Abstract
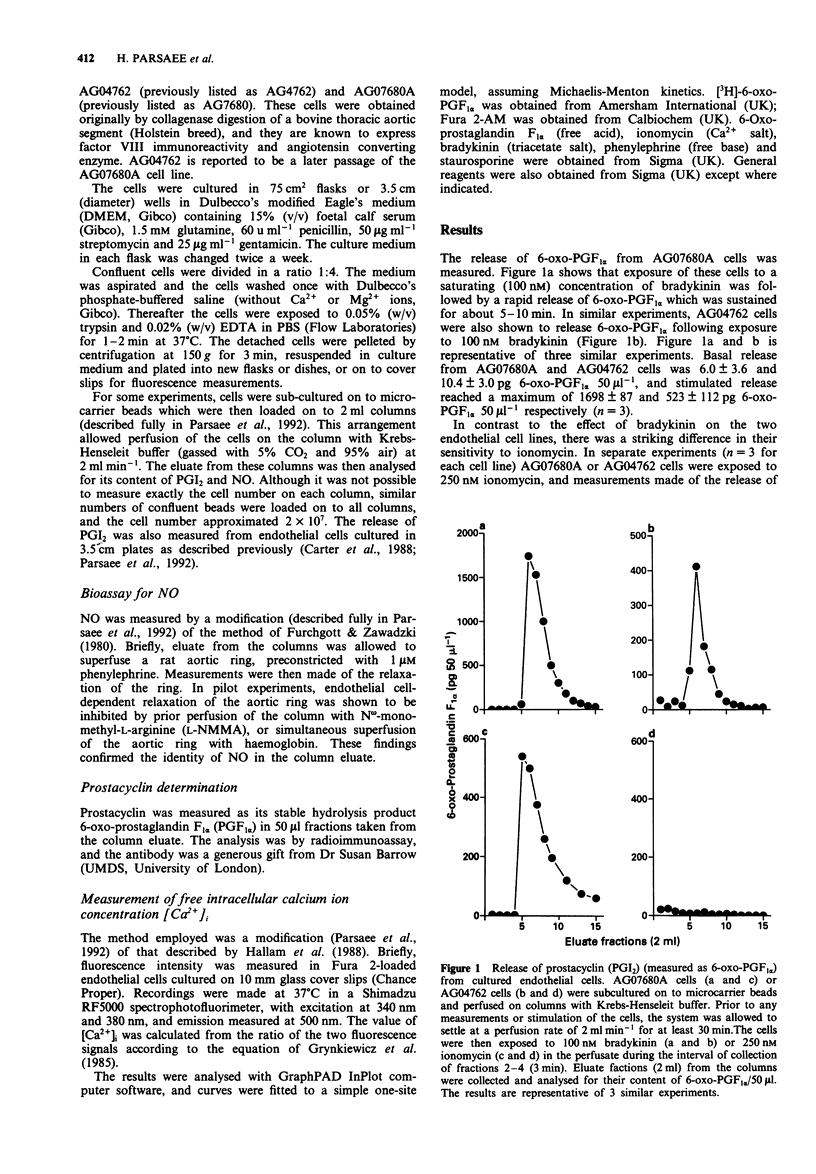
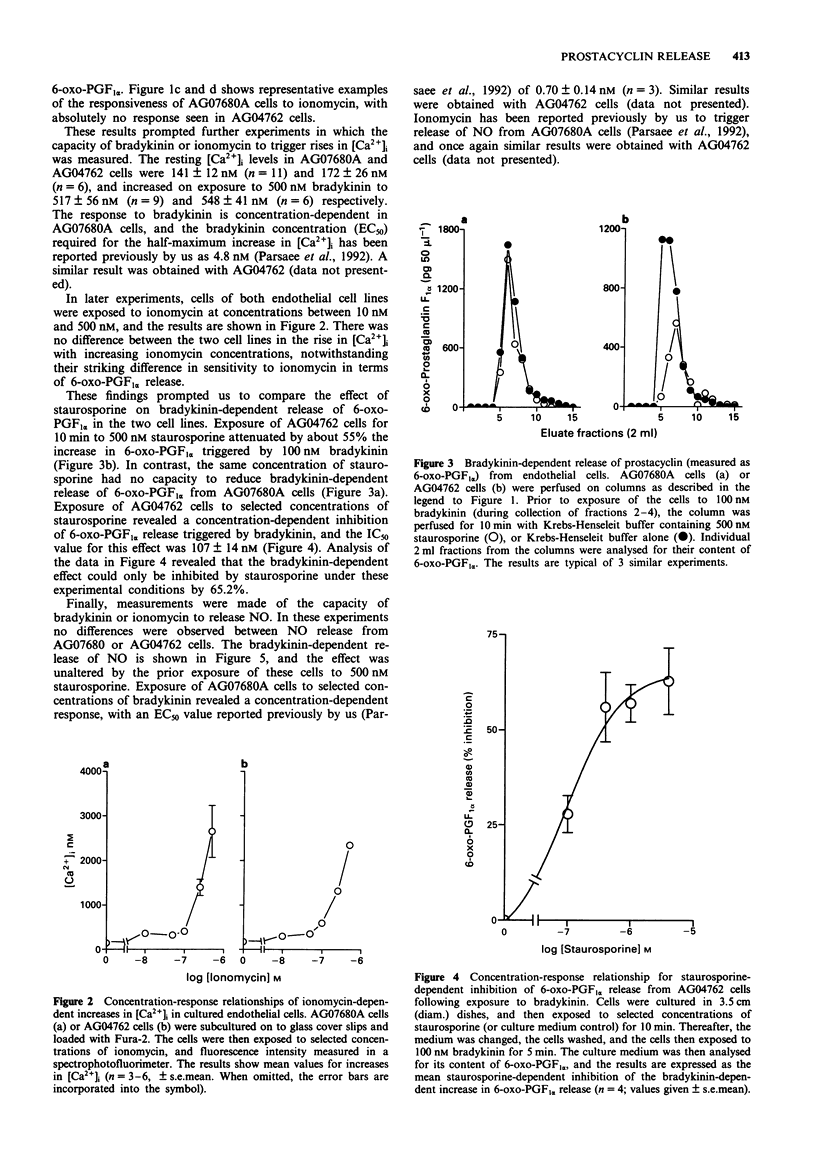
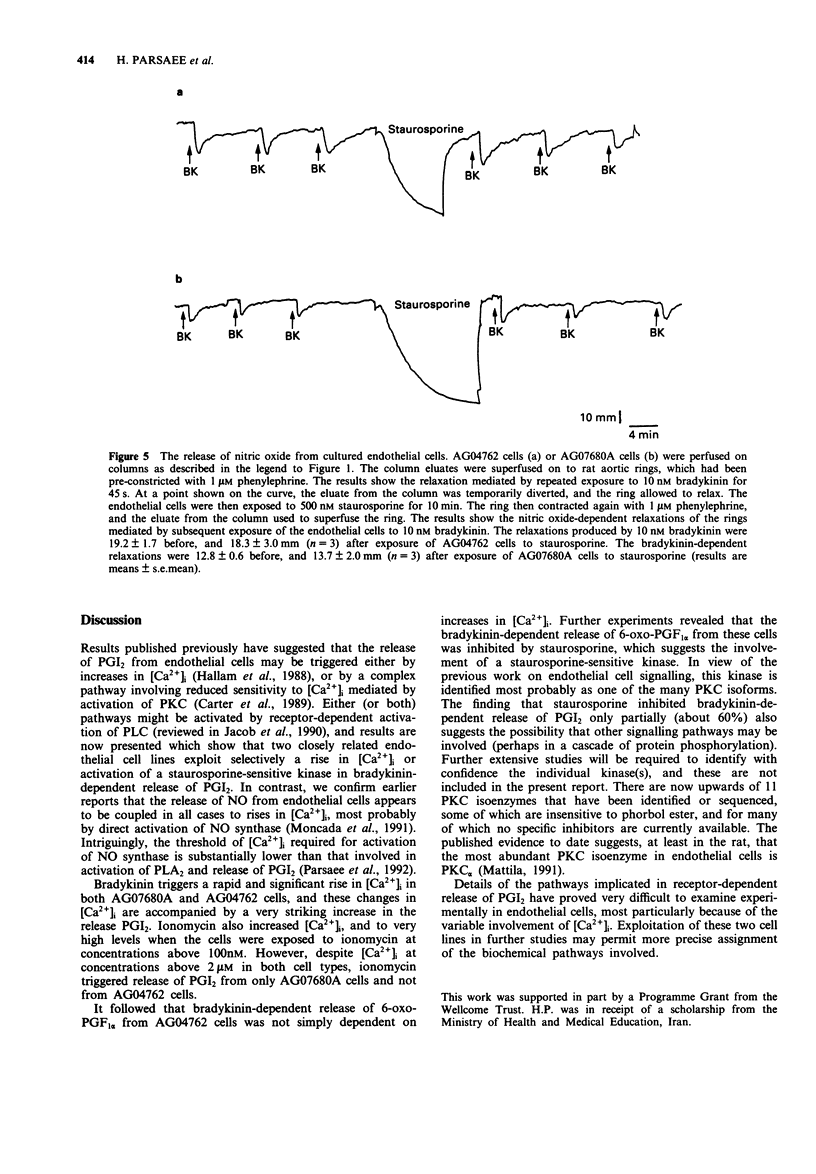
1. Bradykinin (100 nM) triggers release of nitric oxide and prostacyclin from both AG07680A and AG04762 bovine cultured aortic endothelial cells. The exposure of these cells to bradykinin is in each case associated with a striking rise in intracellular calcium ion concentration. 2. Exposure of AG07680A cells to 250 nM ionomycin was followed also by a significant release of prostacyclin, whereas 250 nM ionomycin had no capacity to stimulate release of prostacyclin from AG04762 cells. 3. There was a similar concentration-dependent increase in intracellular calcium ion concentration on exposure of AG07680A and AG04762 cells to ionomycin. 4. Exposure of AG04762 cells for 10 min to staurosporine produced a concentration-dependent inhibition (IC50 = 107 +/- 14 nM) in bradykinin-stimulated prostacyclin release. There was no similar inhibitory effect of staurosporine in AG07680A cells. 5. Bradykinin (10 nM) triggered release of nitric oxide from both AG07680A and AG04762 cells, and the effect was not inhibited by 500 nM staurosporine. There was a similar ionomycin-dependent release of nitric oxide from both cell types. 6. These results identify a common pathway for bradykinin-dependent nitric oxide release from both AG07680A and AG04762 cells, involving increases in intracellular calcium ion concentration. In contrast, the bradykinin-dependent release of prostacyclin may involve one of two pathways (involving an increase in intracellular calcium or activation of a staurosporine-sensitive kinase), and the two pathways are selectively exploited in AG07680A and AG04762 cells, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Cusack N. J., Pearson J. D. Regulation of P2y-purinoceptor-mediated prostacyclin release from human endothelial cells by cytoplasmic calcium concentration. Br J Pharmacol. 1988 Dec;95(4):1181–1190. doi: 10.1111/j.1476-5381.1988.tb11754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Hallam T. J., Pearson J. D. Protein kinase C activation alters the sensitivity of agonist-stimulated endothelial-cell prostacyclin production to intracellular Ca2+. Biochem J. 1989 Sep 1;262(2):431–437. doi: 10.1042/bj2620431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D., Needham L. A. Thrombin-stimulated elevation of human endothelial-cell cytoplasmic free calcium concentration causes prostacyclin production. Biochem J. 1988 Apr 1;251(1):243–249. doi: 10.1042/bj2510243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Mattila P. Protein kinase C subtypes in endothelial cells. FEBS Lett. 1991 Sep 2;289(1):86–90. doi: 10.1016/0014-5793(91)80914-o. [DOI] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Parsaee H., McEwan J. R., Joseph S., MacDermot J. Differential sensitivities of the prostacyclin and nitric oxide biosynthetic pathways to cytosolic calcium in bovine aortic endothelial cells. Br J Pharmacol. 1992 Dec;107(4):1013–1019. doi: 10.1111/j.1476-5381.1992.tb13400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]


