Abstract
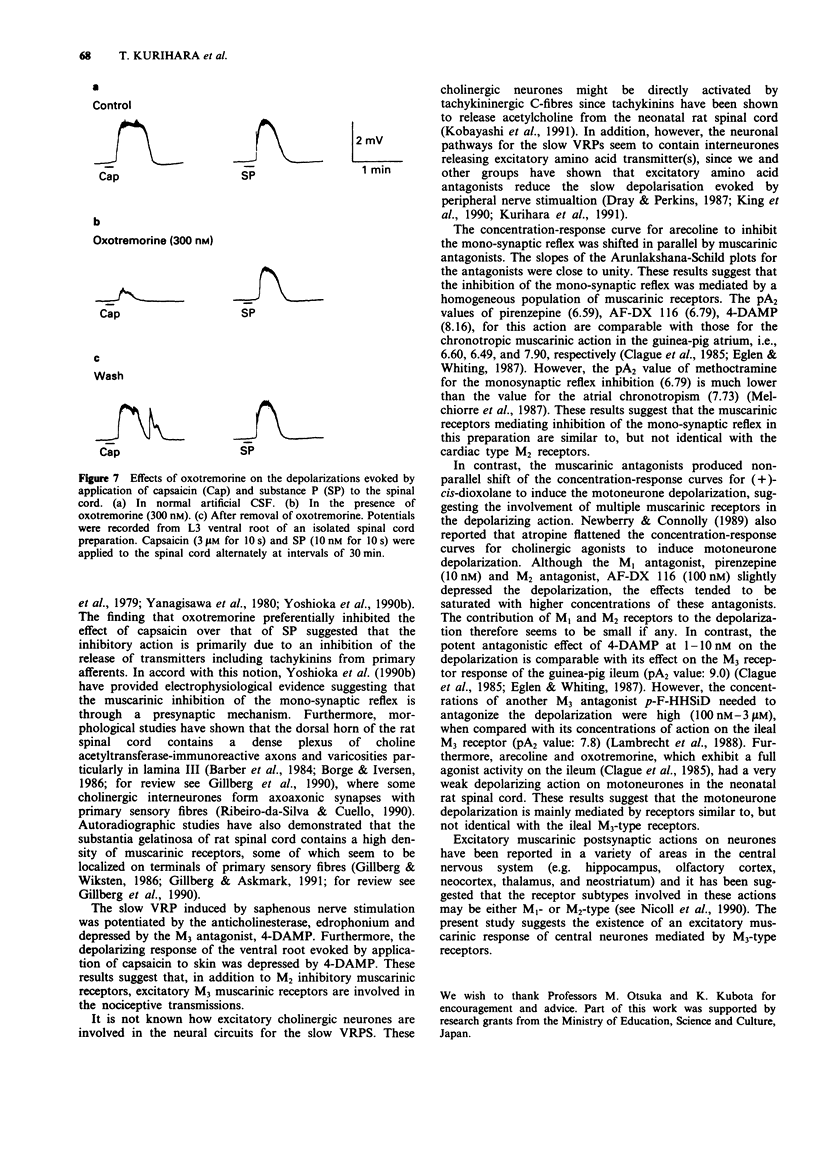
1. The involvement of acetylcholine and muscarinic receptors in spinal synaptic responses evoked by electrical and noxious sensory stimuli was investigated in the neonatal rat spinal cord in vitro. 2. Potentials were recorded extracellularly from a ventral root (L3-L5) of the isolated spinal cord, spinal cord-cutaneous nerve, and spinal cord-skin preparations of 1- to 4-day-old rats. Spinal reflexes were elicited by electrical stimulation of the ipsilateral dorsal root or the cutaneous saphenous nerve, or by noxious skin stimulation. 3. Single shock stimulation of supramaximum intensity of a dorsal root induced a mono-synaptic reflex in the corresponding ventral root. Bath-application of the muscarinic agonists, muscarine (0.3-30 microM) and (+)-cis-dioxolane (0.1-100 microM), produced an inhibition of the mono-synaptic reflex and a depolarization of motoneurones. Other muscarinic agonists, arecoline (10 nM-10 microM) and oxotremorine (10 nM-1 microM), inhibited the mono-synaptic reflex with little or no depolarization of motoneurones. Repetitive stimulation of the saphenous nerve at C-fibre strength induced a slow depolarizing response lasting about 30 s of the L3 ventral root. This slow ventral root potential (VRP) was also inhibited by arecoline (10 nM-10 microM) and oxotremorine (10 nM-1 microM). 4. In the spinal cord-saphenous nerve-skin preparation, a slow VRP was evoked by application of capsaicin (0.5 microM), bradykinin (3 microM), or noxious heat (47 degrees C) to skin. This slow VRP was depressed by the muscarinic agonists, arecoline (3 microM) and oxotremorine (1 microM). 5. Of the (+)-cis-dioxolane-induced inhibition of mono-synaptic reflex and motoneurone depolarization, the M2 antagonists, AF-DX 116 (0.1-1 microM) and methoctramine (100-300 nM), preferentially blocked the former response, whereas the M3 antagonists, 4-DAMP (3-10 nM) and p-F-HHSiD (0.3-3 microM), preferentially blocked the latter response. AF-DX 116 (0.1-1 microM) and methoctramine (100-300 nM) also effectively antagonized the arecoline- and oxotremorine-induced inhibition of the slow VRP. The pA2 values of AF-DX 116 and methoctramine against the arecoline-induced inhibition of the mono-synaptic reflex were both 6.79, and that of 4-DAMP against the (+)-cis-dioxolane-induced motoneurone depolarization was 8.16. 6. In the spinal cord-cutaneous nerve preparation, the saphenous nerve-evoked slow VRP was augmented by the anticholinesterase, edrophonium (5 microM). AF-DX 116 (1 microM) and methoctramine (100 nM) also potentiated the slow VRP, whereas 4-DAMP (10 nM) depressed the response.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

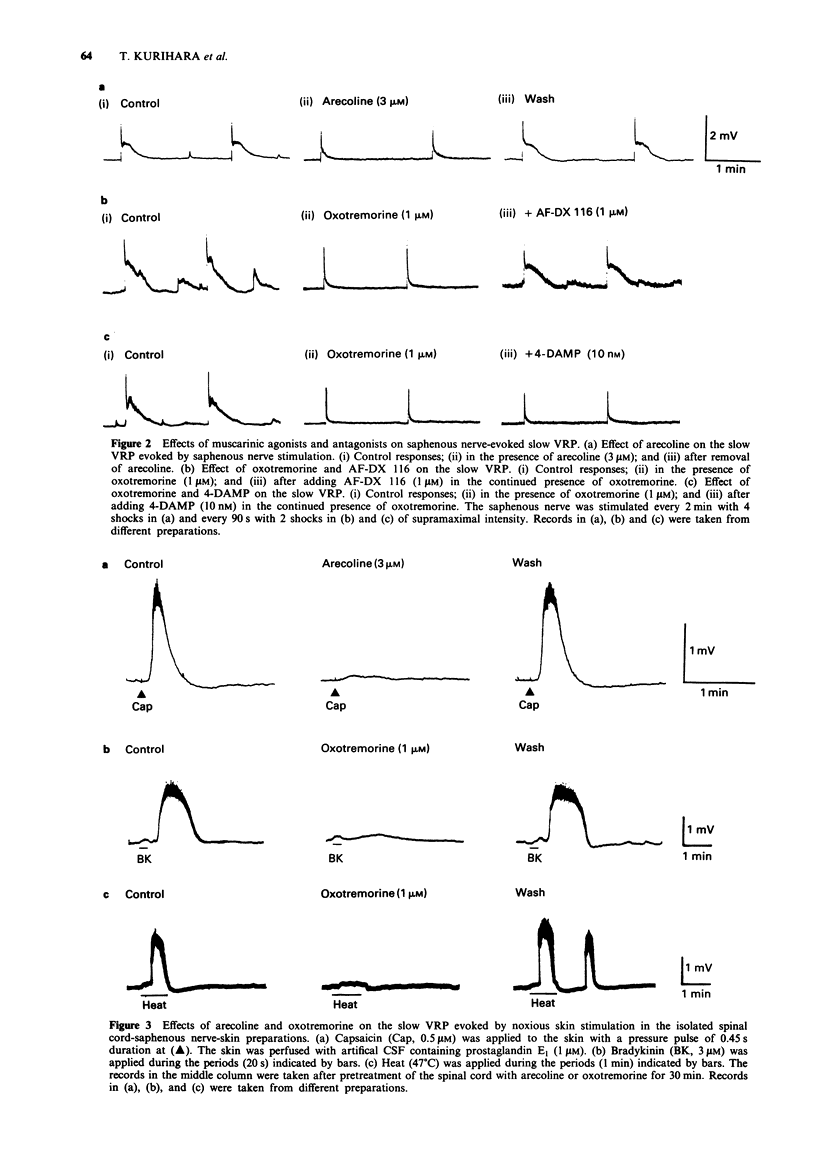
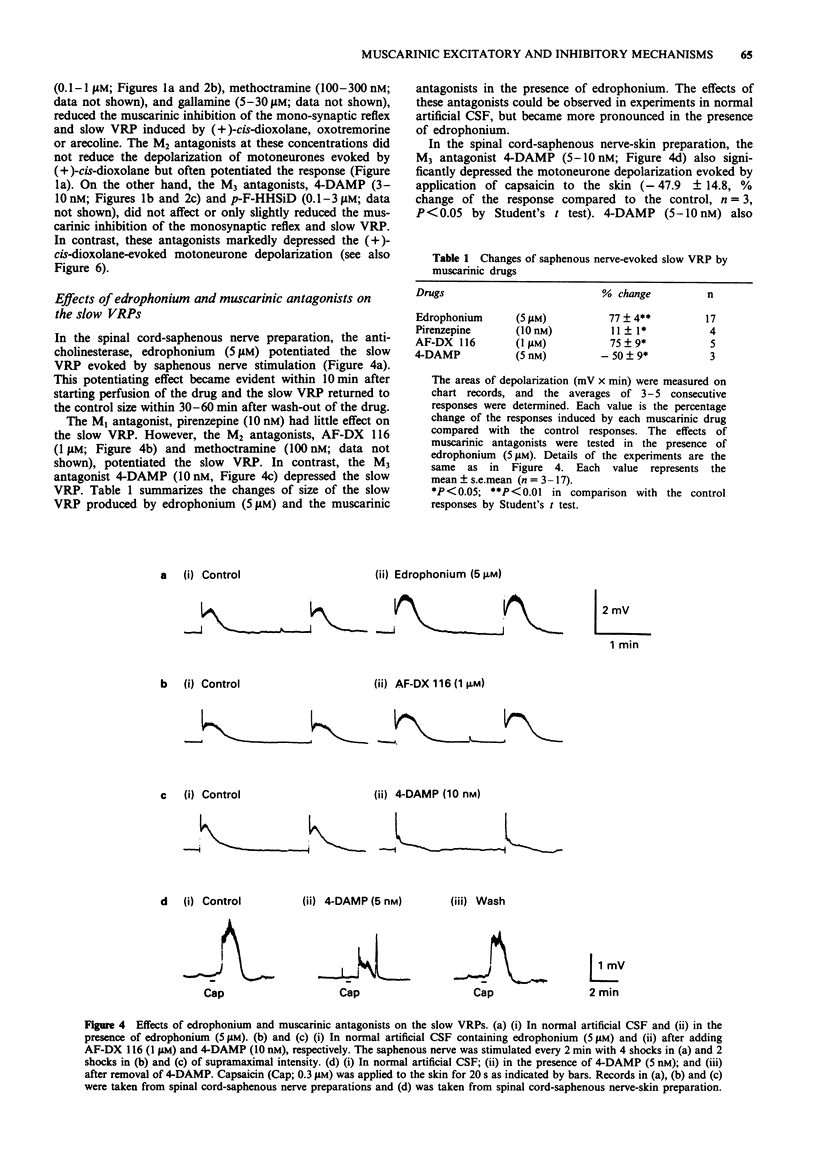


Selected References
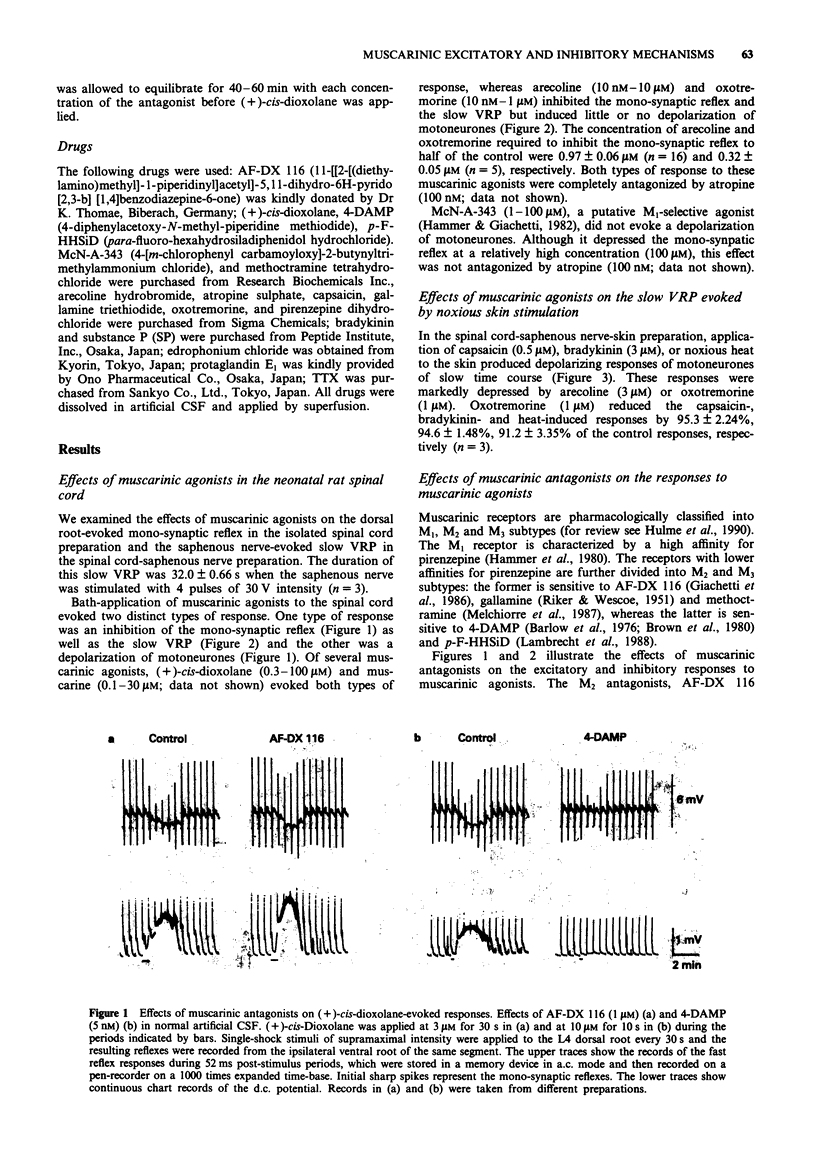
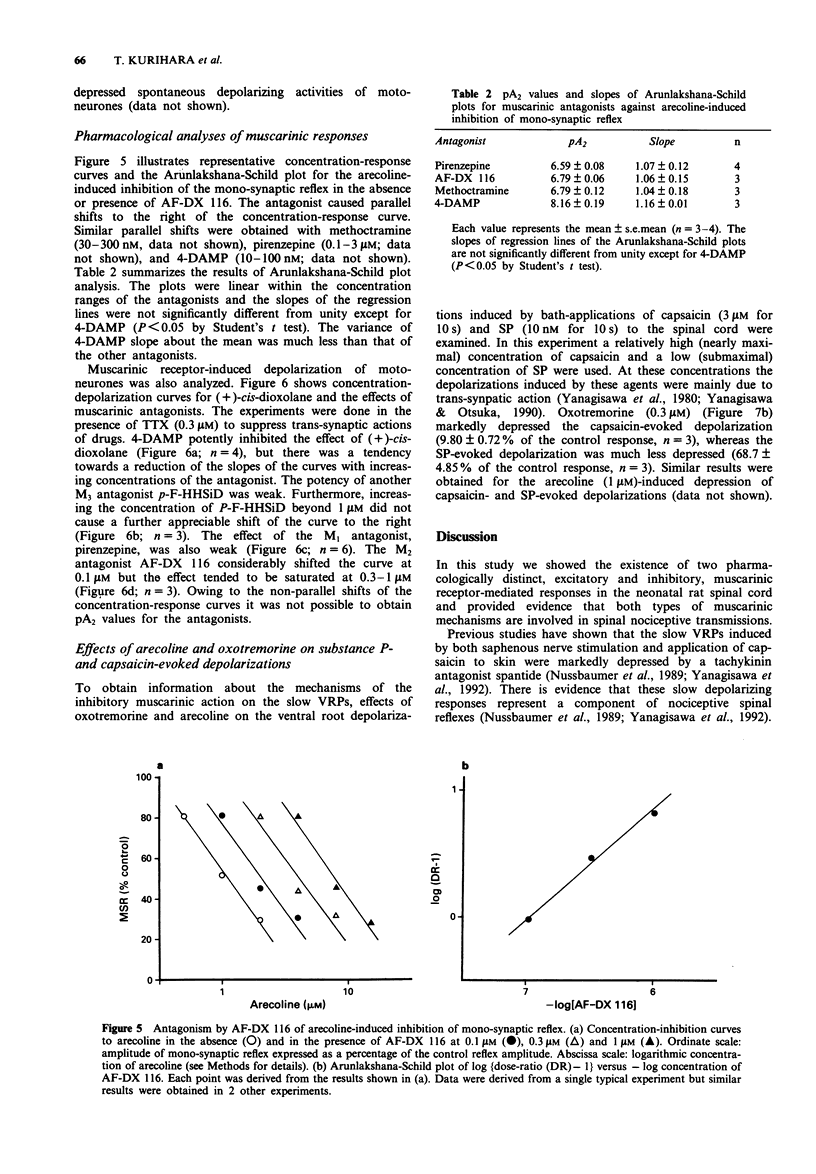
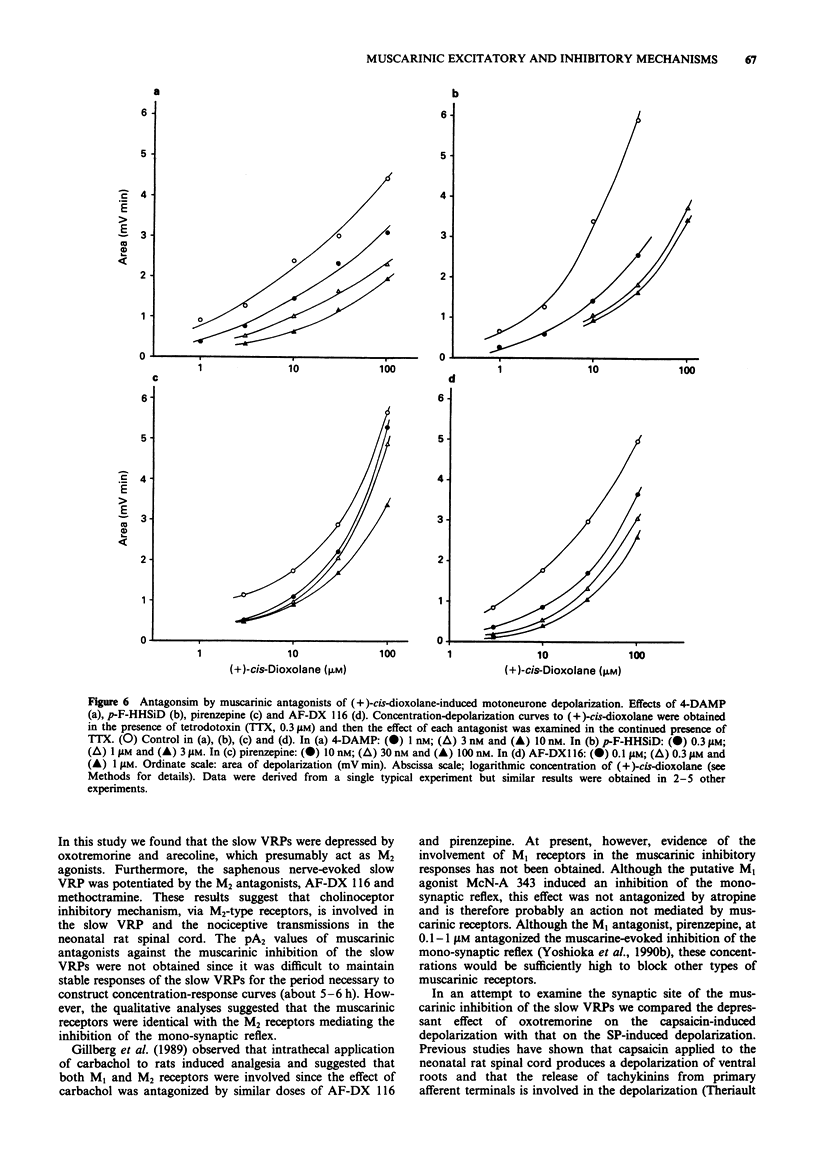
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi H., Konishi S., Otsuka M., Yanagisawa M. The role of substance P as a neurotransmitter in the reflexes of slow time courses in the neonatal rat spinal cord. Br J Pharmacol. 1985 Mar;84(3):663–673. doi: 10.1111/j.1476-5381.1985.tb16148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R. P., Phelps P. E., Houser C. R., Crawford G. D., Salvaterra P. M., Vaughn J. E. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984 Nov 1;229(3):329–346. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- Barlow R. B., Berry K. J., Glenton P. A., Nilolaou N. M., Soh K. S. A comparison of affinity constants for muscarine-sensitive acetylcholine receptors in guinea-pig atrial pacemaker cells at 29 degrees C and in ileum at 29 degrees C and 37 degrees C. Br J Pharmacol. 1976 Dec;58(4):613–620. doi: 10.1111/j.1476-5381.1976.tb08631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges L. F., Iversen S. D. Topography of choline acetyltransferase immunoreactive neurons and fibers in the rat spinal cord. Brain Res. 1986 Jan 1;362(1):140–148. doi: 10.1016/0006-8993(86)91407-1. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Forward A., Marsh S. Antagonist discrimination between ganglionic and ileal muscarinic receptors. Br J Pharmacol. 1980;71(2):362–364. doi: 10.1111/j.1476-5381.1980.tb10948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clague R. U., Eglen R. M., Strachan A. C., Whiting R. L. Action of agonists and antagonists at muscarinic receptors present on ileum and atria in vitro. Br J Pharmacol. 1985 Sep;86(1):163–170. doi: 10.1111/j.1476-5381.1985.tb09446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen R., Nijhuis G. M. The relevance of cholinergic transmission at the spinal level to opiate effectiveness. Eur J Pharmacol. 1983 Jul 22;91(2-3):215–221. doi: 10.1016/0014-2999(83)90467-3. [DOI] [PubMed] [Google Scholar]
- Eglen R. M., Whiting R. L. Competitive and non-competitive antagonism exhibited by 'selective' antagonists at atrial and ileal muscarinic receptor subtypes. Br J Pharmacol. 1987 Apr;90(4):701–707. doi: 10.1111/j.1476-5381.1987.tb11223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert F. J., Dumont Y., Roeske W. R., Yamamura H. I. Muscarinic receptor binding in rat brain using the agonist, [3H]cis methyldioxolane. Life Sci. 1980 Mar 24;26(12):961–967. doi: 10.1016/0024-3205(80)90117-4. [DOI] [PubMed] [Google Scholar]
- Evans R. H. Cholinoceptive properties of motoneurons of the immature rat spinal cord maintained in vitro. Neuropharmacology. 1978 Apr-May;17(4-5):277–279. doi: 10.1016/0028-3908(78)90112-0. [DOI] [PubMed] [Google Scholar]
- Giachetti A., Micheletti R., Montagna E. Cardioselective profile of AF-DX 116, a muscarine M2 receptor antagonist. Life Sci. 1986 May 5;38(18):1663–1672. doi: 10.1016/0024-3205(86)90410-8. [DOI] [PubMed] [Google Scholar]
- Gillberg P. G., Askmark H., Aquilonius S. M. Spinal cholinergic mechanisms. Prog Brain Res. 1990;84:361–370. [PubMed] [Google Scholar]
- Gillberg P. G., Askmark H. Changes in cholinergic and opioid receptors in the rat spinal cord, dorsal root and sciatic nerve after ventral and dorsal root lesion. J Neural Transm Gen Sect. 1991;85(1):31–39. doi: 10.1007/BF01244655. [DOI] [PubMed] [Google Scholar]
- Gillberg P. G., Gordh T., Jr, Hartvig P., Jansson I., Pettersson J., Post C. Characterization of the antinociception induced by intrathecally administered carbachol. Pharmacol Toxicol. 1989 Apr;64(4):340–343. doi: 10.1111/j.1600-0773.1989.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Gillberg P. G., Wiksten B. Effects of spinal cord lesions and rhizotomies on cholinergic and opiate receptor binding sites in rat spinal cord. Acta Physiol Scand. 1986 Apr;126(4):575–582. doi: 10.1111/j.1748-1716.1986.tb07857.x. [DOI] [PubMed] [Google Scholar]
- Green P. G., Kitchen I. Antinociception opioids and the cholinergic system. Prog Neurobiol. 1986;26(2):119–146. doi: 10.1016/0301-0082(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Hammer R., Berrie C. P., Birdsall N. J., Burgen A. S., Hulme E. C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980 Jan 3;283(5742):90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- Hammer R., Giachetti A. Muscarinic receptor subtypes: M1 and M2 biochemical and functional characterization. Life Sci. 1982 Dec 27;31(26):2991–2998. doi: 10.1016/0024-3205(82)90066-2. [DOI] [PubMed] [Google Scholar]
- Hartvig P., Gillberg P. G., Gordh T., Jr, Post C. Cholinergic mechanisms in pain and analgesia. Trends Pharmacol Sci. 1989 Dec;Suppl:75–79. [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Jiang Z. G., Dun N. J. Presynaptic suppression of excitatory postsynaptic potentials in rat ventral horn neurons by muscarinic agonists. Brain Res. 1986 Aug 27;381(1):182–186. doi: 10.1016/0006-8993(86)90710-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Sakuma M., Yoshioka K., Onishi Y., Yanagisawa M., Kawashima K., Otsuka M. Substance P-evoked release of acetylcholine from isolated spinal cord of the newborn rat. Neuroscience. 1991;45(2):331–337. doi: 10.1016/0306-4522(91)90230-l. [DOI] [PubMed] [Google Scholar]
- Lambrecht G., Feifel R., Forth B., Strohmann C., Tacke R., Mutschler E. p-fluoro-hexahydro-sila-difenidol: the first M2 beta-selective muscarinic antagonist. Eur J Pharmacol. 1988 Jul 26;152(1-2):193–194. doi: 10.1016/0014-2999(88)90856-4. [DOI] [PubMed] [Google Scholar]
- Melchiorre C., Angeli P., Lambrecht G., Mutschler E., Picchio M. T., Wess J. Antimuscarinic action of methoctramine, a new cardioselective M-2 muscarinic receptor antagonist, alone and in combination with atropine and gallamine. Eur J Pharmacol. 1987 Dec 1;144(2):117–124. doi: 10.1016/0014-2999(87)90509-7. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Connolly G. P. Muscarinic pharmacology of the spinal cord of the neonatal rat in vitro. Neuropharmacology. 1989 Feb;28(2):149–152. doi: 10.1016/0028-3908(89)90051-8. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Malenka R. C., Kauer J. A. Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev. 1990 Apr;70(2):513–565. doi: 10.1152/physrev.1990.70.2.513. [DOI] [PubMed] [Google Scholar]
- Nussbaumer J. C., Yanagisawa M., Otsuka M. Pharmacological properties of a C-fibre response evoked by saphenous nerve stimulation in an isolated spinal cord-nerve preparation of the newborn rat. Br J Pharmacol. 1989 Oct;98(2):373–382. doi: 10.1111/j.1476-5381.1989.tb12607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Electrophysiology of mammalian spinal cord in vitro. Nature. 1974 Dec 20;252(5485):733–734. doi: 10.1038/252733a0. [DOI] [PubMed] [Google Scholar]
- Otsuka M., Yanagisawa M. Effect of a tachykinin antagonist on a nociceptive reflex in the isolated spinal cord-tail preparation of the newborn rat. J Physiol. 1988 Jan;395:255–270. doi: 10.1113/jphysiol.1988.sp016917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIKER W. F., Jr, WESCOE W. C. The pharmacology of Flaxedil, with observations on certain analogs. Ann N Y Acad Sci. 1951 Oct;54(3):373–394. doi: 10.1111/j.1749-6632.1951.tb39932.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A., Cuello A. C. Choline acetyltransferase-immunoreactive profiles are presynaptic to primary sensory fibers in the rat superficial dorsal horn. J Comp Neurol. 1990 May 15;295(3):370–384. doi: 10.1002/cne.902950303. [DOI] [PubMed] [Google Scholar]
- Smith M. D., Yang X. H., Nha J. Y., Buccafusco J. J. Antinociceptive effect of spinal cholinergic stimulation: interaction with substance P. Life Sci. 1989;45(14):1255–1261. doi: 10.1016/0024-3205(89)90127-6. [DOI] [PubMed] [Google Scholar]
- Taylor J. E., Yaksh T. L., Richelson E. Agonist regulation of muscarinic acetylcholine receptors in rat spinal cord. J Neurochem. 1982 Aug;39(2):521–524. doi: 10.1111/j.1471-4159.1982.tb03975.x. [DOI] [PubMed] [Google Scholar]
- Theriault E., Otsuka M., Jessell T. Capsaicin-evoked release of substance P from primary sensory neurons. Brain Res. 1979 Jul 6;170(1):209–213. doi: 10.1016/0006-8993(79)90957-0. [DOI] [PubMed] [Google Scholar]
- Yaksh T. L., Dirksen R., Harty G. J. Antinociceptive effects of intrathecally injected cholinomimetic drugs in the rat and cat. Eur J Pharmacol. 1985 Oct 29;117(1):81–88. doi: 10.1016/0014-2999(85)90474-1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Hosoki R., Otsuka M. The isolated spinal cord-skin preparation of the newborn rat and effects of some algogenic and analgesic substances. Eur J Pharmacol. 1992 Sep 22;220(2-3):111–117. doi: 10.1016/0014-2999(92)90737-o. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Otsuka M., Konishi S., Akagi H., Folkers K., Rosell S. A substance P antagonist inhibits a slow reflex response in the spinal cord of the newborn rat. Acta Physiol Scand. 1982 Sep;116(1):109–112. doi: 10.1111/j.1748-1716.1982.tb10608.x. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Otsuka M. Pharmacological profile of a tachykinin antagonist, spantide, as examined on rat spinal motoneurones. Br J Pharmacol. 1990 Aug;100(4):711–716. doi: 10.1111/j.1476-5381.1990.tb14080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K., Sakuma M., Otsuka M. Cutaneous nerve-evoked cholinergic inhibition of monosynaptic reflex in the neonatal rat spinal cord: involvement on M2 receptors and tachykininergic primary afferents. Neuroscience. 1990;38(1):195–203. doi: 10.1016/0306-4522(90)90385-h. [DOI] [PubMed] [Google Scholar]


