Abstract
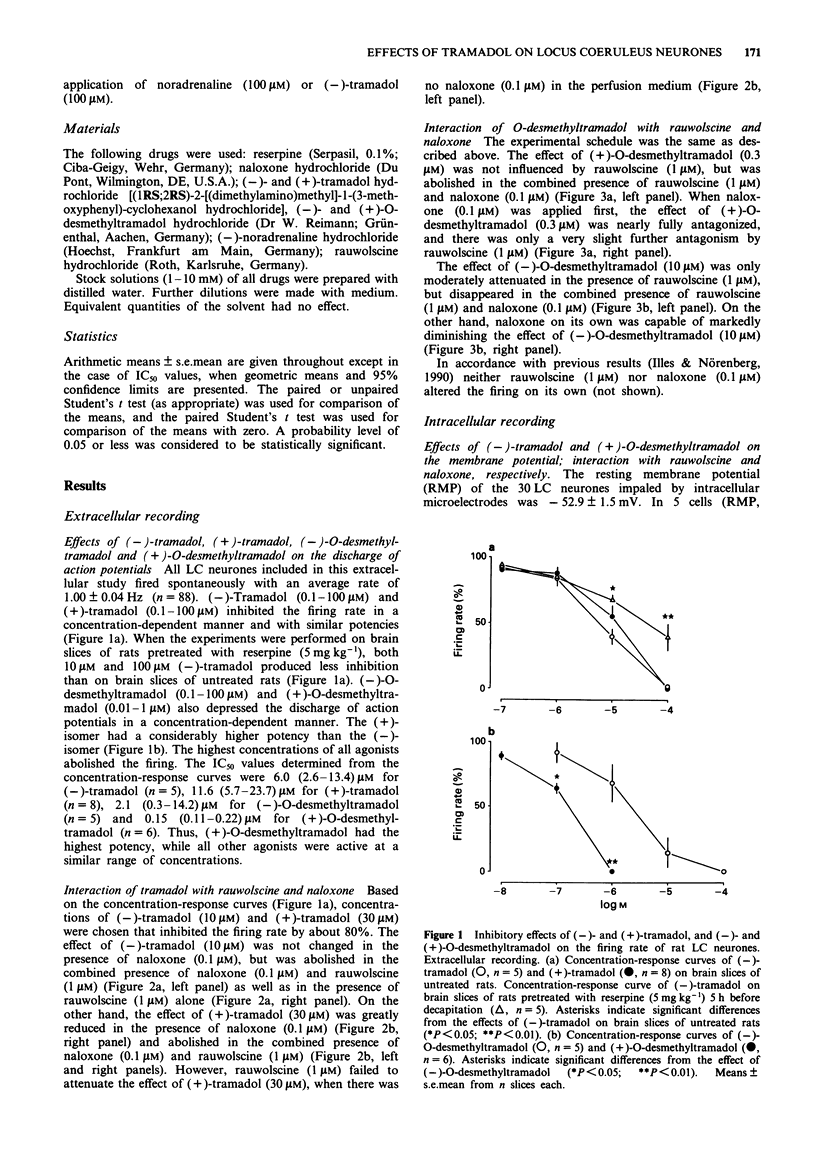
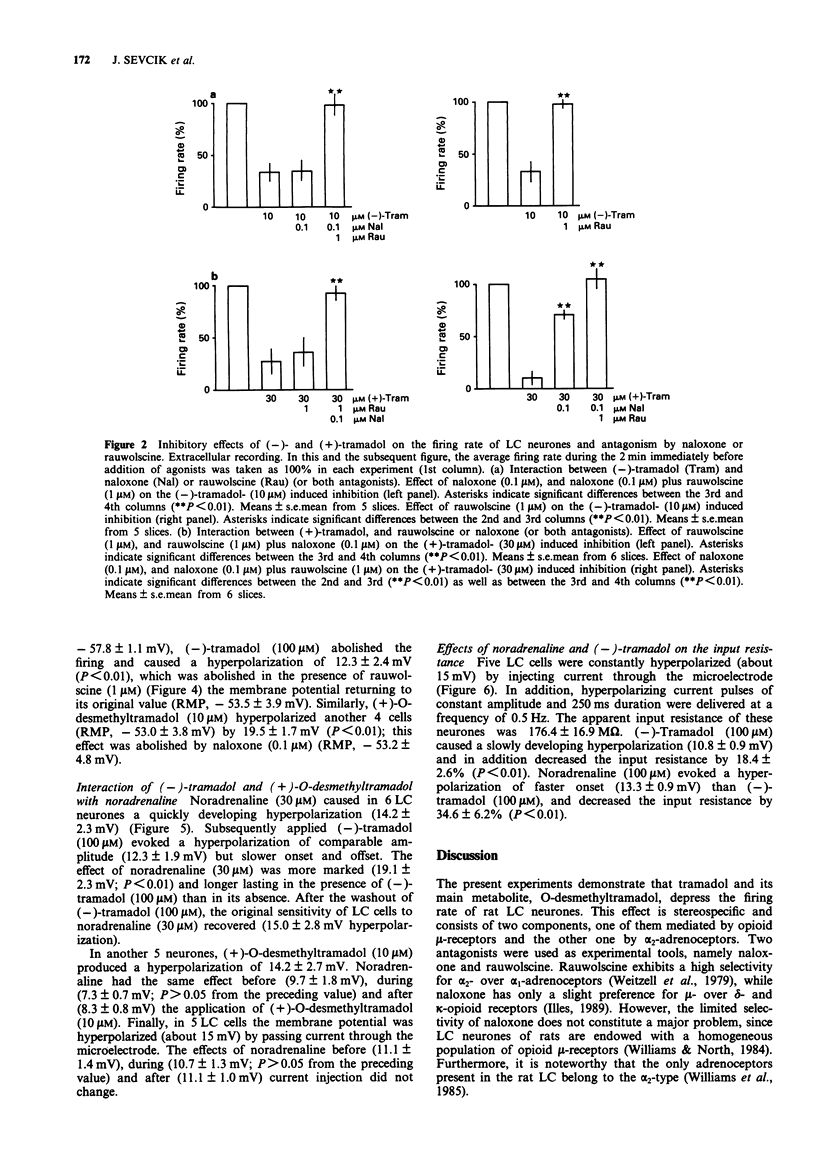
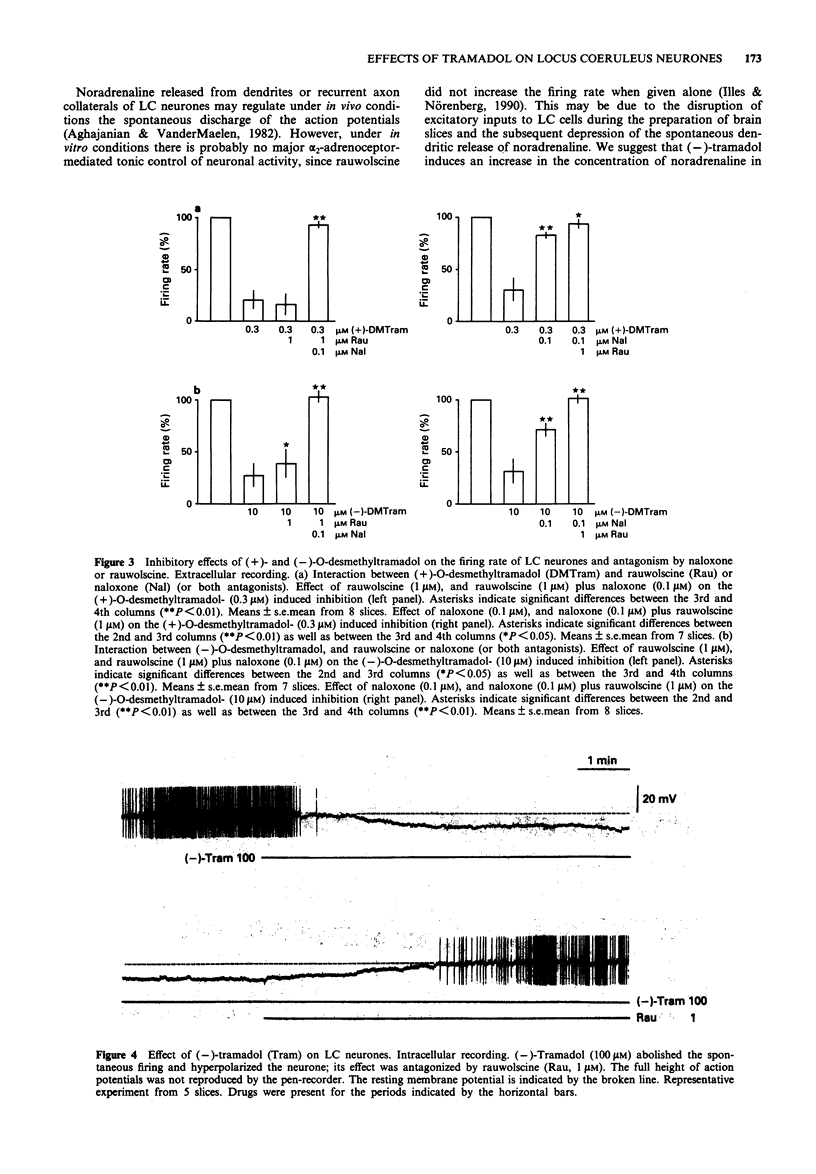
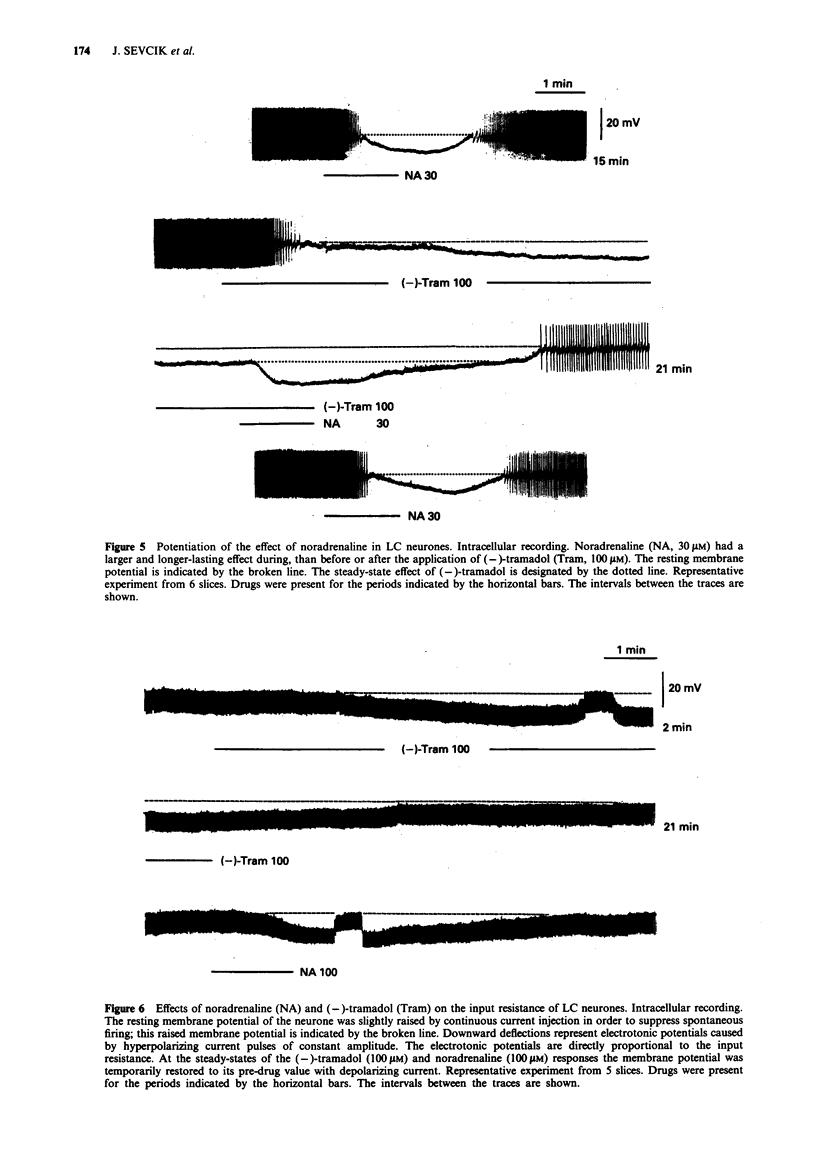
1. Tramadol is a centrally acting analgesic with low opioid receptor affinity and, therefore, presumably additional mechanisms of analgesic action. Tramadol and its main metabolite O-desmethyltramadol were tested on rat central noradrenergic neurones of the nucleus locus coeruleus (LC), which are involved in the modulation of nociceptive afferent stimuli. 2. In pontine slices of the rat brain the spontaneous discharge of action potentials of LC cells was recorded extracellularly. (-)-Tramadol (0.1-100 microM), (+)-tramadol (0.1-100 microM), (-)-O-desmethyl-tramadol (0.1-100 microM) and (+)-O-desmethyltramadol (0.01-1 microM) inhibited the firing rate in a concentration-dependent manner. (+)-O-desmethyltramadol had the highest potency, while all other agonists were active at a similar range of concentrations. 3. (-)-Tramadol (10, 100 microM) was less inhibitory in brain slices of rats pretreated with reserpine (5 mg kg-1, 5 h before decapitation) than in controls. 4. The effect of (-)-tramadol (10 microM) was abolished in the presence of the alpha 2-adrenoceptor antagonist, rauwolscine (1 microM), whilst that of (+)-O-desmethyltramadol (0.3 microM) virtually disappeared in the presence of the opioid antagonist, naloxone (0.1 microM). (+)-Tramadol (30 microM) and (-)-O-desmethyl-tramadol (10 microM) became inactive only in the combined presence of naloxone (0.1 microM) and rauwolscine (1 microM). 5. In another series of experiments, the membrane potential of LC neurones was determined with intracellular microelectrodes. (-)-Tramadol (100 microM) inhibited the spontaneous firing and hyper-polarized the cells; this effect was abolished by rauwolscine (1 microM).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., VanderMaelen C. P. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982 Mar 12;215(4538):1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Besson J. M., Chaouch A. Descending serotoninergic systems. Pain Headache. 1987;9:64–100. [PubMed] [Google Scholar]
- Bird S. J., Kuhar M. J. Iontophoretic application of opiates to the locus coeruleus. Brain Res. 1977 Feb 25;122(3):523–533. doi: 10.1016/0006-8993(77)90462-0. [DOI] [PubMed] [Google Scholar]
- Carlsson K. H., Jurna I. Effects of tramadol on motor and sensory responses of the spinal nociceptive system in the rat. Eur J Pharmacol. 1987 Jul 2;139(1):1–10. doi: 10.1016/0014-2999(87)90491-2. [DOI] [PubMed] [Google Scholar]
- Cherubini E., North R. A., Williams J. T. Synaptic potentials in rat locus coeruleus neurones. J Physiol. 1988 Dec;406:431–442. doi: 10.1113/jphysiol.1988.sp017389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen B., Reimann W., Giertz H. Effects of the central analgesic tramadol on the uptake and release of noradrenaline and dopamine in vitro. Br J Pharmacol. 1993 Mar;108(3):806–811. doi: 10.1111/j.1476-5381.1993.tb12882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen B., Reimann W. Interaction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitro. Br J Pharmacol. 1992 Jan;105(1):147–151. doi: 10.1111/j.1476-5381.1992.tb14226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. M., Henderson G., North R. A., Williams J. T. Noradrenaline-mediated synaptic inhibition in rat locus coeruleus neurones. J Physiol. 1983 Dec;345:477–488. doi: 10.1113/jphysiol.1983.sp014990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flohé L., Arend I., Cogal A., Richter W., Simon W. Klinische Prüfung der Abhängigkeitsentwicklung nach Langzeitapplikation von Tramadol. Arzneimittelforschung. 1978;28(1A):213–217. [PubMed] [Google Scholar]
- Foote S. L., Bloom F. E., Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983 Jul;63(3):844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Friderichs E., Felgenhauer F., Jongschaap P., Osterloh G. Pharmakologische Untersuchungen zur Analgesie, Abhängigkeits- und Toleranzentwicklung von Tramadol, einem stark wirkenden Analgetikum. Arzneimittelforschung. 1978;28(1A):122–134. [PubMed] [Google Scholar]
- Henderson G., Pepper C. M., Shefner S. A. Electrophysiological properties of neurons contained in the locus coeruleus and mesencephalic nucleus of the trigeminal nerve in vitro. Exp Brain Res. 1982;45(1-2):29–37. doi: 10.1007/BF00235760. [DOI] [PubMed] [Google Scholar]
- Hennies H. H., Friderichs E., Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung. 1988 Jul;38(7):877–880. [PubMed] [Google Scholar]
- Illes P. Modulation of transmitter and hormone release by multiple neuronal opioid receptors. Rev Physiol Biochem Pharmacol. 1989;112:139–233. doi: 10.1007/BFb0027497. [DOI] [PubMed] [Google Scholar]
- Illes P., Nörenberg W. Blockade of alpha 2-adrenoceptors increases opioid mu-receptor-mediated inhibition of the firing rate of rat locus coeruleus neurones. Naunyn Schmiedebergs Arch Pharmacol. 1990 Nov;342(5):490–496. doi: 10.1007/BF00169034. [DOI] [PubMed] [Google Scholar]
- Illes P., Weber H. D., Neuburger J., Bucher B., Regenold J. T., Nörenberg W. Receptor interactions at noradrenergic neurones. Ann N Y Acad Sci. 1990;604:197–210. doi: 10.1111/j.1749-6632.1990.tb31994.x. [DOI] [PubMed] [Google Scholar]
- Jones S. L. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- Jones S. L., Gebhart G. F. Inhibition of spinal nociceptive transmission from the midbrain, pons and medulla in the rat: activation of descending inhibition by morphine, glutamate and electrical stimulation. Brain Res. 1988 Sep 20;460(2):281–296. doi: 10.1016/0006-8993(88)90373-3. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y., Satoh M., Takagi H. The descending noradrenergic system and analgesia. Pain Headache. 1987;9:101–128. [PubMed] [Google Scholar]
- Lintz W., Erlaçin S., Frankus E., Uragg H. Metabolismus von Tramadol bei Mensch und Tier. Arzneimittelforschung. 1981;31(11):1932–1943. [PubMed] [Google Scholar]
- North R. A., Williams J. T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985 Jul;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper C. M., Henderson G. Opiates and opioid peptides hyperpolarize locus coeruleus neurons in vitro. Science. 1980 Jul 18;209(4454):394–395. doi: 10.1126/science.7384811. [DOI] [PubMed] [Google Scholar]
- Raffa R. B., Friderichs E., Reimann W., Shank R. P., Codd E. E., Vaught J. L. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an 'atypical' opioid analgesic. J Pharmacol Exp Ther. 1992 Jan;260(1):275–285. [PubMed] [Google Scholar]
- Reddy S. V., Maderdrut J. L., Yaksh T. L. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980 Jun;213(3):525–533. [PubMed] [Google Scholar]
- Regenold J. T., Illes P. Inhibitory adenosine A1-receptors on rat locus coeruleus neurones. An intracellular electrophysiological study. Naunyn Schmiedebergs Arch Pharmacol. 1990 Mar;341(3):225–231. doi: 10.1007/BF00169735. [DOI] [PubMed] [Google Scholar]
- Segal M., Sandberg D. Analgesia produced by electrical stimulation of catecholamine nuclei in the rat brain. Brain Res. 1977 Mar 11;123(2):369–372. doi: 10.1016/0006-8993(77)90488-7. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Williams J. T. Inhibitory synaptic potentials recorded from mammalian neurones prolonged by blockade of noradrenaline uptake. J Physiol. 1987 Jan;382:87–103. doi: 10.1113/jphysiol.1987.sp016357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W., Burchardi H., Sihler K., Valic L. Uber die Wirkung von Tramadol auf Atmung und Kreislauf. Arzneimittelforschung. 1978;28(1A):183–186. [PubMed] [Google Scholar]
- Weitzell R., Tanaka T., Starke K. Pre- and postsynaptic effects of yohimbine stereoisomers on noradrenergic transmission in the pulmonary artery of the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1979 Aug;308(2):127–136. doi: 10.1007/BF00499054. [DOI] [PubMed] [Google Scholar]
- Williams J. T., Henderson G., North R. A. Characterization of alpha 2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. Neuroscience. 1985 Jan;14(1):95–101. doi: 10.1016/0306-4522(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Williams J. T., North R. A. Opiate-receptor interactions on single locus coeruleus neurones. Mol Pharmacol. 1984 Nov;26(3):489–497. [PubMed] [Google Scholar]
- Williams J. T., North R. A., Shefner S. A., Nishi S., Egan T. M. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984 Sep;13(1):137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]


