Abstract
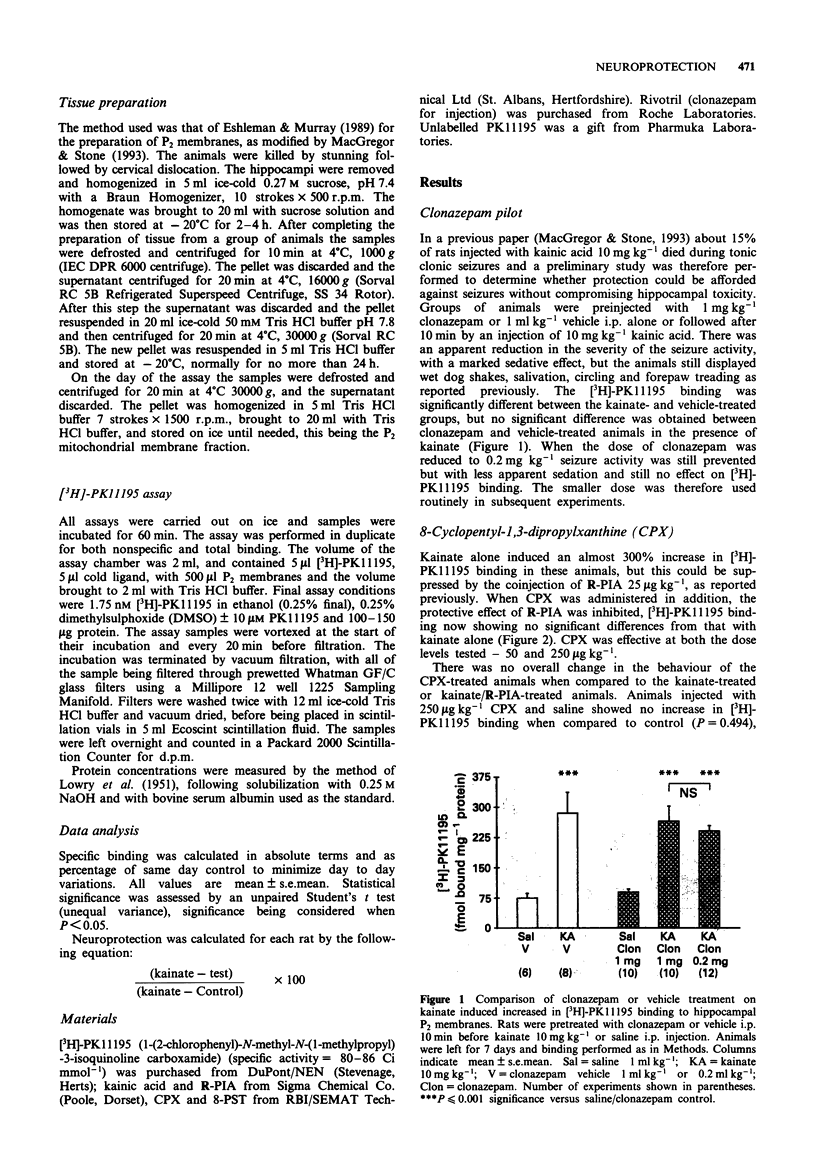
1. Systemic injections of kainic acid, 10 mg kg-1, into adult rats resulted in lesions in the hippocampus, as assessed by peripheral benzodiazepine ligand binding. Co-administration of clonazepam at 1 mg kg-1 or 0.2 mg kg-1 prevented major seizures associated with kainate injections, but did not alter significantly the production of hippocampal damage. 2. The co-administration of the adenosine A1 agonist R-phenylisopropyladenosine (R-PIA, 25 micrograms kg-1, i.p.) abolished the lesions induced by kainic acid. 3. The presence of the selective A1 antagonist, 8-cyclopentyl-1,3-dipropylxanthine (250 or 50 micrograms kg-1, i.p.) abolished the R-PIA neuroprotective action. 4. The A1/A2 antagonist, 8-(p-sulphophenyl)theophylline (20 mg kg-1, i.p.) which cannot cross the blood brain barrier, did not alter significantly the neuroprotective action of R-PIA, indicating that the neuroprotective action of the purine may be predominantly central. 5. The time course of the neuroprotection was also examined. R-PIA was effective when administered 2 h before or after kainate administration. 6. The results emphasise the potential utility of systemically active adenosine A1 receptor ligands in reducing CNS gliosis induced by the activation of excitatory amino acid receptors.
Full text
PDF
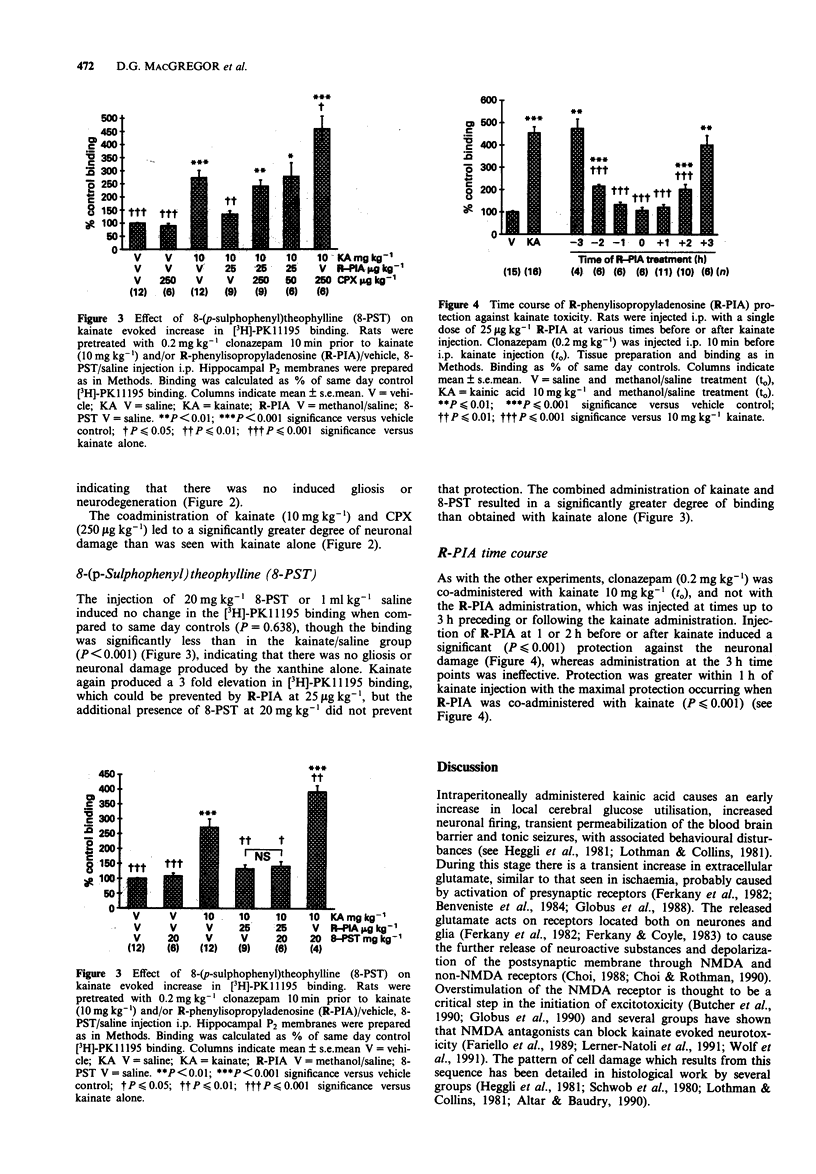




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altar C. A., Baudry M. Systemic injection of kainic acid: gliosis in olfactory and limbic brain regions quantified with [3H]PK 11195 binding autoradiography. Exp Neurol. 1990 Sep;109(3):333–341. doi: 10.1016/s0014-4886(05)80024-x. [DOI] [PubMed] [Google Scholar]
- Alzheimer C., Kargl L., ten Bruggencate G. Adenosinergic inhibition in hippocampus is mediated by adenosine A1 receptors very similar to those of peripheral tissues. Eur J Pharmacol. 1991 Apr 24;196(3):313–317. doi: 10.1016/0014-2999(91)90445-v. [DOI] [PubMed] [Google Scholar]
- Ameri A., Jurna I. Adenosine A1 and non-A1 receptors: intracellular analysis of the actions of adenosine agonists and antagonists in rat hippocampal neurons. Brain Res. 1991 Apr 12;546(1):69–78. doi: 10.1016/0006-8993(91)91160-3. [DOI] [PubMed] [Google Scholar]
- Arvin B., Neville L. F., Pan J., Roberts P. J. 2-chloroadenosine attenuates kainic acid-induced toxicity within the rat straitum: relationship to release of glutamate and Ca2+ influx. Br J Pharmacol. 1989 Sep;98(1):225–235. doi: 10.1111/j.1476-5381.1989.tb16886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A. J., Zornow M. H., Grafe M. R., Scheller M. S., Skilling S. R., Smullin D. H., Larson A. A. Hypothermia prevents ischemia-induced increases in hippocampal glycine concentrations in rabbits. Stroke. 1991 May;22(5):666–673. doi: 10.1161/01.str.22.5.666. [DOI] [PubMed] [Google Scholar]
- Baumgold J., Nikodijevic O., Jacobson K. A. Penetration of adenosine antagonists into mouse brain as determined by ex vivo binding. Biochem Pharmacol. 1992 Feb 18;43(4):889–894. doi: 10.1016/0006-2952(92)90257-j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Tremblay E., Ottersen O. P., Naquet R. Evidence suggesting secondary epileptogenic lesion after kainic acid: pre treatment with diazepam reduces distant but not local brain damage. Brain Res. 1979 Apr 13;165(2):362–365. doi: 10.1016/0006-8993(79)90571-7. [DOI] [PubMed] [Google Scholar]
- Benveniste H., Drejer J., Schousboe A., Diemer N. H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984 Nov;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Braun D. E., Freed W. J. Effect of nifedipine and anticonvulsants on kainic acid-induced seizures in mice. Brain Res. 1990 Nov 12;533(1):157–160. doi: 10.1016/0006-8993(90)91810-4. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hartman J. D., Hays S. J., Huang C. C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Bullock R., Fujisawa H. The role of glutamate antagonists for the treatment of CNS injury. J Neurotrauma. 1992 May;9 (Suppl 2):S443–S462. [PubMed] [Google Scholar]
- Butcher S. P., Bullock R., Graham D. I., McCulloch J. Correlation between amino acid release and neuropathologic outcome in rat brain following middle cerebral artery occlusion. Stroke. 1990 Dec;21(12):1727–1733. doi: 10.1161/01.str.21.12.1727. [DOI] [PubMed] [Google Scholar]
- Cantor S. L., Zornow M. H., Miller L. P., Yaksh T. L. The effect of cyclohexyladenosine on the periischemic increases of hippocampal glutamate and glycine in the rabbit. J Neurochem. 1992 Nov;59(5):1884–1892. doi: 10.1111/j.1471-4159.1992.tb11024.x. [DOI] [PubMed] [Google Scholar]
- Carlà V., Moroni F. General anaesthetics inhibit the responses induced by glutamate receptor agonists in the mouse cortex. Neurosci Lett. 1992 Oct 26;146(1):21–24. doi: 10.1016/0304-3940(92)90162-z. [DOI] [PubMed] [Google Scholar]
- Chen Y., Graham D. I., Stone T. W. Release of endogenous adenosine and its metabolites by the activation of NMDA receptors in the rat hippocampus in vivo. Br J Pharmacol. 1992 Jul;106(3):632–638. doi: 10.1111/j.1476-5381.1992.tb14387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988 Oct;11(10):465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Rothman S. M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Connick J. H., Stone T. W. Quinolinic acid neurotoxicity: protection by intracerebral phenylisopropyladenosine (PIA) and potentiation by hypotension. Neurosci Lett. 1989 Jun 19;101(2):191–196. doi: 10.1016/0304-3940(89)90529-6. [DOI] [PubMed] [Google Scholar]
- Coyle J. T. Kainic acid: insights into excitatory mechanisms causing selective neuronal degeneration. Ciba Found Symp. 1987;126:186–203. doi: 10.1002/9780470513422.ch12. [DOI] [PubMed] [Google Scholar]
- Deckert J., Jorgensen M. B. Evidence for pre- and postsynaptic localization of adenosine A1 receptors in the CA1 region of rat hippocampus: a quantitative autoradiographic study. Brain Res. 1988 Apr 12;446(1):161–164. doi: 10.1016/0006-8993(88)91308-x. [DOI] [PubMed] [Google Scholar]
- Eshleman A. J., Murray T. F. Differential binding properties of the peripheral-type benzodiazepine ligands [3H]PK 11195 and [3H]Ro 5-4864 in trout and mouse brain membranes. J Neurochem. 1989 Aug;53(2):494–502. doi: 10.1111/j.1471-4159.1989.tb07361.x. [DOI] [PubMed] [Google Scholar]
- Fariello R. G., Golden G. T., Smith G. G., Reyes P. F. Potentiation of kainic acid epileptogenicity and sparing from neuronal damage by an NMDA receptor antagonist. Epilepsy Res. 1989 May-Jun;3(3):206–213. doi: 10.1016/0920-1211(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Fastbom J., Fredholm B. B. Inhibition of [3H] glutamate release from rat hippocampal slices by L-phenylisopropyladenosine. Acta Physiol Scand. 1985 Sep;125(1):121–123. doi: 10.1111/j.1748-1716.1985.tb07698.x. [DOI] [PubMed] [Google Scholar]
- Ferkany J. W., Coyle J. T. Kainic acid selectively stimulates the release of endogenous excitatory acidic amino acids. J Pharmacol Exp Ther. 1983 May;225(2):399–406. [PubMed] [Google Scholar]
- Ferkany J. W., Zaczek R., Coyle J. T. Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature. 1982 Aug 19;298(5876):757–759. doi: 10.1038/298757a0. [DOI] [PubMed] [Google Scholar]
- Finn S. F., Swartz K. J., Beal M. F. 2-Chloroadenosine attenuates NMDA, kainate, and quisqualate toxicity. Neurosci Lett. 1991 May 27;126(2):191–194. doi: 10.1016/0304-3940(91)90551-4. [DOI] [PubMed] [Google Scholar]
- Franck J. E., Roberts D. L. Combined kainate and ischemia produces 'mesial temporal sclerosis'. Neurosci Lett. 1990 Oct 16;118(2):159–163. doi: 10.1016/0304-3940(90)90616-h. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Dunwiddie T. V. How does adenosine inhibit transmitter release? Trends Pharmacol Sci. 1988 Apr;9(4):130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Ylinen A., Miettinen R., Pitkänen A., Lahtinen H., Baimbridge K. G., Riekkinen P. J. Pattern of neuronal death in the rat hippocampus after status epilepticus. Relationship to calcium binding protein content and ischemic vulnerability. Brain Res Bull. 1992 Jan;28(1):27–38. doi: 10.1016/0361-9230(92)90227-o. [DOI] [PubMed] [Google Scholar]
- Fuller T. A., Olney J. W. Only certain anticonvulsants protect against kainate neurotoxicity. Neurobehav Toxicol Teratol. 1981 Fall;3(3):355–361. [PubMed] [Google Scholar]
- Globus M. Y., Busto R., Dietrich W. D., Martinez E., Valdes I., Ginsberg M. D. Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and gamma-aminobutyric acid studied by intracerebral microdialysis. J Neurochem. 1988 Nov;51(5):1455–1464. doi: 10.1111/j.1471-4159.1988.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Globus M. Y., Busto R., Martinez E., Valdés I., Dietrich W. D., Ginsberg M. D. Comparative effect of transient global ischemia on extracellular levels of glutamate, glycine, and gamma-aminobutyric acid in vulnerable and nonvulnerable brain regions in the rat. J Neurochem. 1991 Aug;57(2):470–478. doi: 10.1111/j.1471-4159.1991.tb03775.x. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Synder S. H. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982 Sep;2(9):1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haba K., Ogawa N., Mizukawa K., Mori A. Time course of changes in lipid peroxidation, pre- and postsynaptic cholinergic indices, NMDA receptor binding and neuronal death in the gerbil hippocampus following transient ischemia. Brain Res. 1991 Feb 1;540(1-2):116–122. doi: 10.1016/0006-8993(91)90497-j. [DOI] [PubMed] [Google Scholar]
- Heggli D. E., Aamodt A., Malthe-Sørenssen D. Kainic acid neurotoxicity; effect of systemic injection on neurotransmitter markers in different brain regions. Brain Res. 1981 Dec 28;230(1-2):253–262. doi: 10.1016/0006-8993(81)90405-4. [DOI] [PubMed] [Google Scholar]
- Heggli D. E., Malthe-Sørenssen D. Systemic injection of kainic acid: effect on neurotransmitter markers in piriform cortex, amygdaloid complex and hippocampus and protection by cortical lesioning and anticonvulsants. Neuroscience. 1982 May;7(5):1257–1264. doi: 10.1016/0306-4522(82)91132-0. [DOI] [PubMed] [Google Scholar]
- Hoehn K., White T. D. Glutamate-evoked release of endogenous adenosine from rat cortical synaptosomes is mediated by glutamate uptake and not by receptors. J Neurochem. 1990 May;54(5):1716–1724. doi: 10.1111/j.1471-4159.1990.tb01226.x. [DOI] [PubMed] [Google Scholar]
- Héron A., Lasbennes F., Seylaz J. Effect of two different routes of administration of R-PIA on glutamate release during ischemia. Neurosci Lett. 1992 Dec 7;147(2):205–208. doi: 10.1016/0304-3940(92)90596-y. [DOI] [PubMed] [Google Scholar]
- Januszewicz von Lubitz D. K., Dambrosia J. M., Redmond D. J. Protective effect of cyclohexyl adenosine in treatment of cerebral ischemia in gerbils. Neuroscience. 1989;30(2):451–462. doi: 10.1016/0306-4522(89)90265-0. [DOI] [PubMed] [Google Scholar]
- Kendall T. J., Minchin M. C. The effects of anaesthetics on the uptake and release of amino acid neurotransmitters in thalamic slices. Br J Pharmacol. 1982 Jan;75(1):219–227. doi: 10.1111/j.1476-5381.1982.tb08776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh J. Y., Choi D. W. Effect of anticonvulsant drugs on glutamate neurotoxicity in cortical cell culture. Neurology. 1987 Feb;37(2):319–322. doi: 10.1212/wnl.37.2.319. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leranth C., Ribak C. E. Calcium-binding proteins are concentrated in the CA2 field of the monkey hippocampus: a possible key to this region's resistance to epileptic damage. Exp Brain Res. 1991;85(1):129–136. doi: 10.1007/BF00229993. [DOI] [PubMed] [Google Scholar]
- Lerma J., Zukin R. S., Bennett M. V. Glycine decreases desensitization of N-methyl-D-aspartate (NMDA) receptors expressed in Xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner-Natoli M., Rondouin G., Belaidi M., Baldy-Moulinier M., Kamenka J. M. N-[1-(2-thienyl)cyclohexyl]-piperidine (TCP) does not block kainic acid-induced status epilepticus but reduces secondary hippocampal damage. Neurosci Lett. 1991 Jan 28;122(2):174–178. doi: 10.1016/0304-3940(91)90851-j. [DOI] [PubMed] [Google Scholar]
- Lin B. W., Dietrich W. D., Busto R., Ginsberg M. D. (S)-emopamil protects against global ischemic brain injury in rats. Stroke. 1990 Dec;21(12):1734–1739. doi: 10.1161/01.str.21.12.1734. [DOI] [PubMed] [Google Scholar]
- Lothman E. W., Collins R. C. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. 1981 Aug 10;218(1-2):299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- MacGregor D. G., Stone T. W. Inhibition by the adenosine analogue, (R-)-N6-phenylisopropyladenosine, of kainic acid neurotoxicity in rat hippocampus after systemic administration. Br J Pharmacol. 1993 Jun;109(2):316–321. doi: 10.1111/j.1476-5381.1993.tb13572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. P., Hsu C. Therapeutic potential for adenosine receptor activation in ischemic brain injury. J Neurotrauma. 1992 May;9 (Suppl 2):S563–S577. [PubMed] [Google Scholar]
- Nakano S., Kogure K., Fujikura H. Ischemia-induced slowly progressive neuronal damage in the rat brain. Neuroscience. 1990;38(1):115–124. doi: 10.1016/0306-4522(90)90378-h. [DOI] [PubMed] [Google Scholar]
- Nehlig A., Pereira de Vasconcelos A., Collignon A., Boyet S. Comparative effects of caffeine and L-phenylisopropyladenosine on local cerebral glucose utilization in the rat. Eur J Pharmacol. 1988 Nov 15;157(1):1–11. doi: 10.1016/0014-2999(88)90464-5. [DOI] [PubMed] [Google Scholar]
- Notman H., Whitney R., Jhamandas K. Kainic acid evoked release of D-[3H]aspartate from rat striatum in vitro: characterization and pharmacological modulation. Can J Physiol Pharmacol. 1984 Sep;62(9):1070–1077. doi: 10.1139/y84-179. [DOI] [PubMed] [Google Scholar]
- Ohta S., Smith M. L., Siesjö B. K. The effect of a dihydropyridine calcium antagonist (isradipine) on selective neuronal necrosis. J Neurol Sci. 1991 May;103(1):109–115. doi: 10.1016/0022-510x(91)90293-g. [DOI] [PubMed] [Google Scholar]
- Palmer A. M., Reiter C. T., Botscheller M. Comparison of the release of exogenous and endogenous excitatory amino acids from rat cerebral cortex. Ann N Y Acad Sci. 1992 May 11;648:361–364. doi: 10.1111/j.1749-6632.1992.tb24583.x. [DOI] [PubMed] [Google Scholar]
- Patel J., Zinkand W. C., Thompson C., Keith R., Salama A. Role of glycine in the N-methyl-D-aspartate-mediated neuronal cytotoxicity. J Neurochem. 1990 Mar;54(3):849–854. doi: 10.1111/j.1471-4159.1990.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Walter G. A., O'Regan M. H., Stair R. E. Increases in cerebral cortical perfusate adenosine and inosine concentrations during hypoxia and ischemia. J Cereb Blood Flow Metab. 1987 Dec;7(6):679–686. doi: 10.1038/jcbfm.1987.122. [DOI] [PubMed] [Google Scholar]
- Richards C. D. Actions of general anaesthetics on synaptic transmission in the CNS. Br J Anaesth. 1983 Mar;55(3):201–207. doi: 10.1093/bja/55.3.201. [DOI] [PubMed] [Google Scholar]
- Rudolphi K. A., Keil M., Hinze H. J. Effect of theophylline on ischemically induced hippocampal damage in Mongolian gerbils: a behavioral and histopathological study. J Cereb Blood Flow Metab. 1987 Feb;7(1):74–81. doi: 10.1038/jcbfm.1987.11. [DOI] [PubMed] [Google Scholar]
- Rudolphi K. A., Schubert P., Parkinson F. E., Fredholm B. B. Neuroprotective role of adenosine in cerebral ischaemia. Trends Pharmacol Sci. 1992 Dec;13(12):439–445. doi: 10.1016/0165-6147(92)90141-r. [DOI] [PubMed] [Google Scholar]
- Saija A., Princi P., Pisani A., Santoro G., De Pasquale R., Massi M., Costa G. Blood-brain barrier dysfunctions following systemic injection of kainic acid in the rat. Life Sci. 1992;51(7):467–477. doi: 10.1016/0024-3205(92)90023-i. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R., Freund T. F. Selective vulnerability of the hippocampus in brain ischemia. Neuroscience. 1991;40(3):599–636. doi: 10.1016/0306-4522(91)90001-5. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Foster A. C., French E. D., Whetsell W. O., Jr, Köhler C. Excitotoxic models for neurodegenerative disorders. Life Sci. 1984 Jul 2;35(1):19–32. doi: 10.1016/0024-3205(84)90148-6. [DOI] [PubMed] [Google Scholar]
- Schwob J. E., Fuller T., Price J. L., Olney J. W. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5(6):991–1014. doi: 10.1016/0306-4522(80)90181-5. [DOI] [PubMed] [Google Scholar]
- Simpson R. E., O'Regan M. H., Perkins L. M., Phillis J. W. Excitatory transmitter amino acid release from the ischemic rat cerebral cortex: effects of adenosine receptor agonists and antagonists. J Neurochem. 1992 May;58(5):1683–1690. doi: 10.1111/j.1471-4159.1992.tb10041.x. [DOI] [PubMed] [Google Scholar]
- Stewart G. R., Zorumski C. F., Price M. T., Olney J. W. Domoic acid: a dementia-inducing excitotoxic food poison with kainic acid receptor specificity. Exp Neurol. 1990 Oct;110(1):127–138. doi: 10.1016/0014-4886(90)90057-y. [DOI] [PubMed] [Google Scholar]
- Sutula T., Cavazos J., Golarai G. Alteration of long-lasting structural and functional effects of kainic acid in the hippocampus by brief treatment with phenobarbital. J Neurosci. 1992 Nov;12(11):4173–4187. doi: 10.1523/JNEUROSCI.12-11-04173.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M., Haas H. L., Gähwiler B. H. Comparison of the actions of adenosine at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:347–363. doi: 10.1113/jphysiol.1992.sp019168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K., Shibata S., Watanabe S. A neuroprotective effect of adenosine A1-receptor agonists on ischemia-induced decrease in 2-deoxyglucose uptake in rat hippocampal slices. Neurosci Lett. 1992 Sep 28;145(1):67–70. doi: 10.1016/0304-3940(92)90205-l. [DOI] [PubMed] [Google Scholar]
- Uematsu D., Araki N., Greenberg J. H., Sladky J., Reivich M. Combined therapy with MK-801 and nimodipine for protection of ischemic brain damage. Neurology. 1991 Jan;41(1):88–94. doi: 10.1212/wnl.41.1.88. [DOI] [PubMed] [Google Scholar]
- Voll C. L., Auer R. N. Postischemic seizures and necrotizing ischemic brain damage: neuroprotective effect of postischemic diazepam and insulin. Neurology. 1991 Mar;41(3):423–428. doi: 10.1212/wnl.41.3.423. [DOI] [PubMed] [Google Scholar]
- Wolf G., Fischer S., Hass P., Abicht K., Keilhoff G. Magnesium sulphate subcutaneously injected protects against kainate-induced convulsions and neurodegeneration: in vivo study on the rat hippocampus. Neuroscience. 1991;43(1):31–34. doi: 10.1016/0306-4522(91)90413-i. [DOI] [PubMed] [Google Scholar]
- Wood E. R., Bussey T. J., Phillips A. G. A glycine antagonist reduces ischemia-induced CA1 cell loss in vivo. Neurosci Lett. 1992 Sep 28;145(1):10–14. doi: 10.1016/0304-3940(92)90191-9. [DOI] [PubMed] [Google Scholar]
- Zaczek R., Nelson M. F., Coyle J. T. Effects of anaesthetics and anticonvulsants on the action of kainic acid in the rat hippocampus. Eur J Pharmacol. 1978 Dec 1;52(3-4):323–327. doi: 10.1016/0014-2999(78)90285-6. [DOI] [PubMed] [Google Scholar]
- Zucker D. K., Wooten G. F., Lothman E. W. Blood-brain barrier changes with kainic acid-induced limbic seizures. Exp Neurol. 1983 Feb;79(2):422–433. doi: 10.1016/0014-4886(83)90223-6. [DOI] [PubMed] [Google Scholar]


