Abstract
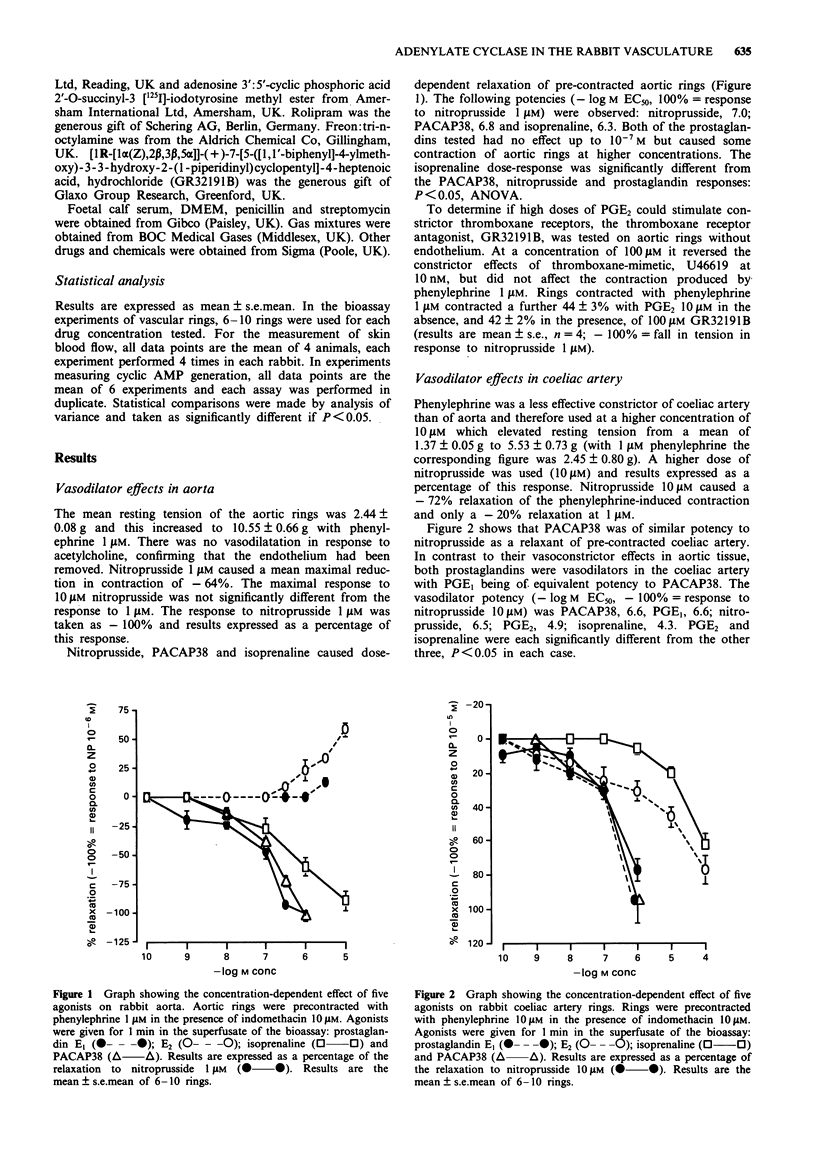
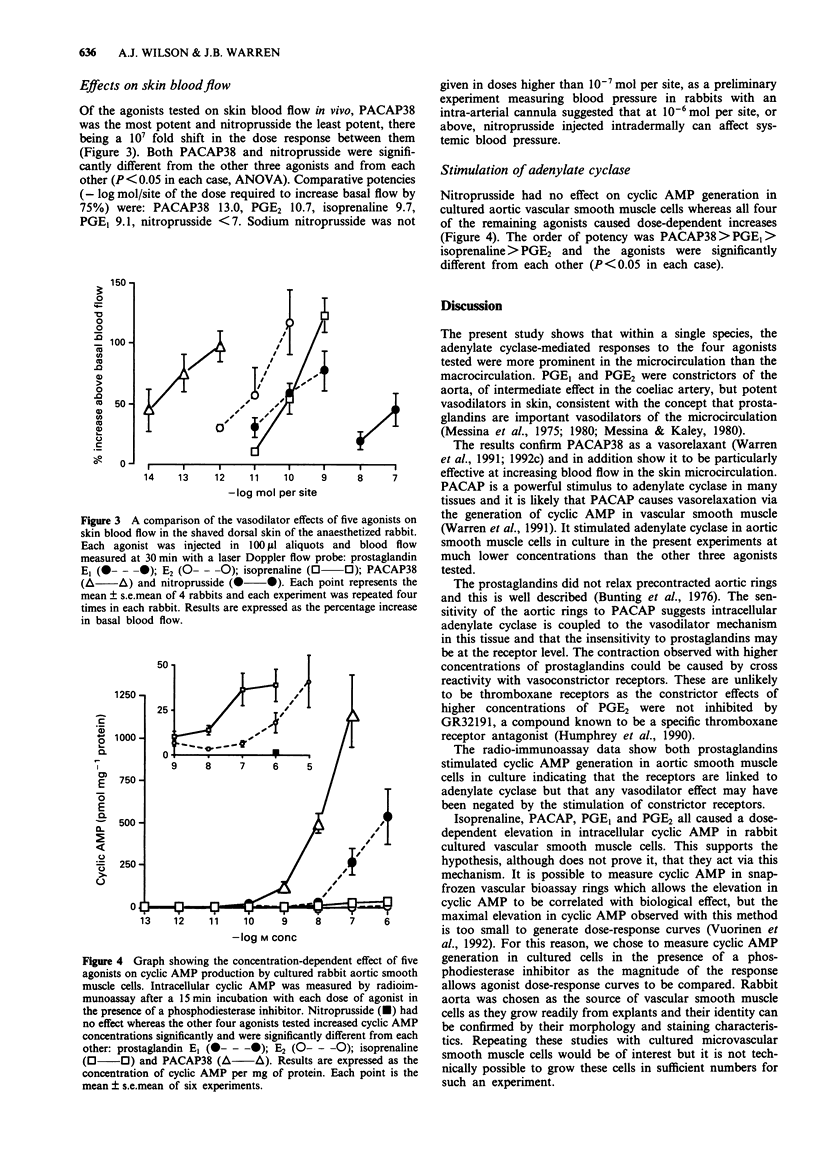
1. The importance of adenylate cyclase-mediated vascular relaxation in the macro and microcirculation was assessed in rabbit aortic and coeliac artery bioassay rings in vitro and skin microvessels in vivo. 2. The neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP38), the beta-agonist, isoprenaline, and the prostaglandins, PGE1 and PGE2, were compared with the activity of nitroprusside, which acts by stimulating guanylate cyclase. 3. In aortic tissue the relative relaxant potencies were (-log M EC50, 100% = response to nitroprusside 10(-6) M): nitroprusside 7.0, PACAP38 6.8, isoprenaline 6.3; PGE1 and PGE2 were weak constrictors. In coeliac artery rings relative potencies were (-log M EC50, 100% = response to nitroprusside 10(-5) M): PACAP38 6.6, PGE1 6.6, nitroprusside 6.5, PGE2 4.9, and isoprenaline 4.3. 4. Comparative potencies when injected into anaesthetized rabbit skin in vivo were (-log mol/site required to increase blood red cell flux by 75%): PACAP38 13.0, PGE2 10.7, isoprenaline 9.7, PGE1 9.1, nitroprusside < 7. 5. Nitroprusside, the most effective relaxant tested in the aorta, was 10(7) fold less potent than PACAP in its effect on skin blood flow. PGE1 and PGE2 were constrictors of the aorta, of intermediate effect in the coeliac artery, but potent vasodilators of the microcirculation. 6. In this model, the importance of adenylate cyclase-mediated vascular relaxation increases with decreasing vessel size.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson R. Role of cyclic AMP and Ca ++ in mechanical and metabolic events in isometrically contracting vascular smooth muscle. Acta Physiol Scand. 1973 Jan;87(1):84–95. doi: 10.1111/j.1748-1716.1973.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Barsony J., Marx S. J. Immunocytology on microwave-fixed cells reveals rapid and agonist-specific changes in subcellular accumulation patterns for cAMP or cGMP. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1188–1192. doi: 10.1073/pnas.87.3.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting S., Gryglewski R., Moncada S., Vane J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins. 1976 Dec;12(6):897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Moncada S., Palmer R. M. Bioassay of prostacyclin and endothelium-derived relaxing factor (EDRF) from porcine aortic endothelial cells. Br J Pharmacol. 1986 Apr;87(4):685–694. doi: 10.1111/j.1476-5381.1986.tb14586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesen B. J., De Mey J. G. Effects of cyclic AMP-affecting agents on contractile reactivity of isolated mesenteric and renal resistance arteries of the rat. Br J Pharmacol. 1990 Dec;101(4):859–864. doi: 10.1111/j.1476-5381.1990.tb14171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner W. L., Svensjö E., Arfors K. E. Simultaneous measurements of macromolecular leakage and arteriolar blood flow as altered by PGE1 and beta 2-receptor stimulant in the hamster cheek pouch. Microvasc Res. 1979 Nov;18(3):301–310. doi: 10.1016/0026-2862(79)90038-4. [DOI] [PubMed] [Google Scholar]
- Messina E. J., Kaley G. Microcirculatory responses to prostacyclin and PGE2 in the rat cremaster muscle. Adv Prostaglandin Thromboxane Res. 1980;7:719–722. [PubMed] [Google Scholar]
- Messina E. J., Rodenburg J., Slomiany B. L., Roberts A. M., Hintze T. H., Kaley G. Microcirculatory effects of arachidonic acid and a prostaglandin endoperoxide (PGH2). Microvasc Res. 1980 May;19(3):288–296. doi: 10.1016/0026-2862(80)90049-7. [DOI] [PubMed] [Google Scholar]
- Messina E. J., Weiner R., Kaley G. Inhibition of bradykinin vasodilation and potentiation of norepinephrine and angiotensin vasoconstriction by inhibitors of prostaglandin synthesis in skeletal muscle of the rat. Circ Res. 1975 Oct;37(4):430–437. doi: 10.1161/01.res.37.4.430. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Newman C. M., Warren J. B., Taylor G. W., Boobis A. R., Davies D. S. Rapid tolerance to the hypotensive effects of glyceryl trinitrate in the rat: prevention by N-acetyl-L- but not N-acetyl-D-cysteine. Br J Pharmacol. 1990 Apr;99(4):825–829. doi: 10.1111/j.1476-5381.1990.tb13014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson M. G., Gustafsson L. E., Wiklund N. P., Hedqvist P., Moncada S. Endogenous nitric oxide as a modulator of rabbit skeletal muscle microcirculation in vivo. Br J Pharmacol. 1990 Jul;100(3):463–466. doi: 10.1111/j.1476-5381.1990.tb15829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter J. M., Doktor H. S., Cragoe E. J., Jr Actions of bradykinin and related peptides on rabbit coeliac artery rings. Br J Pharmacol. 1989 Jan;96(1):23–28. doi: 10.1111/j.1476-5381.1989.tb11779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sata T., Linden J., Liu L. W., Kubota E., Said S. I. Vasoactive intestinal peptide evokes endothelium-dependent relaxation and cyclic AMP accumulation in rat aorta. Peptides. 1988 Jul-Aug;9(4):853–858. doi: 10.1016/0196-9781(88)90133-7. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Honeyman T. W., Fay F. S. Mechanism of beta-adrenergic relaxation of smooth muscle. Nature. 1979 Jan 4;277(5691):32–36. doi: 10.1038/277032a0. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Lugnier C., Demesy-Waeldele F., Stoclet J. C. Role of cyclic AMP- and cyclic GMP-phosphodiesterases in the control of cyclic nucleotide levels and smooth muscle tone in rat isolated aorta. A study with selective inhibitors. Biochem Pharmacol. 1987 Nov 15;36(22):3965–3972. doi: 10.1016/0006-2952(87)90465-5. [DOI] [PubMed] [Google Scholar]
- Vuorinen P., Pörsti I., Metsä-Ketelä T., Manninen V., Vapaatalo H., Laustiola K. E. Endothelium-dependent and -independent effects of exogenous ATP, adenosine, GTP and guanosine on vascular tone and cyclic nucleotide accumulation of rat mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):279–284. doi: 10.1111/j.1476-5381.1992.tb14246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanless R. B., Anand I. S., Gurden J., Harris P., Poole-Wilson P. A. Regional blood flow and hemodynamics in the rabbit with adriamycin cardiomyopathy: effects of isosorbide dinitrate, dobutamine and captopril. J Pharmacol Exp Ther. 1987 Dec;243(3):1101–1106. [PubMed] [Google Scholar]
- Warren J. B., Brady A. J., Taylor G. W. Vascular smooth muscle influences the release of endothelium-derived relaxing factor. Proc Biol Sci. 1990 Aug 22;241(1301):127–131. doi: 10.1098/rspb.1990.0076. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Cockcroft J. R., Larkin S. W., Kajekar R., Macrae A., Ghatei M. A., Bloom S. R. Pituitary adenylate cyclase activating polypeptide is a potent vasodilator in humans. J Cardiovasc Pharmacol. 1992 Jul;20(1):83–87. [PubMed] [Google Scholar]
- Warren J. B., Coughlan M. L., Williams T. J. Endotoxin-induced vasodilatation in anaesthetized rat skin involves nitric oxide and prostaglandin synthesis. Br J Pharmacol. 1992 Aug;106(4):953–957. doi: 10.1111/j.1476-5381.1992.tb14441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. B., Donnelly L. E., Cullen S., Robertson B. E., Ghatei M. A., Bloom S. R., MacDermot J. Pituitary adenylate cyclase-activating polypeptide: a novel, long-lasting, endothelium-independent vasorelaxant. Eur J Pharmacol. 1991 May 17;197(2-3):131–134. doi: 10.1016/0014-2999(91)90511-n. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Larkin S. W., Coughlan M., Kajekar R., Williams T. J. Pituitary adenylate cyclase activating polypeptide is a potent vasodilator and oedema potentiator in rabbit skin in vivo. Br J Pharmacol. 1992 Jun;106(2):331–334. doi: 10.1111/j.1476-5381.1992.tb14336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. B., Loi R. K., Coughlan M. L. Involvement of nitric oxide synthase in the delayed vasodilator response to ultraviolet light irradiation of rat skin in vivo. Br J Pharmacol. 1993 Jul;109(3):802–806. doi: 10.1111/j.1476-5381.1993.tb13645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. J., Peck M. J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- Williams T. J. Vasoactive intestinal polypeptide is more potent than prostaglandin E2 as a vasodilator and oedema potentiator in rabbit skin. Br J Pharmacol. 1982 Nov;77(3):505–509. doi: 10.1111/j.1476-5381.1982.tb09324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L. M., Owen D. A. A comparison of vasodilator activity of agents activating cyclic nucleotides with those inhibiting their metabolism in rabbit isolated ear artery. Br J Pharmacol. 1989 Mar;96(3):718–724. doi: 10.1111/j.1476-5381.1989.tb11873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


