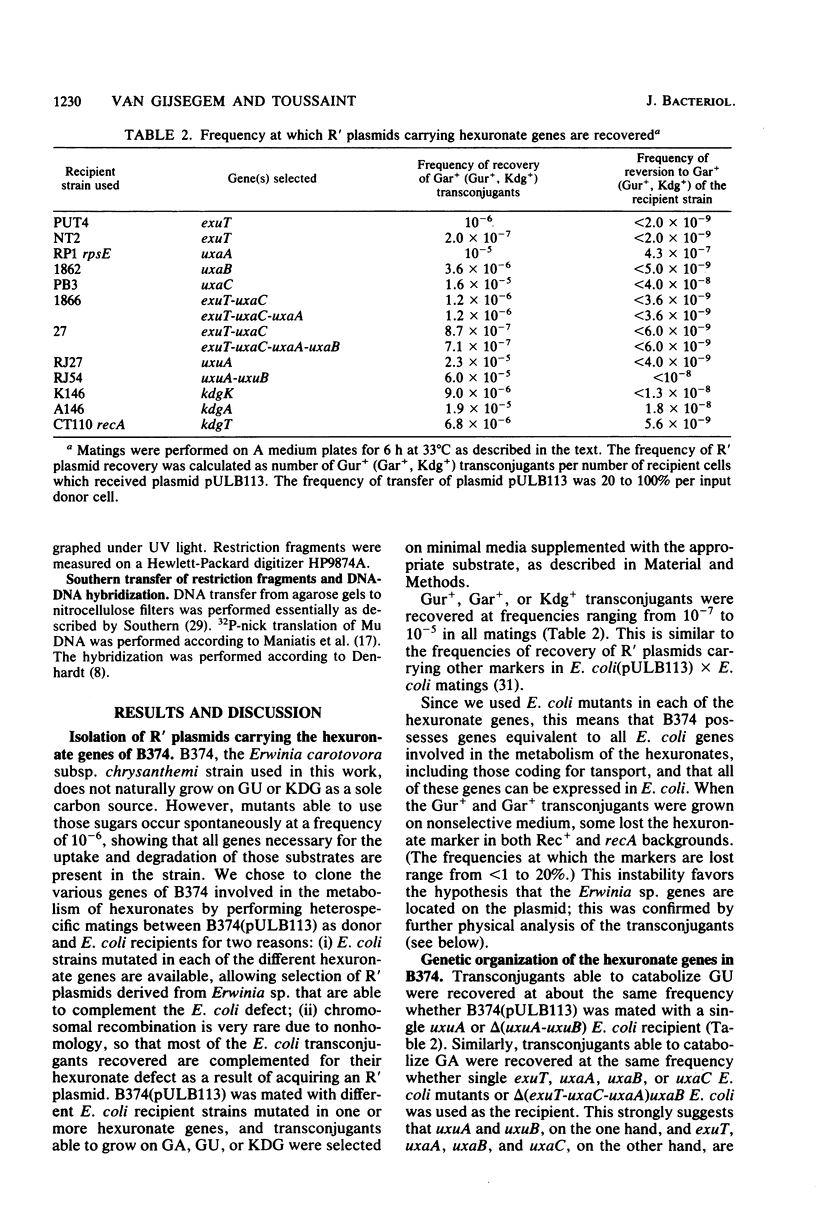
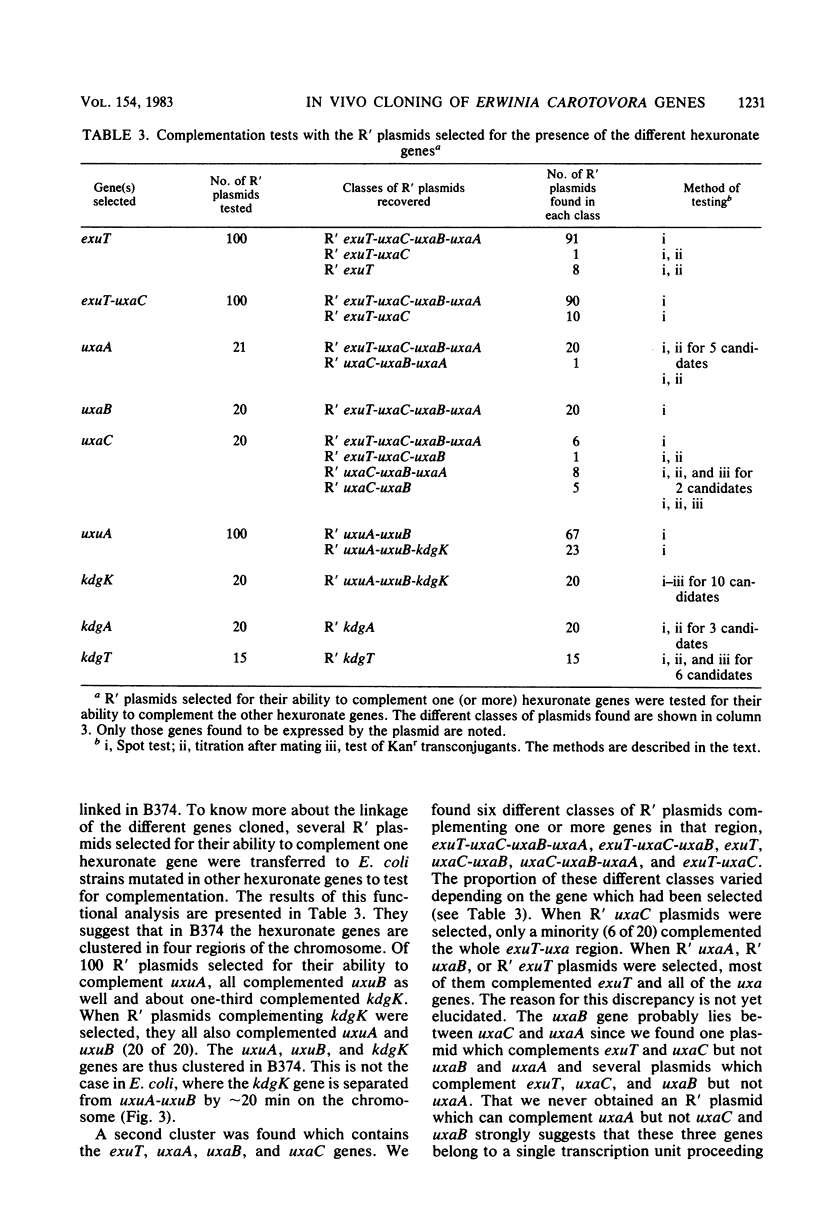
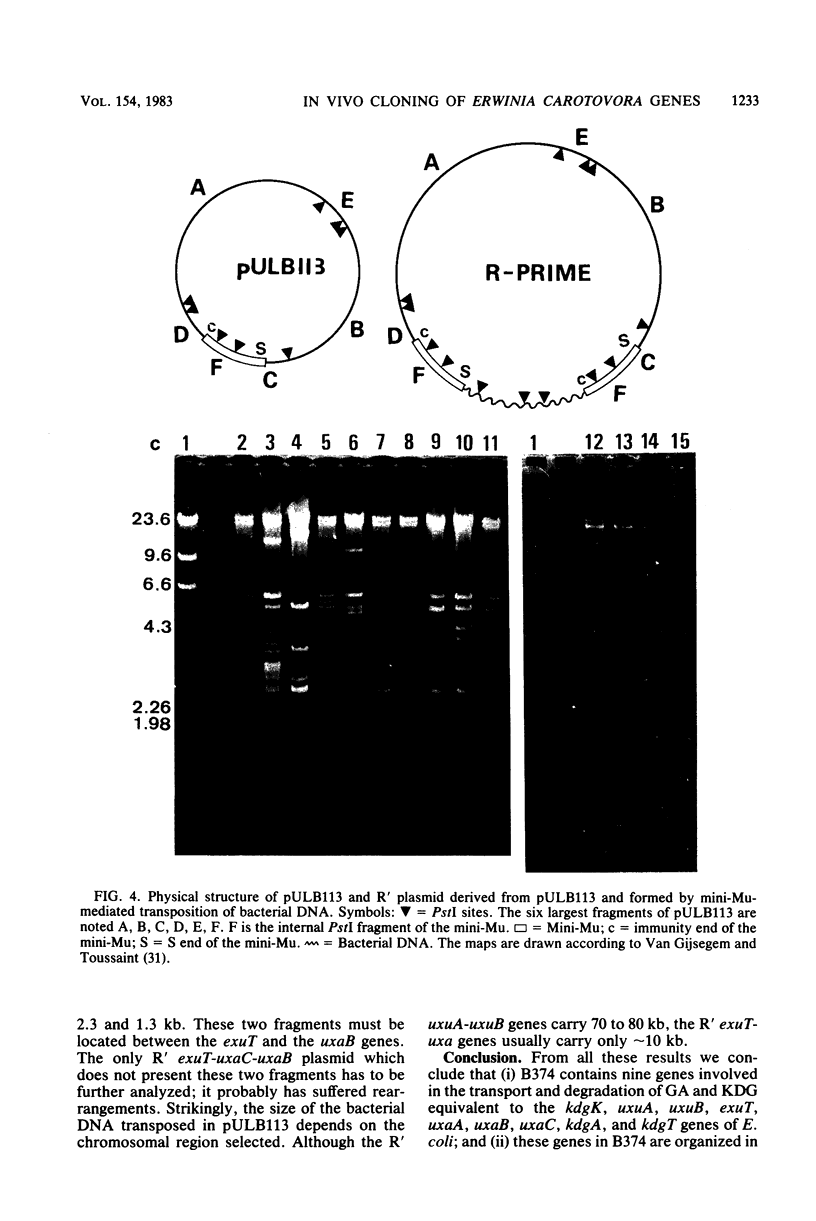
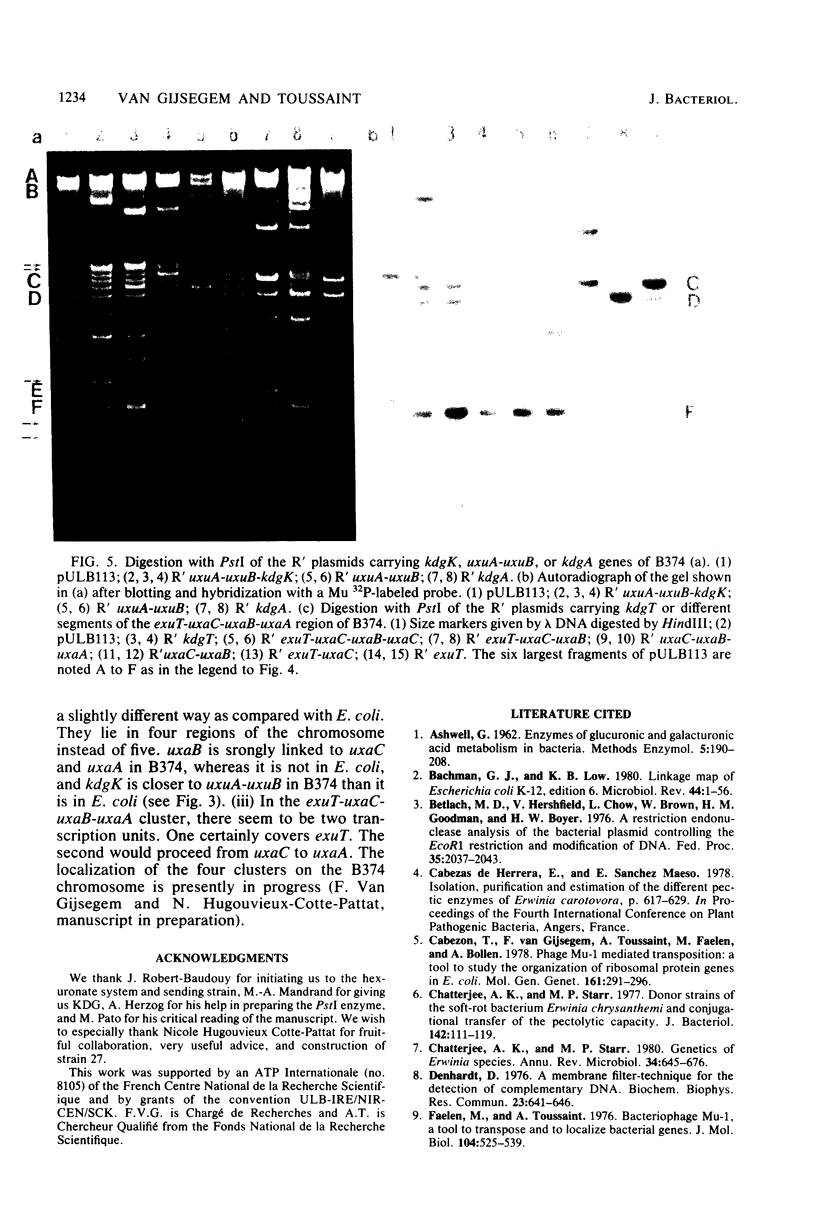
Abstract
Using the RP4::mini-Mu pULB113 plasmid, an RP4 derivative carrying a deleted Mu prophage which allows the plasmid to pick up any chromosomal DNA segment to form R' plasmids, we cloned all of the genes of Erwinia carotovora involved in the catabolism of the hexuronates and in the transport of these substrates. With the R' plasmids we isolated, we performed complementation analysis and found that, in the Erwinia carotovora strain we used, the genes involved in the catabolism of the hexuronates are clustered in four regions of the chromosome. This genetic organization is compared with that of Escherichia coli K-12.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betlach M., Hershfield V., Chow L., Brown W., Goodman H., Boyer H. W. A restriction endonuclease analysis of the bacterial plasmid controlling the ecoRI restriction and modification of DNA. Fed Proc. 1976 Jul;35(9):2037–2043. [PubMed] [Google Scholar]
- Cabezón T., Gijsegem F. V., Toussaint A., Faelen M., Bollen A. Phage Mu-1 mediated transposition: a tool to study the organization of ribosomal protein genes in Escherichia coli. Mol Gen Genet. 1978 May 31;161(3):291–296. doi: 10.1007/BF00331003. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K. Acceptance by Erwinia spp. of R plasmid R68.45 and its ability to mobilize the chromosome of Erwinia chrysanthemi. J Bacteriol. 1980 Apr;142(1):111–119. doi: 10.1128/jb.142.1.111-119.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Starr M. P. Genetics of Erwinia species. Annu Rev Microbiol. 1980;34:645–676. doi: 10.1146/annurev.mi.34.100180.003241. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- FRY B. A. Conditions for the infection of Escherichia coli with lambda phage and for the establishment of lysogeny. J Gen Microbiol. 1959 Dec;21:676–684. doi: 10.1099/00221287-21-3-676. [DOI] [PubMed] [Google Scholar]
- Faelen M., Toussaint A. Bacteriophage Mu-1: a tool to transpose and to localize bacterial genes. J Mol Biol. 1976 Jul 5;104(3):525–539. doi: 10.1016/0022-2836(76)90118-2. [DOI] [PubMed] [Google Scholar]
- HAMON Y., PERON Y. [The reciprocal antagonistic properties among the Erwinia. Discussion of the taxonomic position of this genus]. C R Hebd Seances Acad Sci. 1961 Jul 31;253:913–915. [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Isolation of fusions between the lac genes and several genes of the exu regulon: analysis of their regulation, determination of the transcription direction of the uxaC-uxaA operon, in Escherichia coli K-12. Mol Gen Genet. 1981;182(2):279–287. doi: 10.1007/BF00269671. [DOI] [PubMed] [Google Scholar]
- KILGORE W. W., STARR M. P. Catabolism of galacturonic and glucuronic acids by Erwinia carotovora. J Biol Chem. 1959 Sep;234:2227–2235. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Stoeber F. R. Escherichia coli K-12 structural kdgT mutants exhibiting thermosensitive 2-keto-3-deoxy-D-gluconate uptake. J Bacteriol. 1977 Feb;129(2):606–615. doi: 10.1128/jb.129.2.606-615.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
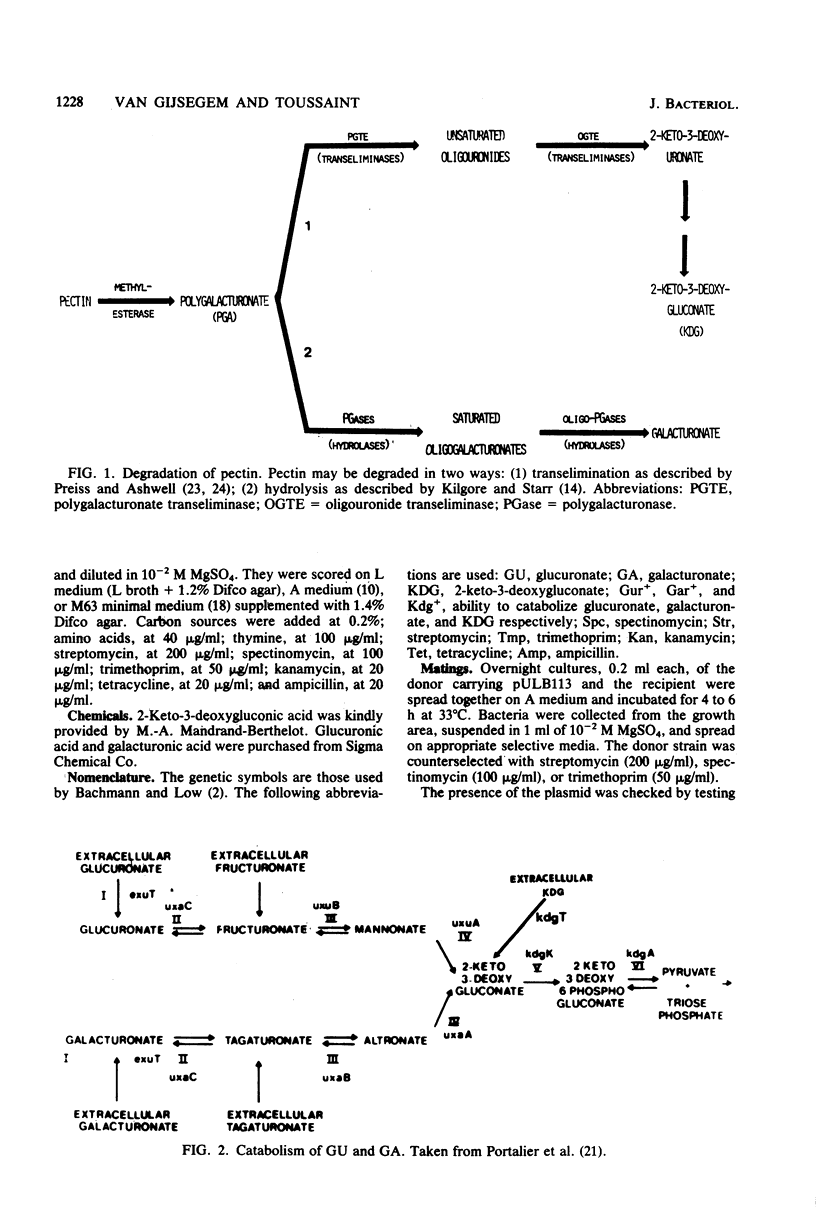
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. I. Enzymatic formation of 4-deoxy-L-threo-5-hexoseulose uronic acid. J Biol Chem. 1963 May;238:1571–1583. [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. II. Formation and metabolism of 3-deoxy-D-glycero-2, 5-hexodiulosonic acid. J Biol Chem. 1963 May;238:1577–1583. [PubMed] [Google Scholar]
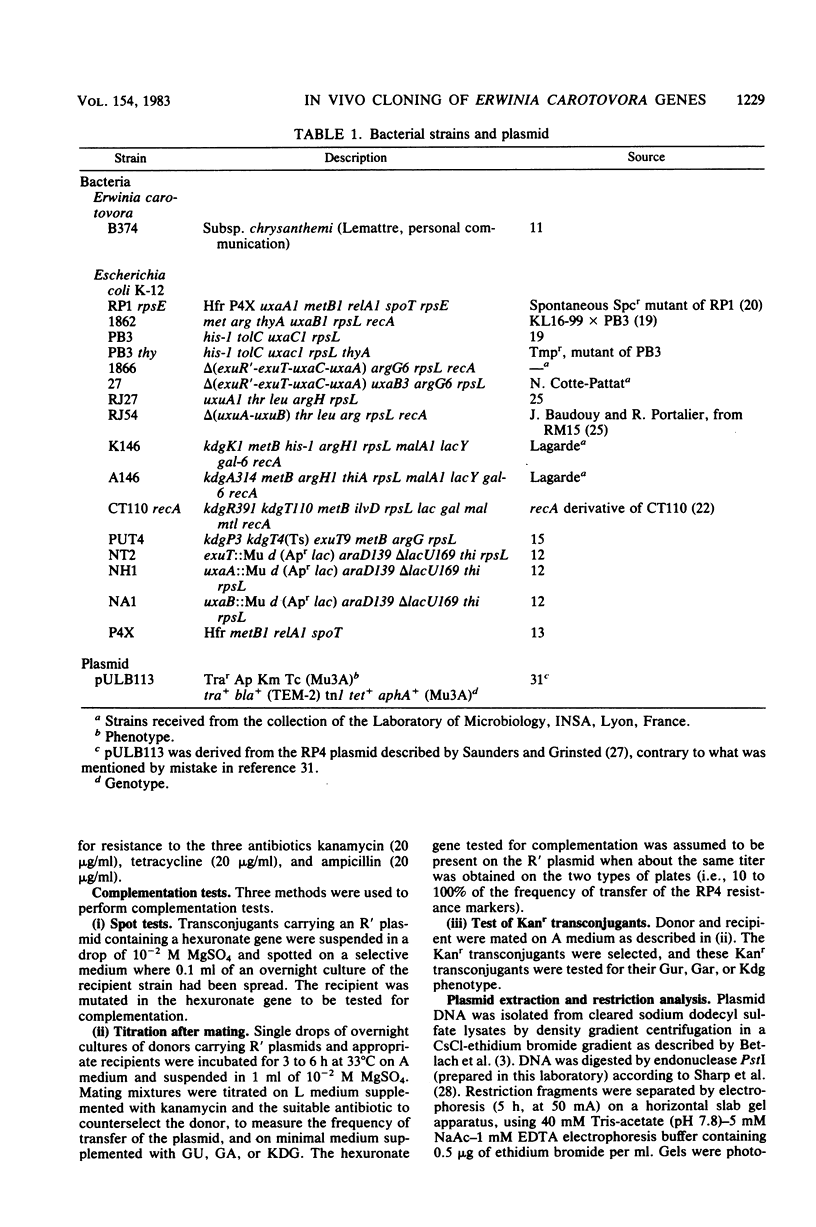
- Portalier R. C., Robert-Baudouy J. M., Némoz G. M. Etudes de mutations affectant les gènes de structure de l'isomerase uronique et de l'oxydoreductase altronique chez Escherichia coli K 12. Mol Gen Genet. 1974;128(4):301–319. doi: 10.1007/BF00268518. [DOI] [PubMed] [Google Scholar]
- Portalier R. C., Robert-Baudouy J. M., Stoeber F. R. Localisation génétique et caractérisation biochimique de mutations affectant le gène de structure de l'hydrolyase altronique chez Escherichia coli K 12. Mol Gen Genet. 1972;118(4):335–350. doi: 10.1007/BF00333569. [DOI] [PubMed] [Google Scholar]
- Portalier R., Robert-Baudouy J., Stoeber F. Regulation of Escherichia coli K-12 hexuronate system genes: exu regulon. J Bacteriol. 1980 Sep;143(3):1095–1107. doi: 10.1128/jb.143.3.1095-1107.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Lagarde A. Système de transport du 2-céto-3-désoxy-gluconate chez E. coli K 12: localisation d'un gène de structure et de son opérateur. Mol Gen Genet. 1973 Mar 1;121(2):163–180. doi: 10.1007/BF00277530. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J. M., Portalier R. C. Mutations affectant le catabolisme du glucuronate chez Escherichia coli K12. Mol Gen Genet. 1974;131(1):31–46. doi: 10.1007/BF00269385. [DOI] [PubMed] [Google Scholar]
- Robert-Baudouy J., Portalier R., Stoeber F. Regulation of hexuronate system genes in Escherichia coli K-12: multiple regulation of the uxu operon by exuR and uxuR gene products. J Bacteriol. 1981 Jan;145(1):211–220. doi: 10.1128/jb.145.1.211-220.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. R., Grinsted J. Properties of RP4, an R factor which originated in Pseudomonas aeruginosa S8. J Bacteriol. 1972 Nov;112(2):690–696. doi: 10.1128/jb.112.2.690-696.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Chatterjee A. K. The genus Erwinia: enterobacteria pathogenic to plants and animals. Annu Rev Microbiol. 1972;26:389–426. doi: 10.1146/annurev.mi.26.100172.002133. [DOI] [PubMed] [Google Scholar]
- Van Gijsegem F., Toussaint A. Chromosome transfer and R-prime formation by an RP4::mini-Mu derivative in Escherichia coli, Salmonella typhimurium, Klebsiella pneumoniae, and Proteus mirabilis. Plasmid. 1982 Jan;7(1):30–44. doi: 10.1016/0147-619x(82)90024-5. [DOI] [PubMed] [Google Scholar]