Abstract
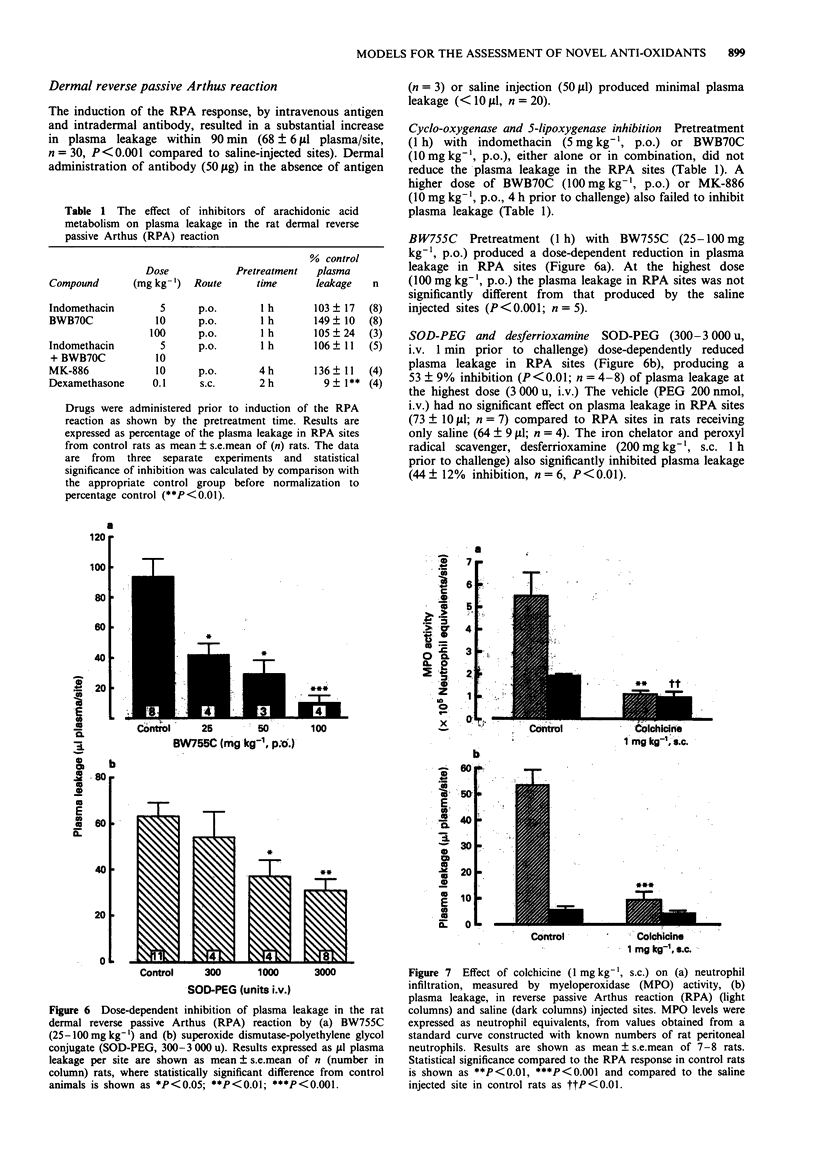
1. The role of arachidonic acid metabolites and oxygen radicals in carrageenin-induced rat paw oedema and dermal reverse passive Arthus reaction (RPA) have been investigated. 2. Indomethacin (10 mg kg-1, p.o.) inhibited carrageenin paw oedema when administered 30 min before, but not 2 h after carrageenin. BWB70C (10 mg kg-1, p.o.), a selective inhibitor of 5-lipoxygenase, had no effect whether administered before or after carrageenin. Administration of both indomethacin and BWB70C had no greater anti-inflammatory effect than indomethacin alone. 3. BW755C (20 mg kg-1, p.o.), which inhibits the cyclo-oxygenase and lipoxygenase pathways of arachidonic acid metabolism, or superoxide dismutase-polyethylene glycol conjugate (SOD-PEG, 3000 u, i.v.) inhibited carrageenin paw oedema whether administered either 30 min before, or 2 h after carrageenin. 4. Pretreatment with dexamethasone (0.1 mg kg-1) or colchicine (2 mg kg-1), likewise suppressed carrageenin paw oedema. 5. BW755C (25-100 mg kg-1, p.o.) dose-dependently reduced plasma leakage in the RPA, whereas indomethacin (5 mg kg-1, p.o.) or BWB70C either alone or in combination, did not. 6. SOD-PEG (300-3000 u, i.v.) dose-dependently inhibited plasma leakage in the RPA. In addition, the iron chelator and peroxyl radical scavenger, desferrioxamine (200 mg kg-1, s.c.) also inhibited plasma leakage. 7. Pretreatment with dexamethasone (0.1 mg kg-1) or colchicine (1 mg kg-1) reduced the plasma leakage in RPA, whereas MK-886 (10 mg kg-1) had no effect.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boughton-Smith N. K., Whittle B. J. Failure of the inhibition of rat gastric mucosal 5-lipoxygenase by novel acetohydroxamic acids to prevent ethanol-induced damage. Br J Pharmacol. 1988 Sep;95(1):155–162. doi: 10.1111/j.1476-5381.1988.tb16559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Carter G. W., Martin M. K., Krause R. A., Young P. R. The effects of anti-inflammatory and other agents on the rat dermal arthus reaction. Res Commun Chem Pathol Pharmacol. 1982 Feb;35(2):189–207. [PubMed] [Google Scholar]
- Chang Y. H., Otterness I. G. Effects of pharmacologic agents on the reversed passive Arthus reaction in the rat. Eur J Pharmacol. 1981 Jan 16;69(2):155–164. doi: 10.1016/0014-2999(81)90410-6. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Fligiel S. E., Ward P. A., Johnson K. J., Till G. O. Evidence for a role of hydroxyl radical in immune-complex-induced vasculitis. Am J Pathol. 1984 Jun;115(3):375–382. [PMC free article] [PubMed] [Google Scholar]
- Flower R. Lipocortin. Biochem Soc Trans. 1989 Apr;17(2):276–278. doi: 10.1042/bst0170276. [DOI] [PubMed] [Google Scholar]
- Gillard J., Ford-Hutchinson A. W., Chan C., Charleson S., Denis D., Foster A., Fortin R., Leger S., McFarlane C. S., Morton H. L-663,536 (MK-886) (3-[1-(4-chlorobenzyl)-3-t-butyl-thio-5-isopropylindol-2-yl]-2,2 - dimethylpropanoic acid), a novel, orally active leukotriene biosynthesis inhibitor. Can J Physiol Pharmacol. 1989 May;67(5):456–464. doi: 10.1139/y89-073. [DOI] [PubMed] [Google Scholar]
- HUMPHREY J. H. The mechanism of Arthus reactions. II. The role of polymorphonuclear leucocytes and platelets in reversed passive reactions in the guinea-pig. Br J Exp Pathol. 1955 Jun;36(3):283–289. [PMC free article] [PubMed] [Google Scholar]
- Hellewell P. G., Williams T. J. A specific antagonist of platelet-activating factor suppresses oedema formation in an Arthus reaction but not oedema induced by leukocyte chemoattractants in rabbit skin. J Immunol. 1986 Jul 1;137(1):302–307. [PubMed] [Google Scholar]
- Higgs G. A., Flower R. J., Vane J. R. A new approach to anti-inflammatory drugs. Biochem Pharmacol. 1979 Jun 15;28(12):1959–1961. doi: 10.1016/0006-2952(79)90651-8. [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Follenfant R. L., Garland L. G. Selective inhibition of arachidonate 5-lipoxygenase by novel acetohydroxamic acids: effects on acute inflammatory responses. Br J Pharmacol. 1988 Jun;94(2):547–551. doi: 10.1111/j.1476-5381.1988.tb11559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs G. A., Moncada S., Vane J. R. Eicosanoids in inflammation. Ann Clin Res. 1984;16(5-6):287–299. [PubMed] [Google Scholar]
- Holsapple M. P., Yim G. K. Therapeutic reduction of ongoing carrageenin-induced inflammation by lipoxygenase, but not cyclooxygenase inhibitors. Inflammation. 1984 Sep;8(3):223–230. doi: 10.1007/BF00916412. [DOI] [PubMed] [Google Scholar]
- Hughes S. R., Williams T. J., Brain S. D. Evidence that endogenous nitric oxide modulates oedema formation induced by substance P. Eur J Pharmacol. 1990 Dec 4;191(3):481–484. doi: 10.1016/0014-2999(90)94184-y. [DOI] [PubMed] [Google Scholar]
- Ialenti A., Ianaro A., Moncada S., Di Rosa M. Modulation of acute inflammation by endogenous nitric oxide. Eur J Pharmacol. 1992 Feb 11;211(2):177–182. doi: 10.1016/0014-2999(92)90526-a. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Kopp D. E., Esser B., Tashoff T., Goldman D. W., Goetzl E. J., Lemanske R. F., Jr In vivo and in vitro assessment of the role of leukotriene B4 as a mediator of rat cutaneous late-phase reactions. J Allergy Clin Immunol. 1986 Feb;77(2):302–308. doi: 10.1016/s0091-6749(86)80108-7. [DOI] [PubMed] [Google Scholar]
- Kreisle R. A., Parker C. W., Griffin G. L., Senior R. M., Stenson W. F. Studies of leukotriene B4-specific binding and function in rat polymorphonuclear leukocytes: absence of a chemotactic response. J Immunol. 1985 May;134(5):3356–3363. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Soberman R. J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990 Sep 6;323(10):645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Marnett L. J., Siedlik P. H., Fung L. W. Oxidation of phenidone and BW755C by prostaglandin endoperoxide synthetase. J Biol Chem. 1982 Jun 25;257(12):6957–6964. [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. R., Harkin M. M., Johnson K. J., Ward P. A. Suppression by superoxide dismutase of immune-complex--induced pulmonary alveolitis and dermal inflammation. Am J Pathol. 1981 Jan;102(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Namiki M., Igarashi Y., Sakamoto K., Nakamura T., Koga Y. Pharmacological profiles of a potential LTB4-antagonist, SM-9064. Biochem Biophys Res Commun. 1986 Jul 31;138(2):540–546. doi: 10.1016/s0006-291x(86)80530-7. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Participation of superoxide anions at the prostaglandin phase of carrageenan foot-oedema. Biochem Pharmacol. 1976 Jul 1;25(13):1465–1472. [PubMed] [Google Scholar]
- Pekoe G., Van Dyke K., Peden D., Mengoli H., English D. Antioxidation theory of non-steroidal anti-inflammatory drugs based upon the inhibition of luminol-enhanced chemiluminescence from the myeloperoxidase reaction. Agents Actions. 1982 Jul;12(3):371–376. doi: 10.1007/BF01965406. [DOI] [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflum L. R., Graeme M. L. The Arthus reaction in rats, a possible test for anti-inflammatory and antiheumatic drugs. Agents Actions. 1979 Jun;9(2):184–189. doi: 10.1007/BF02024732. [DOI] [PubMed] [Google Scholar]
- Randall R. W., Eakins K. E., Higgs G. A., Salmon J. A., Tateson J. E. Inhibition of arachidonic acid cyclo-oxygenase and lipoxygenase activities of leukocytes by indomethacin and compound BW755C. Agents Actions. 1980 Dec;10(6):553–555. doi: 10.1007/BF02024164. [DOI] [PubMed] [Google Scholar]
- Salmon J. A., Jackson W. P., Garland L. G. Inhibition of 5-lipoxygenase: development of hydroxamic acids and hydroxyureas as potential therapeutic agents. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:109–112. [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Jackson J., Cochrane C. C. Oxidant injury of cells. Int J Tissue React. 1987;9(4):317–324. [PubMed] [Google Scholar]
- Ward P. A. Mechanisms of endothelial cell injury. J Lab Clin Med. 1991 Nov;118(5):421–426. [PubMed] [Google Scholar]
- Warren J. S., Ward P. A., Johnson K. J., Ginsburg I. Modulation of acute immune complex-mediated tissue injury by the presence of polyionic substances. Am J Pathol. 1987 Jul;128(1):67–77. [PMC free article] [PubMed] [Google Scholar]
- Warren J. S., Yabroff K. R., Mandel D. M., Johnson K. J., Ward P. A. Role of O2- in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic Biol Med. 1990;8(2):163–172. doi: 10.1016/0891-5849(90)90089-2. [DOI] [PubMed] [Google Scholar]
- Wedmore C. V., Williams T. J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981 Feb 19;289(5799):646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Peck M. J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]


