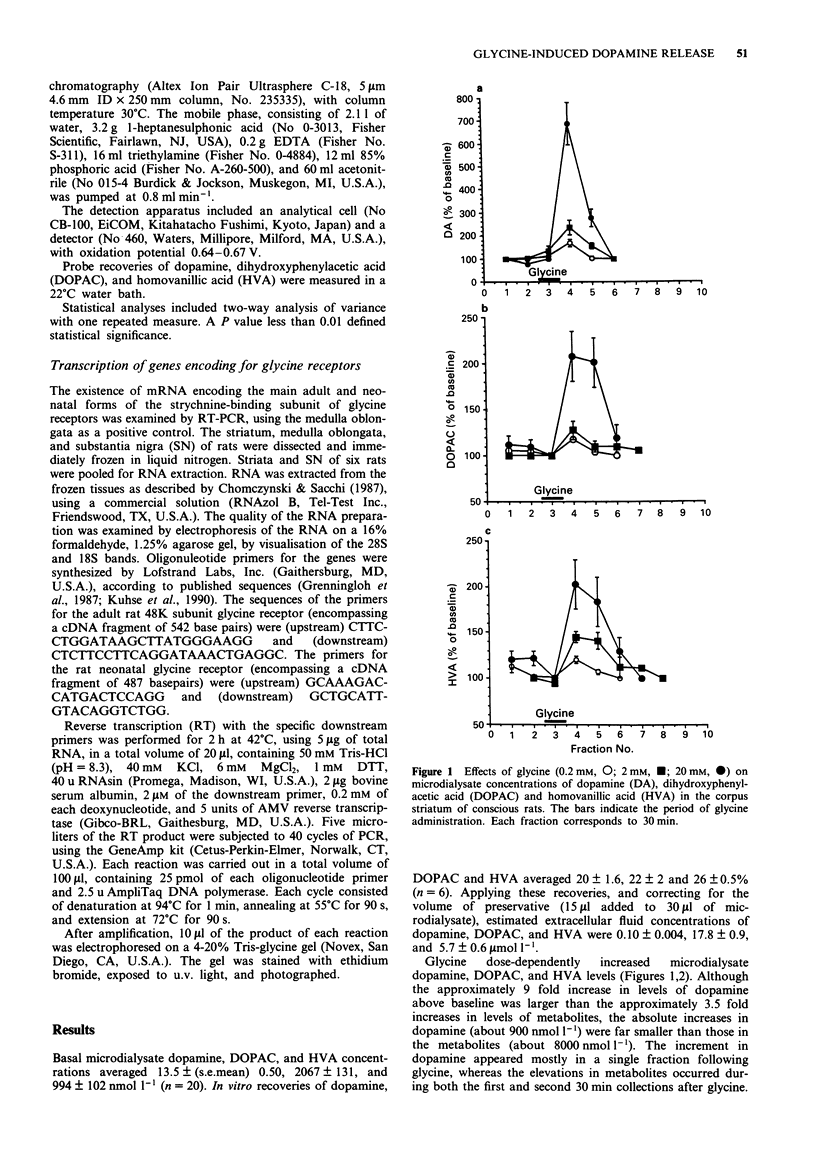
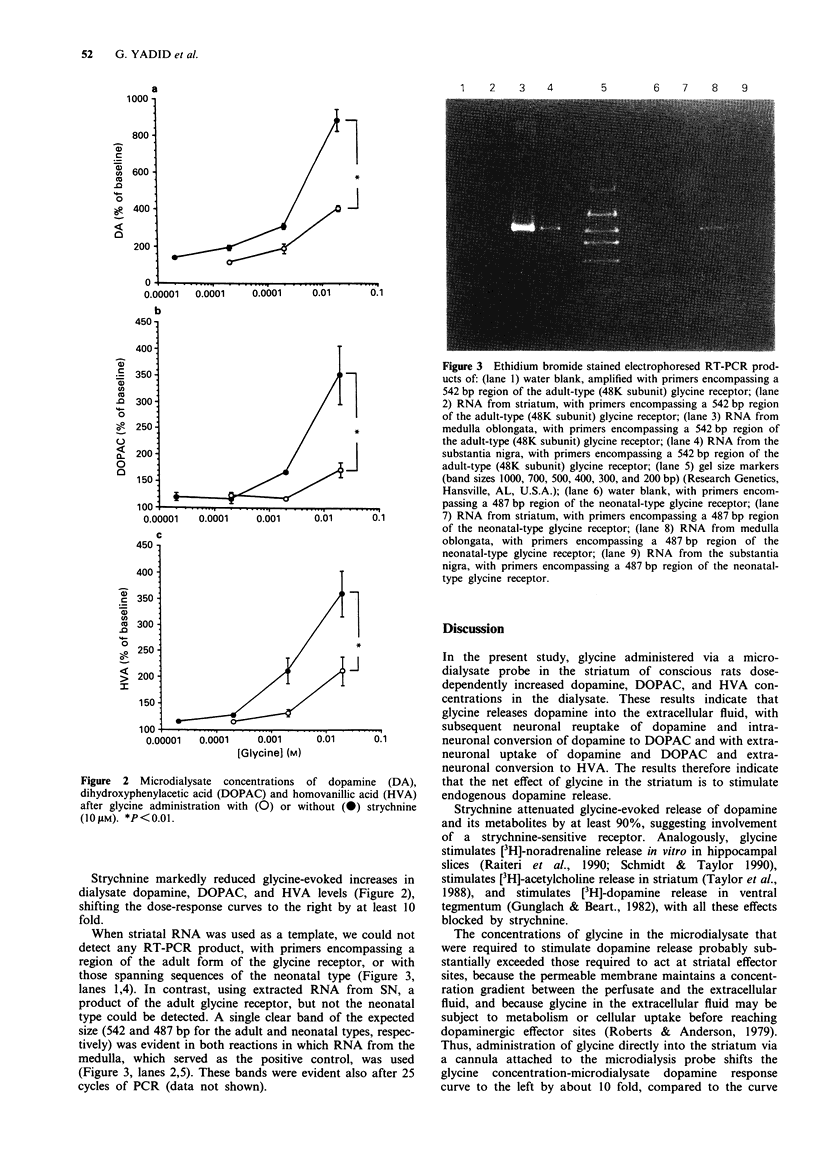
Abstract
1. Glycine is an inhibitory neurotransmitter in the spinal cord and brainstem. The mechanism of this inhibition is via binding of glycine to specific receptors, increasing transmembrane Cl- conductance and hyperpolarizing neurones. Strychnine selectively antagonizes these effects. The role of glycinergic neurones in supraspinal regions is poorly understood. 2. Effects of glycine on release of catecholamines in the striatum were examined by microdialysis in freely-moving rats. Transcription of the genes encoding strychnine-sensitive glycine receptors was assessed in the striatum and substantia nigra, by use of reverse transcription followed by the polymerase chain reaction. 3. Glycine administered via the microdialysis probe dose-dependently increased concentrations of dopamine and its metabolites, dihydroxyphenylacetic acid and homovanillic acid, in the perfusate, indicating increased local release and metabolism of dopamine. Strychnine markedly attenuated these responses. Whereas striatal tissue did not contain mRNA for either the adult or neonatal form of strychnine-sensitive glycine receptor, nigral tissue contained a message for the adult form. 4. The results suggest that dopaminergic cells in the substantia nigra synthesize strychnine-sensitive glycine receptors and transport the receptors to terminals in the striatum. Occupation of the glycine receptors then exerts a net stimulatory effect on striatal dopamine release in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp Brain Res. 1968;6(1):1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- Giorguieff M. F., Kemel M. L., Glowinski J., Besson M. J. Stimulation of dopamine release by GABA in rat striatal slices. Brain Res. 1978 Jan 6;139(1):115–130. doi: 10.1016/0006-8993(78)90064-1. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Gundlach A. L., Beart P. M. Neurochemical studies of the mesolimbic dopaminergic pathway: glycinergic mechanisms and glycinergic-dopaminergic interactions in the rat ventral tegmentum. J Neurochem. 1982 Feb;38(2):574–581. doi: 10.1111/j.1471-4159.1982.tb08665.x. [DOI] [PubMed] [Google Scholar]
- Kerwin R., Pycock C. Effects of omega-amino acids on tritiated dopamine release from rat striatum: evidence for a possible glycinergic mechanism. Biochem Pharmacol. 1979 Jul 15;28(14):2193–2197. doi: 10.1016/0006-2952(79)90203-x. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. A single amino acid exchange alters the pharmacology of neonatal rat glycine receptor subunit. Neuron. 1990 Dec;5(6):867–873. doi: 10.1016/0896-6273(90)90346-h. [DOI] [PubMed] [Google Scholar]
- Llinás R., Greenfield S. A., Jahnsen H. Electrophysiology of pars compacta cells in the in vitro substantia nigra--a possible mechanism for dendritic release. Brain Res. 1984 Feb 27;294(1):127–132. doi: 10.1016/0006-8993(84)91316-7. [DOI] [PubMed] [Google Scholar]
- Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991 Sep;10(9):2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri N. B., Calabresi P., Bernardi G. Effects of glycine on neurons in the rat substantia nigra zona compacta: in vitro electrophysiological study. Synapse. 1990;5(3):190–200. doi: 10.1002/syn.890050304. [DOI] [PubMed] [Google Scholar]
- Pacak K., Armando I., Fukuhara K., Kvetnansky R., Palkovits M., Kopin I. J., Goldstein D. S. Noradrenergic activation in the paraventricular nucleus during acute and chronic immobilization stress in rats: an in vivo microdialysis study. Brain Res. 1992 Aug 28;589(1):91–96. doi: 10.1016/0006-8993(92)91165-b. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Fontana G., Fedele E. Glycine stimulates [3H]noradrenaline release by activating a strychnine-sensitive receptor present in rat hippocampus. Eur J Pharmacol. 1990 Aug 10;184(2-3):239–250. doi: 10.1016/0014-2999(90)90615-d. [DOI] [PubMed] [Google Scholar]
- Roberts P. J., Anderson S. D. Stimulatory effect of L-glutamate and related amino acids on [3H]dopamine release from rat striatum: an in vitro model for glutamate actions. J Neurochem. 1979 May;32(5):1539–1545. doi: 10.1111/j.1471-4159.1979.tb11096.x. [DOI] [PubMed] [Google Scholar]
- Schmidt C. J., Taylor V. L. Strychnine-sensitive, glycine-induced release of [3H]norepinephrine from rat hippocampal slices. J Neurochem. 1990 Jun;54(6):2077–2081. doi: 10.1111/j.1471-4159.1990.tb04913.x. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Depolarizing responses to glycine, beta-alanine and muscimol in isolated optic nerve and cuneate nucleus. Br J Pharmacol. 1983 Jul;79(3):799–806. doi: 10.1111/j.1476-5381.1983.tb10018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. A., Tsai C., Lehmann J. Glycine-evoked release of [3H]acetylcholine from rat striatal slices is independent of the NMDA receptor. Naunyn Schmiedebergs Arch Pharmacol. 1988 May;337(5):552–555. doi: 10.1007/BF00182730. [DOI] [PubMed] [Google Scholar]
- Yadid G., Youdim M. B., Zinder O. High-affinity strychnine binding to adrenal medulla chromaffin cell membranes. Eur J Pharmacol. 1990 Jan 17;175(3):365–366. doi: 10.1016/0014-2999(90)90579-u. [DOI] [PubMed] [Google Scholar]
- Yadid G., Youdim M. B., Zinder O. Preferential release of epinephrine by glycine from adrenal chromaffin cells. Eur J Pharmacol. 1992 Oct 20;221(2-3):389–391. doi: 10.1016/0014-2999(92)90729-n. [DOI] [PubMed] [Google Scholar]
- Yadid G., Zinder O., Youdim M. B. Effects of the glycine prodrug milacemide (2-N-pentylaminoacetamide) on catecholamine secretion from isolated adrenal medulla chromaffin cells. Br J Pharmacol. 1991 Nov;104(3):760–764. doi: 10.1111/j.1476-5381.1991.tb12501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. Strychnine binding associated with glycine receptors of the central nervous system. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]