Abstract
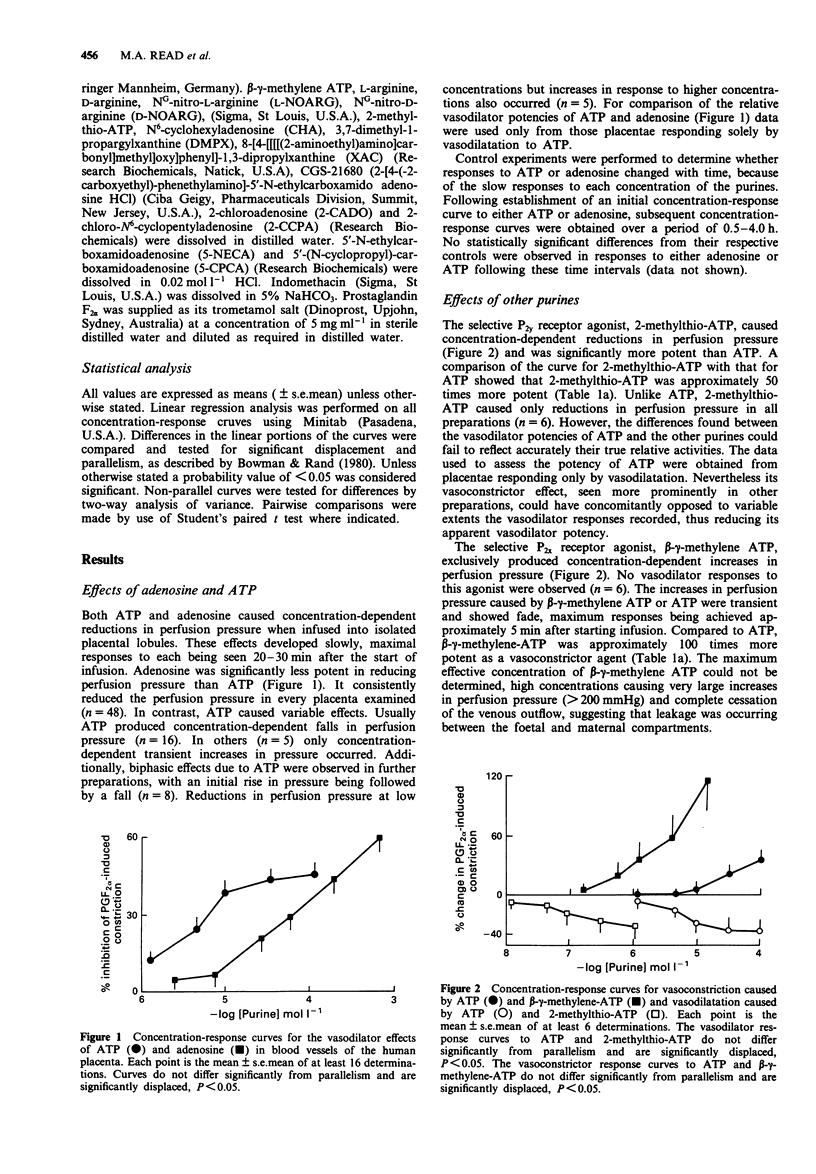
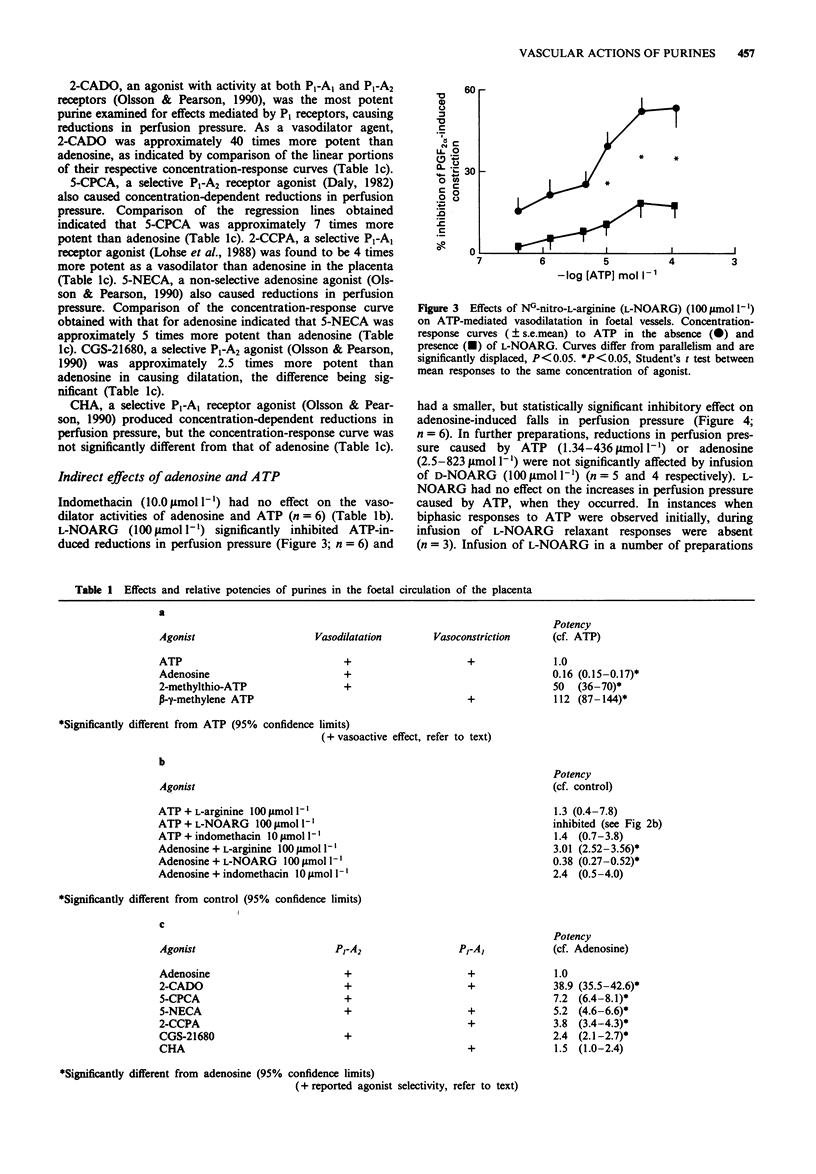
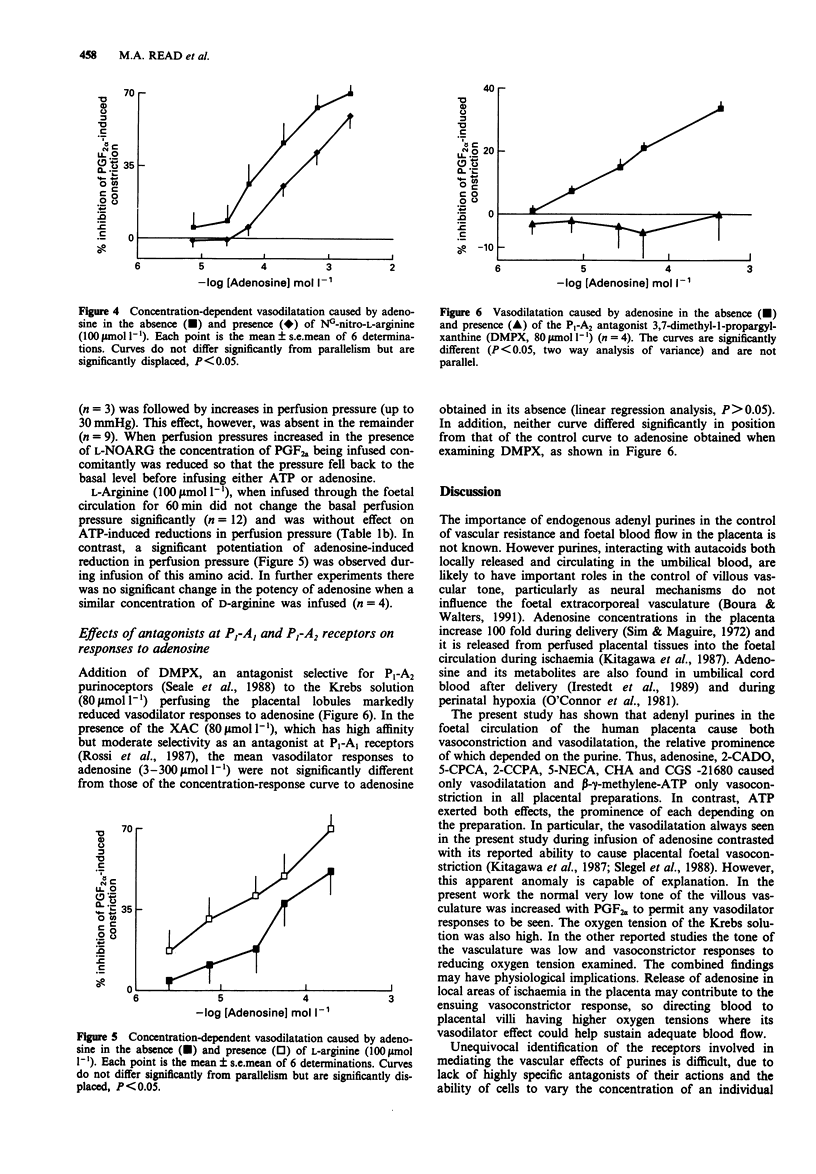
1. The vasoactive effects of adenosine triphosphate (ATP), adenosine and other purines in the foetal circulation of the human placenta were examined. Single lobules of the placenta were bilaterally perfused in vitro with Krebs buffer (maternal and foetal sides 5 ml min-1 each, 95% O2:5% CO2, 37 degrees C). Changes in foetal vascular tone were assessed by recording perfusion pressure during constant infusion of each purine. To allow recording of the vasodilator effects, submaximal vasoconstriction was induced by concomitant infusion of prostaglandin F2 alpha (0.7-2.0 mumol l-1). 2. ATP (1.0-100 mumol l-1) usually caused concentration-dependent reductions in perfusion pressure. However, biphasic with initial transient increases, or only increases in pressure were sometimes observed. Falls in pressure caused by ATP were significantly reduced by addition to the perfusate of NG-nitro-L-arginine (L-NOARG) (100 mumol l-1) but not NG-nitro-D-arginine (D-NOARG) (100 mumol l-1). They were not influenced by addition of indomethacin (10 mumol l-1) or L-arginine (100 mumol l-1). 3. Adenosine (0.01-1.0 mmol l-1) consistently caused concentration-dependent reductions in perfusion pressure, this effect not being influenced by indomethacin. L-NOARG, but not D-NOARG, reduced the potency of adenosine approximately three fold. L-Arginine, but not D-arginine enhanced its potency by a similar amount. 4. 2-Methylthio-ATP, a selective P2 gamma agonist was approximately 50 times more potent than ATP as a vasodilator agent, always causing decreases in perfusion pressure.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Boura A. L., Walters W. A. Autacoids and the control of vascular tone in the human umbilical-placental circulation. Placenta. 1991 Sep-Oct;12(5):453–477. doi: 10.1016/0143-4004(91)90023-9. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Cusack N. J., Pearson J. D. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988 Dec;95(4):1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L., Fredholm B. B. Characterization of adenosine receptors in isolated cerebral arteries of cat. Br J Pharmacol. 1983 Dec;80(4):631–637. doi: 10.1111/j.1476-5381.1983.tb10052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox I. H., Kurpis L. Binding characteristics of an adenosine receptor in human placenta. J Biol Chem. 1983 Jun 10;258(11):6952–6955. [PubMed] [Google Scholar]
- Gude N. M., Boura A. L., King R. G., Brennecke S. P., Jamal O. S., Smith R., Walters W. A. Evidence for inhibition by endothelium-derived relaxing factor of thromboxane A2 receptor-mediated vasoconstriction in the fetal vessels of the human perfused placenta. Placenta. 1992 Nov-Dec;13(6):597–605. doi: 10.1016/0143-4004(92)90022-l. [DOI] [PubMed] [Google Scholar]
- Gude N. M., King R. G., Brennecke S. P. Role of endothelium-derived nitric oxide in maintenance of low fetal vascular resistance in placenta. Lancet. 1990 Dec 22;336(8730):1589–1590. doi: 10.1016/0140-6736(90)93374-x. [DOI] [PubMed] [Google Scholar]
- Houston D. A., Burnstock G., Vanhoutte P. M. Different P2-purinergic receptor subtypes of endothelium and smooth muscle in canine blood vessels. J Pharmacol Exp Ther. 1987 May;241(2):501–506. [PubMed] [Google Scholar]
- Howard R. B., Hosokawa T., Maguire M. H. Hypoxia-induced fetoplacental vasoconstriction in perfused human placental cotyledons. Am J Obstet Gynecol. 1987 Nov;157(5):1261–1266. doi: 10.1016/s0002-9378(87)80307-1. [DOI] [PubMed] [Google Scholar]
- Hutchison A. J., Webb R. L., Oei H. H., Ghai G. R., Zimmerman M. B., Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J Pharmacol Exp Ther. 1989 Oct;251(1):47–55. [PubMed] [Google Scholar]
- Irestedt L., Dahlin I., Hertzberg T., Sollevi A., Lagercrantz H. Adenosine concentration in umbilical cord blood of newborn infants after vaginal delivery and cesarean section. Pediatr Res. 1989 Aug;26(2):106–108. doi: 10.1203/00006450-198908000-00007. [DOI] [PubMed] [Google Scholar]
- Jarvis M. F., Schulz R., Hutchison A. J., Do U. H., Sills M. A., Williams M. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther. 1989 Dec;251(3):888–893. [PubMed] [Google Scholar]
- Kenakin T. P., Pike N. B. An in vitro analysis of purine-mediated renal vasoconstriction in rat isolated kidney. Br J Pharmacol. 1987 Feb;90(2):373–381. doi: 10.1111/j.1476-5381.1987.tb08967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C., Burnstock G. Evidence for two types of P2-purinoceptor in longitudinal muscle of the rabbit portal vein. Eur J Pharmacol. 1985 Apr 23;111(1):49–56. doi: 10.1016/0014-2999(85)90112-8. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Delbro D., Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985 Jan 2;107(2):161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Kusachi S., Thompson R. D., Olsson R. A. Ligand selectivity of dog coronary adenosine receptor resembles that of adenylate cyclase stimulatory (Ra) receptors. J Pharmacol Exp Ther. 1983 Nov;227(2):316–321. [PubMed] [Google Scholar]
- Leung E., Johnston C. I., Woodcock E. A. An investigation of the receptors involved in the coronary vasodilatory effect of adenosine analogues. Clin Exp Pharmacol Physiol. 1985 Sep-Oct;12(5):515–519. doi: 10.1111/j.1440-1681.1985.tb00902.x. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Schwabe U., Cristalli G., Vittori S., Grifantini M. 2-Chloro-N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1988 Jun;337(6):687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- Mak K. K., Gude N. M., Walters W. A., Boura A. L. Effects of vasoactive autacoids on the human umbilical-fetal placental vasculature. Br J Obstet Gynaecol. 1984 Feb;91(2):99–106. doi: 10.1111/j.1471-0528.1984.tb05890.x. [DOI] [PubMed] [Google Scholar]
- Makujina S. R., Sabouni M. H., Bhatia S., Douglas F. L., Mustafa S. J. Vasodilatory effects of adenosine A2 receptor agonists CGS 21680 and CGS 22492 in human vasculature. Eur J Pharmacol. 1992 Oct 20;221(2-3):243–247. doi: 10.1016/0014-2999(92)90708-c. [DOI] [PubMed] [Google Scholar]
- Martin G. N., Thom S. A., Sever P. S. The effects of adenosine triphosphate (ATP) and related purines on human isolated subcutaneous and omental resistance arteries. Br J Pharmacol. 1991 Mar;102(3):645–650. doi: 10.1111/j.1476-5381.1991.tb12227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathie R. T., Ralevic V., Alexander B., Burnstock G. Nitric oxide is the mediator of ATP-induced dilatation of the rabbit hepatic arterial vascular bed. Br J Pharmacol. 1991 Jun;103(2):1602–1606. doi: 10.1111/j.1476-5381.1991.tb09834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieson J. J., Burnstock G. Purine-mediated relaxation and constriction of isolated rabbit mesenteric artery are not endothelium-dependent. Eur J Pharmacol. 1985 Dec 3;118(3):221–229. doi: 10.1016/0014-2999(85)90132-3. [DOI] [PubMed] [Google Scholar]
- Mustafa S. J., Askar A. O. Evidence suggesting an Ra-type adenosine receptor in bovine coronary arteries. J Pharmacol Exp Ther. 1985 Jan;232(1):49–56. [PubMed] [Google Scholar]
- Mülsch A., Busse R. NG-nitro-L-arginine (N5-[imino(nitroamino)methyl]-L-ornithine) impairs endothelium-dependent dilations by inhibiting cytosolic nitric oxide synthesis from L-arginine. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jan-Feb;341(1-2):143–147. doi: 10.1007/BF00195071. [DOI] [PubMed] [Google Scholar]
- Needham L., Cusack N. J., Pearson J. D., Gordon J. L. Characteristics of the P2 purinoceptor that mediates prostacyclin production by pig aortic endothelial cells. Eur J Pharmacol. 1987 Feb 10;134(2):199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- O'Connor M. C., Harkness R. A., Simmonds R. J., Hytten F. E. The measurement of hypoxanthine, xanthine, inosine and uridine in umbilical cord blood and fetal scalp blood samples as a measure of fetal hypoxia. Br J Obstet Gynaecol. 1981 Apr;88(4):381–390. doi: 10.1111/j.1471-0528.1981.tb01001.x. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Penfold P., Drury L., Simmonds R., Hytten F. E. Studies of a single placental cotyledon in vitro: I. The preparation and its viability. Placenta. 1981 Apr-Jun;2(2):149–154. doi: 10.1016/s0143-4004(81)80018-5. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Mathie R. T., Alexander B., Burnstock G. Characterization of P2X- and P2Y-purinoceptors in the rabbit hepatic arterial vasculature. Br J Pharmacol. 1991 May;103(1):1108–1113. doi: 10.1111/j.1476-5381.1991.tb12308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi N. F., Churchill P. C., Jacobson K. A., Leahy A. E. Further characterization of the renovascular effects of N6-cyclohexyladenosine in the isolated perfused rat kidney. J Pharmacol Exp Ther. 1987 Mar;240(3):911–915. [PMC free article] [PubMed] [Google Scholar]
- Seale T. W., Abla K. A., Shamim M. T., Carney J. M., Daly J. W. 3,7-Dimethyl-1-propargylxanthine: a potent and selective in vivo antagonist of adenosine analogs. Life Sci. 1988;43(21):1671–1684. doi: 10.1016/0024-3205(88)90478-x. [DOI] [PubMed] [Google Scholar]
- Sim M. K., Maguire M. H. Presence of adenosine in the human term placenta. Determination of adenosine content and pathways of adenosine metabolism. Circ Res. 1972 Nov;31(5):779–788. doi: 10.1161/01.res.31.5.779. [DOI] [PubMed] [Google Scholar]
- Slegel P., Kitagawa H., Maguire M. H. Determination of adenosine in fetal perfusates of human placental cotyledons using fluorescence derivatization and reversed-phase high-performance liquid chromatography. Anal Biochem. 1988 May 15;171(1):124–134. doi: 10.1016/0003-2697(88)90132-7. [DOI] [PubMed] [Google Scholar]


