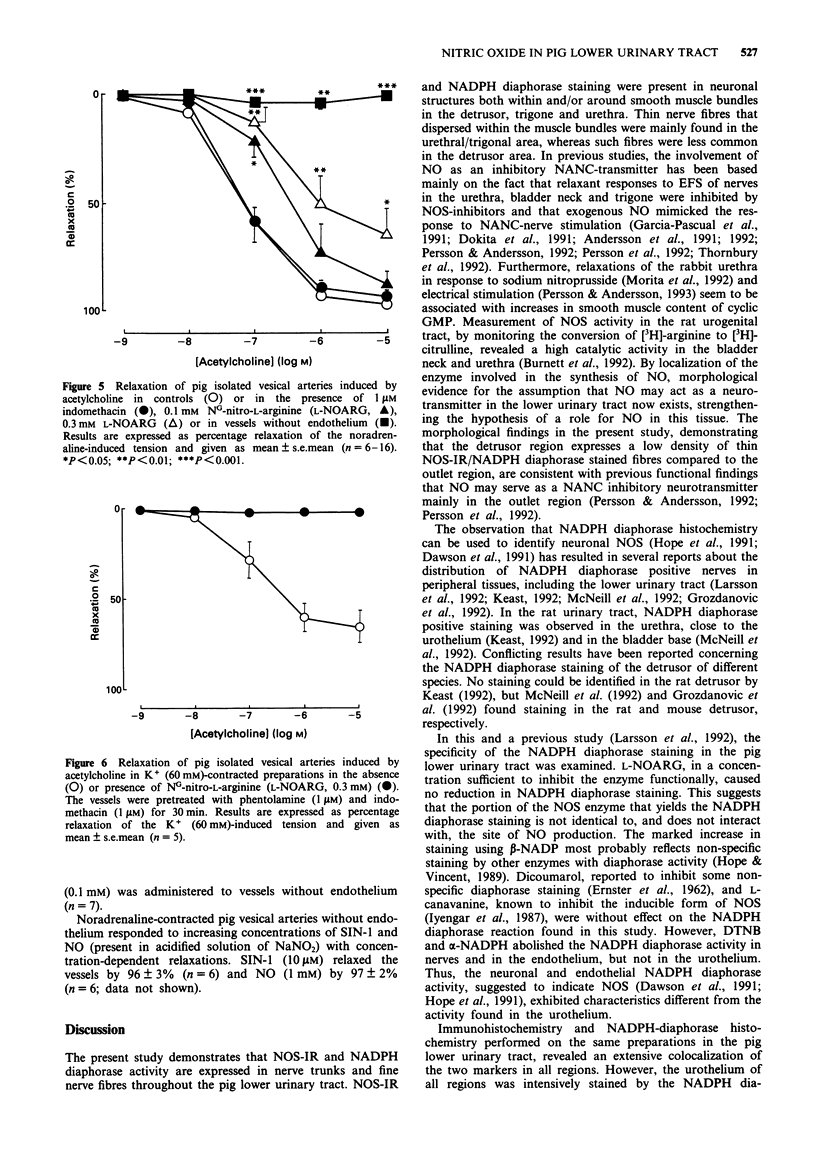
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K. E., Garcia Pascual A., Forman A., Tøttrup A. Non-adrenergic, non-cholinergic nerve-mediated relaxation of rabbit urethra is caused by nitric oxide. Acta Physiol Scand. 1991 Jan;141(1):133–134. doi: 10.1111/j.1748-1716.1991.tb09056.x. [DOI] [PubMed] [Google Scholar]
- Andersson K. E., Garcia Pascual A., Persson K., Forman A., Tøttrup A. Electrically-induced, nerve-mediated relaxation of rabbit urethra involves nitric oxide. J Urol. 1992 Jan;147(1):253–259. doi: 10.1016/s0022-5347(17)37208-7. [DOI] [PubMed] [Google Scholar]
- Belvisi M. G., Stretton D., Barnes P. J. Nitric oxide as an endogenous modulator of cholinergic neurotransmission in guinea-pig airways. Eur J Pharmacol. 1991 Jun 6;198(2-3):219–221. doi: 10.1016/0014-2999(91)90626-2. [DOI] [PubMed] [Google Scholar]
- Brave S. R., Hobbs A. J., Gibson A., Tucker J. F. The influence of L-NG-nitro-arginine on field stimulation induced contractions and acetylcholine release in guinea pig isolated tracheal smooth muscle. Biochem Biophys Res Commun. 1991 Sep 16;179(2):1017–1022. doi: 10.1016/0006-291x(91)91920-8. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Burnett A. L., Lowenstein C. J., Bredt D. S., Chang T. S., Snyder S. H. Nitric oxide: a physiologic mediator of penile erection. Science. 1992 Jul 17;257(5068):401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Chapple C. R., Milner P., Moss H. E., Burnstock G. Loss of sensory neuropeptides in the obstructed human bladder. Br J Urol. 1992 Oct;70(4):373–381. doi: 10.1111/j.1464-410x.1992.tb15791.x. [DOI] [PubMed] [Google Scholar]
- Crowe R., Burnstock G. A histochemical and immunohistochemical study of the autonomic innervation of the lower urinary tract of the female pig. Is the pig a good model for the human bladder and urethra? J Urol. 1989 Feb;141(2):414–422. doi: 10.1016/s0022-5347(17)40785-3. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai K. M., Sessa W. C., Vane J. R. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991 Jun 6;351(6326):477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Dokita S., Morgan W. R., Wheeler M. A., Yoshida M., Latifpour J., Weiss R. M. NG-nitro-L-arginine inhibits non-adrenergic, non-cholinergic relaxation in rabbit urethral smooth muscle. Life Sci. 1991;48(25):2429–2436. doi: 10.1016/0024-3205(91)90377-n. [DOI] [PubMed] [Google Scholar]
- ERNSTER L., DANIELSON L., LJUNGGREN M. DT diaphorase. I. Purification from the soluble fraction of rat-liver cytoplasm, and properties. Biochim Biophys Acta. 1962 Apr 9;58:171–188. doi: 10.1016/0006-3002(62)90997-6. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Pascual A., Costa G., Garcia-Sacristan A., Andersson K. E. Relaxation of sheep urethral muscle induced by electrical stimulation of nerves: involvement of nitric oxide. Acta Physiol Scand. 1991 Apr;141(4):531–539. doi: 10.1111/j.1748-1716.1991.tb09114.x. [DOI] [PubMed] [Google Scholar]
- González C., Estrada C. Nitric oxide mediates the neurogenic vasodilation of bovine cerebral arteries. J Cereb Blood Flow Metab. 1991 May;11(3):366–370. doi: 10.1038/jcbfm.1991.76. [DOI] [PubMed] [Google Scholar]
- Grider J. R., Murthy K. S., Jin J. G., Makhlouf G. M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am J Physiol. 1992 Apr;262(4 Pt 1):G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- Grozdanovic Z., Baumgarten H. G., Brüning G. Histochemistry of NADPH-diaphorase, a marker for neuronal nitric oxide synthase, in the peripheral autonomic nervous system of the mouse. Neuroscience. 1992;48(1):225–235. doi: 10.1016/0306-4522(92)90351-2. [DOI] [PubMed] [Google Scholar]
- Gu J., Blank M. A., Huang W. M., Islam K. N., McGregor G. P., Christofides N., Allen J. M., Bloom S. R., Polak J. M. Peptide-containing nerves in human urinary bladder. Urology. 1984 Oct;24(4):353–357. doi: 10.1016/0090-4295(84)90209-7. [DOI] [PubMed] [Google Scholar]
- Gu J., Restorick J. M., Blank M. A., Huang W. M., Polak J. M., Bloom S. R., Mundy A. R. Vasoactive intestinal polypeptide in the normal and unstable bladder. Br J Urol. 1983 Dec;55(6):645–647. doi: 10.1111/j.1464-410x.1983.tb03396.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Kigoshi S., Muramatsu I. Nitric oxide-dependent and -independent neurogenic relaxation of isolated dog urethra. Eur J Pharmacol. 1993 Feb 9;231(2):209–214. doi: 10.1016/0014-2999(93)90451-m. [DOI] [PubMed] [Google Scholar]
- Hindmarsh J. R., Gosling P. T., Deane A. M. Bladder instability. Is the primary defect in the urethra? Br J Urol. 1983 Dec;55(6):648–651. doi: 10.1111/j.1464-410x.1983.tb03397.x. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope B. T., Vincent S. R. Histochemical characterization of neuronal NADPH-diaphorase. J Histochem Cytochem. 1989 May;37(5):653–661. doi: 10.1177/37.5.2703701. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Keast J. R. A possible neural source of nitric oxide in the rat penis. Neurosci Lett. 1992 Aug 31;143(1-2):69–73. doi: 10.1016/0304-3940(92)90235-y. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W., Fischer A., Mundel P., Mayer B., Hoba B., Philippin B., Preissler U. Nitric oxide synthase in VIP-containing vasodilator nerve fibres in the guinea-pig. Neuroreport. 1992 Jul;3(7):653–655. doi: 10.1097/00001756-199207000-00028. [DOI] [PubMed] [Google Scholar]
- Liu X. R., Gillespie J. S., Gibson I. F., Martin W. Effects of NG-substituted analogues of L-arginine on NANC relaxation of the rat anococcygeus and bovine retractor penis muscles and the bovine penile artery. Br J Pharmacol. 1991 Sep;104(1):53–58. doi: 10.1111/j.1476-5381.1991.tb12384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J. A., Armstrong J. B., Mauger G. M. The unstable urethra in the female. Obstet Gynecol. 1989 Jul;74(1):69–74. [PubMed] [Google Scholar]
- Low J. A. Urethral behavior during the involuntary detrusor contraction. Am J Obstet Gynecol. 1977 May 1;128(1):32–42. doi: 10.1016/0002-9378(77)90292-7. [DOI] [PubMed] [Google Scholar]
- McNeill D. L., Traugh N. E., Jr, Vaidya A. M., Hua H. T., Papka R. E. Origin and distribution of NADPH-diaphorase-positive neurons and fibers innervating the urinary bladder of the rat. Neurosci Lett. 1992 Nov 23;147(1):33–36. doi: 10.1016/0304-3940(92)90768-3. [DOI] [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Morita T., Tsujii T., Dokita S. Regional difference in functional roles of cAMP and cGMP in lower urinary tract smooth muscle contractility. Urol Int. 1992;49(4):191–195. doi: 10.1159/000282424. [DOI] [PubMed] [Google Scholar]
- Nagao T., Vanhoutte P. M. Hyperpolarization as a mechanism for endothelium-dependent relaxations in the porcine coronary artery. J Physiol. 1992 Jan;445:355–367. doi: 10.1113/jphysiol.1992.sp018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Park K. H., Rubin L. E., Gross S. S., Levi R. Nitric oxide is a mediator of hypoxic coronary vasodilatation. Relation to adenosine and cyclooxygenase-derived metabolites. Circ Res. 1992 Oct;71(4):992–1001. doi: 10.1161/01.res.71.4.992. [DOI] [PubMed] [Google Scholar]
- Persson K., Andersson K. E. Nitric oxide and relaxation of pig lower urinary tract. Br J Pharmacol. 1992 Jun;106(2):416–422. doi: 10.1111/j.1476-5381.1992.tb14349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K., Igawa Y., Mattiasson A., Andersson K. E. Effects of inhibition of the L-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br J Pharmacol. 1992 Sep;107(1):178–184. doi: 10.1111/j.1476-5381.1992.tb14483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M. G., Gustafsson L. E., Wiklund N. P., Moncada S., Hedqvist P. Endogenous nitric oxide as a probable modulator of pulmonary circulation and hypoxic pressor response in vivo. Acta Physiol Scand. 1990 Dec;140(4):449–457. doi: 10.1111/j.1748-1716.1990.tb09021.x. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Cellek S., Palmer R. M., Moncada S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: an insight into endotoxin shock. Biochem Biophys Res Commun. 1990 Dec 14;173(2):541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- SCOTT F. B., QUESADA E. M., CARDUS D. STUDIES ON THE DYNAMICS OF MICTURITION: OBSERVATIONS ON HEALTHY MEN. J Urol. 1964 Nov;92:455–463. doi: 10.1016/S0022-5347(17)63987-9. [DOI] [PubMed] [Google Scholar]
- Saffrey M. J., Hassall C. J., Hoyle C. H., Belai A., Moss J., Schmidt H. H., Förstermann U., Murad F., Burnstock G. Colocalization of nitric oxide synthase and NADPH-diaphorase in cultured myenteric neurones. Neuroreport. 1992 Apr;3(4):333–336. doi: 10.1097/00001756-199204000-00011. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Gagne G. D., Nakane M., Pollock J. S., Miller M. F., Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992 Oct;40(10):1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Sheng H., Schmidt H. H., Nakane M., Mitchell J. A., Pollock J. S., Föstermann U., Murad F. Characterization and localization of nitric oxide synthase in non-adrenergic non-cholinergic nerves from bovine retractor penis muscles. Br J Pharmacol. 1992 Aug;106(4):768–773. doi: 10.1111/j.1476-5381.1992.tb14411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanagho E. A., Miller E. R. Initiation of voiding. Br J Urol. 1970 Apr;42(2):175–183. doi: 10.1111/j.1464-410x.1970.tb10019.x. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Hollywood M. A., McHale N. G. Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol. 1992;451:133–144. doi: 10.1113/jphysiol.1992.sp019157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N., Okamura T. Mechanism underlying the response to vasodilator nerve stimulation in isolated dog and monkey cerebral arteries. Am J Physiol. 1990 Nov;259(5 Pt 2):H1511–H1517. doi: 10.1152/ajpheart.1990.259.5.H1511. [DOI] [PubMed] [Google Scholar]
- Ward S. M., Xue C., Shuttleworth C. W., Bredt D. S., Snyder S. H., Sanders K. M. NADPH diaphorase and nitric oxide synthase colocalization in enteric neurons of canine proximal colon. Am J Physiol. 1992 Aug;263(2 Pt 1):G277–G284. doi: 10.1152/ajpgi.1992.263.2.G277. [DOI] [PubMed] [Google Scholar]
- Young H. M., Furness J. B., Shuttleworth C. W., Bredt D. S., Snyder S. H. Co-localization of nitric oxide synthase immunoreactivity and NADPH diaphorase staining in neurons of the guinea-pig intestine. Histochemistry. 1992 May;97(4):375–378. doi: 10.1007/BF00270041. [DOI] [PubMed] [Google Scholar]