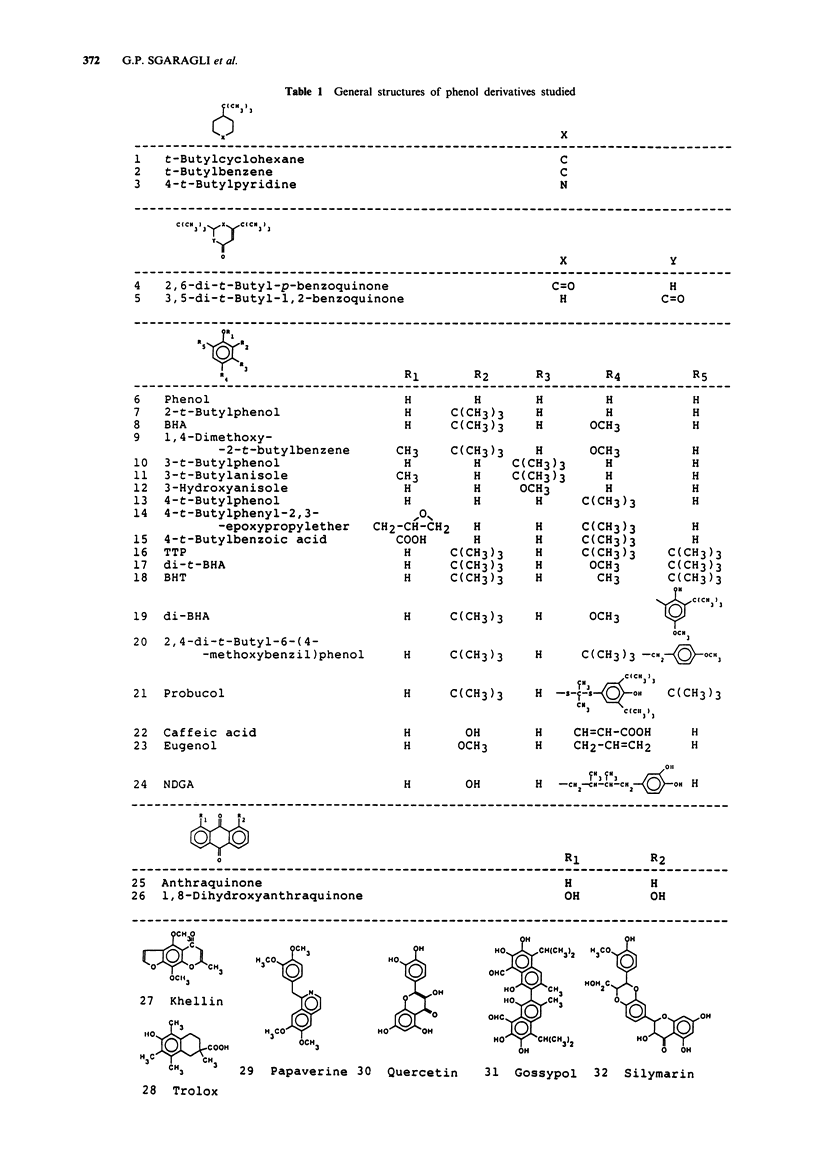
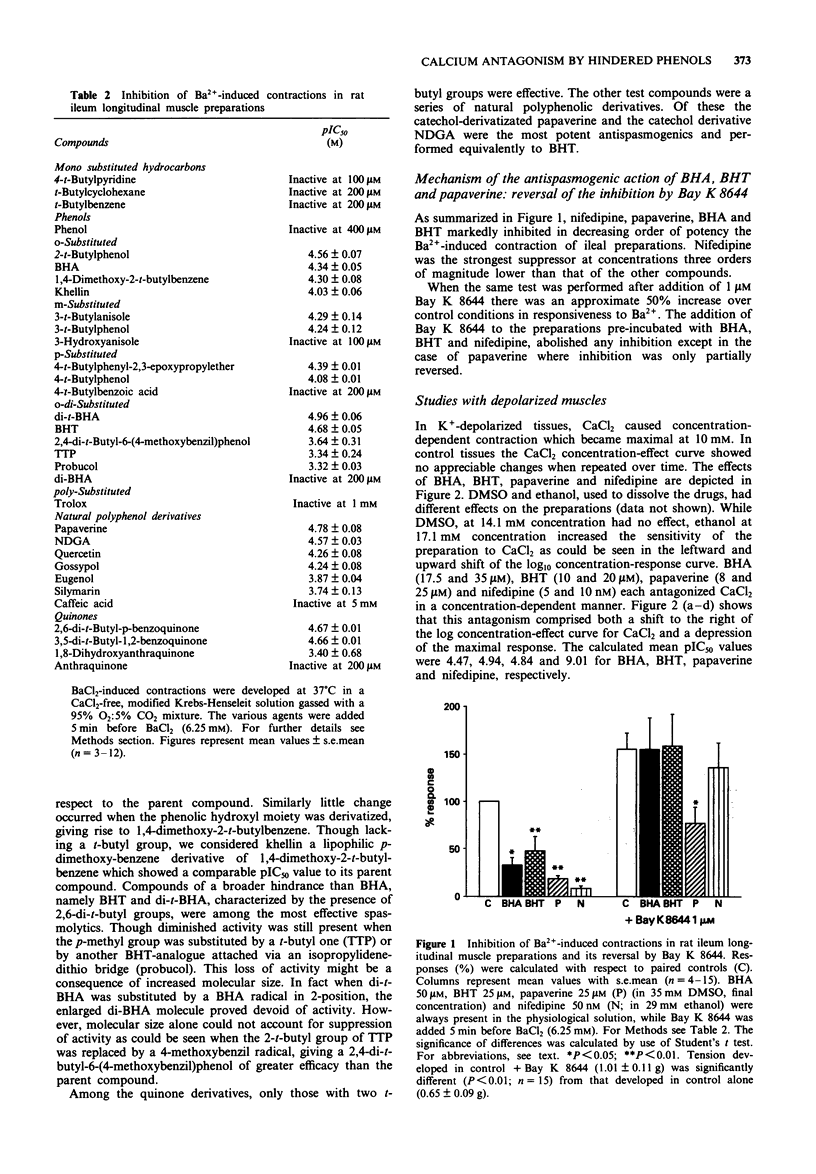
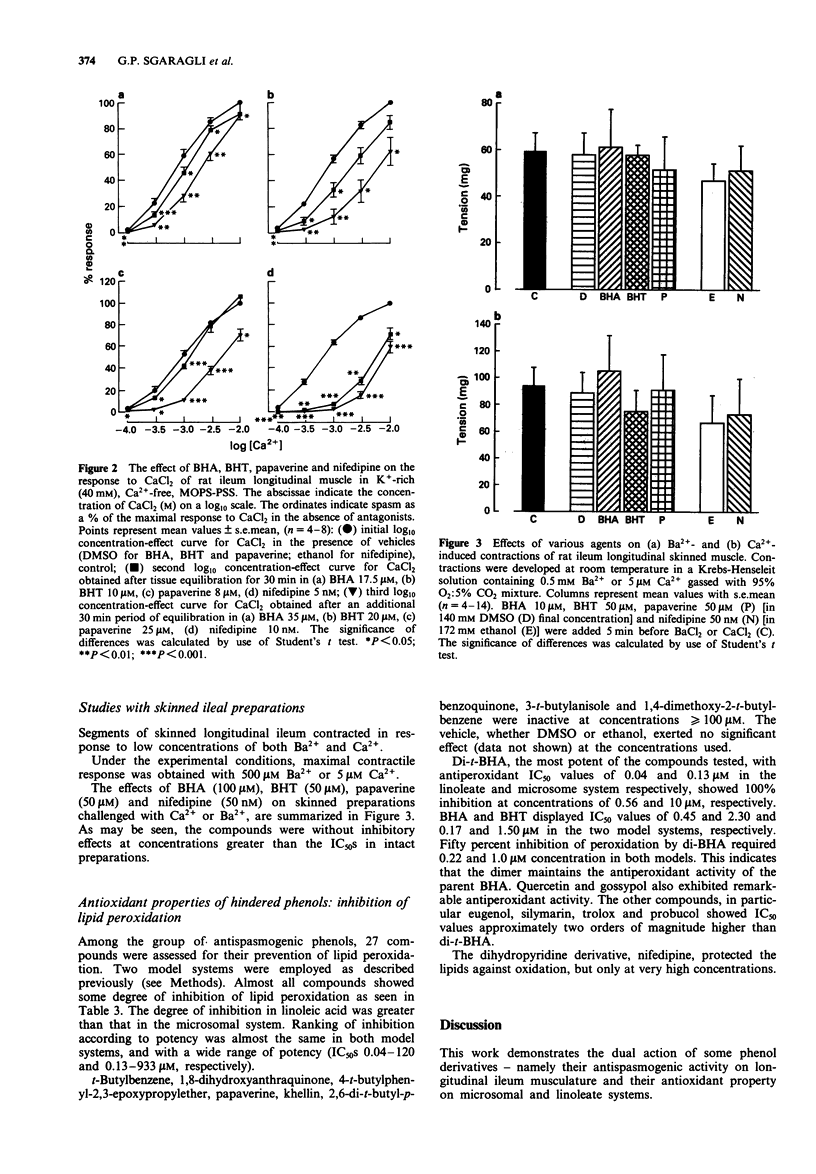
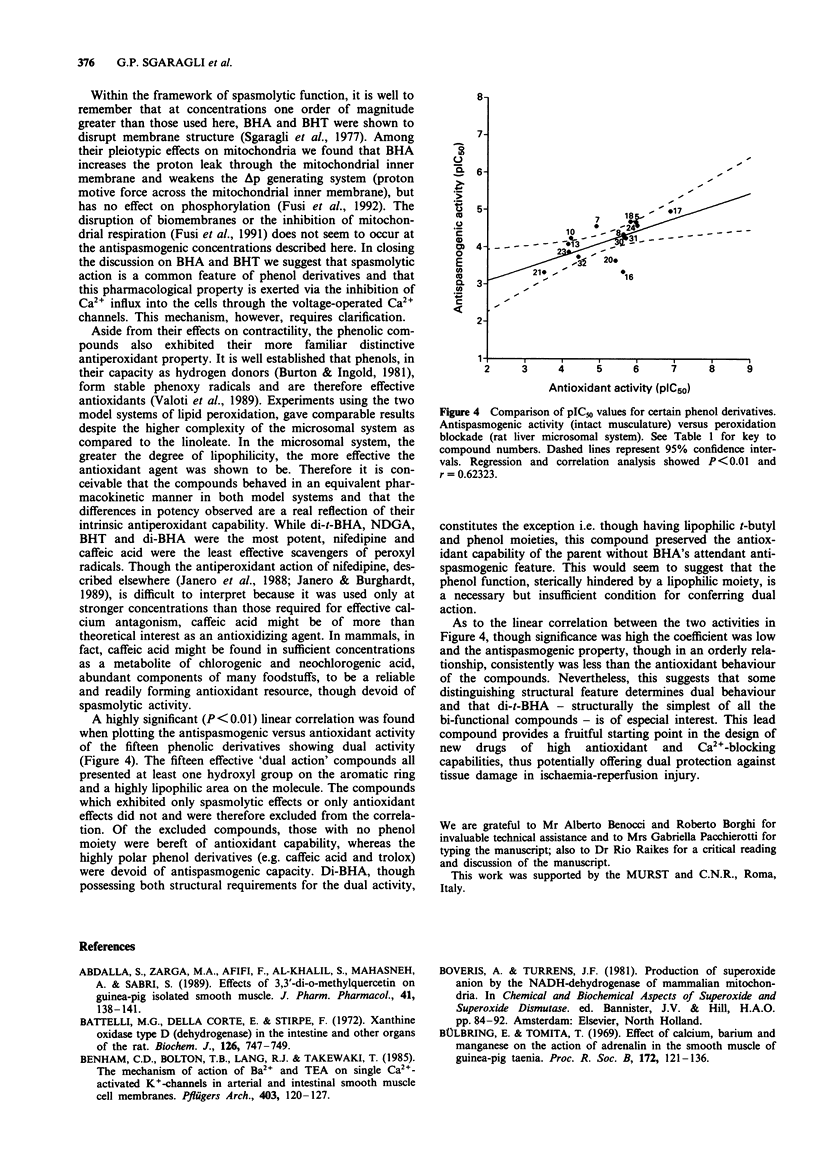
Abstract
1. The calcium antagonist and antioxidant activities of certain synthetic and natural phenols, related to BHA (2-t-butyl-4-methoxyphenol), were evaluated in rat ileal longitudinal muscle and in lipid peroxidation models respectively. 2. Compounds with a phenol or a phenol derivative moiety, with the exception of 2,2'-dihydroxy-3,-3'-di-t-butyl-5,5'-dimethoxydiphenyl (di-BHA), inhibited in a concentration-dependent manner the BaCl2-induced contraction of muscle incubated in a Ca(2+)-free medium. Calculated pIC50 (M) values ranged between 3.32 (probucol) and 4.96 [3,5-di-t-butyl-4-hydroxyanisole (di-t-BHA)], with intermediate activity shown by khellin < gossypol < quercetin < 3-t-butylanisole < BHA < nordihydroguaiaretic acid (NDGA) < 2,6-di-t-butyl-4-methylphenol (BHT) and papaverine. 3. The Ca2+ channel activator Bay K 8644 overcame the inhibition sustained by nifedipine, BHA and BHT, while only partially reversing that of papaverine. 4. BHA, BHT, nifedipine and papaverine also inhibited in a concentration-dependent fashion CaCl2 contractions of muscle depolarized by a K(+)-rich medium. This inhibition appeared to be inversely affected by the Ca(2+)-concentration used. 5. The inhibitory effects of nifedipine, papaverine, BHA and BHT were no longer present when muscle contraction was elicited in skinned fibres by 5 microM Ca2+ or 500 microM Ba2+, suggesting a plasmalemmal involvement of target sites in spasmolysis. 6. Comparative antioxidant capability was assessed in two peroxyl radical scavenging assay systems. These were based either on the oxidation of linoleic acid initiated by a heat labile azo compound or on lipid peroxidation of rat liver microsomes promoted by Fe2+ ions.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdalla S., Zarga M. A., Afifi F., al-Khalil S., Mahasneh A., Sabri S. Effects of 3,3'-di-O-methylquercetin on guinea-pig isolated smooth muscle. J Pharm Pharmacol. 1989 Feb;41(2):138–141. doi: 10.1111/j.2042-7158.1989.tb06413.x. [DOI] [PubMed] [Google Scholar]
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B., Lang R. J., Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+ -channels in arterial and intestinal smooth muscle cell membranes. Pflugers Arch. 1985 Feb;403(2):120–127. doi: 10.1007/BF00584088. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Effect of calcium, barium and manganese on the action of adrenaline in the smooth muscle of the guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):121–136. doi: 10.1098/rspb.1969.0015. [DOI] [PubMed] [Google Scholar]
- Cheung J. Y., Bonventre J. V., Malis C. D., Leaf A. Calcium and ischemic injury. N Engl J Med. 1986 Jun 26;314(26):1670–1676. doi: 10.1056/NEJM198606263142604. [DOI] [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortijo J., Dixon J. S., Foster R. W., Small R. C. Influence of some variables in the Triton X-100 method of skinning the plasmalemmal membrane from guinea pig trachealis muscle. J Pharmacol Methods. 1987 Nov;18(3):253–266. doi: 10.1016/0160-5402(87)90075-1. [DOI] [PubMed] [Google Scholar]
- Deby C., Goutier R. New perspectives on the biochemistry of superoxide anion and the efficiency of superoxide dismutases. Biochem Pharmacol. 1990 Feb 1;39(3):399–405. doi: 10.1016/0006-2952(90)90043-k. [DOI] [PubMed] [Google Scholar]
- Effect of verapamil on mortality and major events after acute myocardial infarction (the Danish Verapamil Infarction Trial II--DAVIT II) Am J Cardiol. 1990 Oct 1;66(10):779–785. doi: 10.1016/0002-9149(90)90351-z. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Fusi F., Sgaragli G., Murphy M. P. Interaction of butylated hydroxyanisole with mitochondrial oxidative phosphorylation. Biochem Pharmacol. 1992 Mar 17;43(6):1203–1208. doi: 10.1016/0006-2952(92)90493-3. [DOI] [PubMed] [Google Scholar]
- Fusi F., Valoti M., Sgaragli G., Murphy M. P. The interaction of antioxidants and structurally related compounds with mitochondrial oxidative phosphorylation. Methods Find Exp Clin Pharmacol. 1991 Nov;13(9):599–603. [PubMed] [Google Scholar]
- Hai C. M., Murphy R. A. Ba2+ induces contraction in swine carotid artery by mobilizing intracellular Ca2+. Am J Physiol. 1987 Apr;252(4 Pt 1):C378–C384. doi: 10.1152/ajpcell.1987.252.4.C378. [DOI] [PubMed] [Google Scholar]
- Hara H., Kogure K. Prevention of hippocampus neuronal damage in ischemic gerbils by a novel lipid peroxidation inhibitor (quinazoline derivative). J Pharmacol Exp Ther. 1990 Nov;255(2):906–913. [PubMed] [Google Scholar]
- Inomata H., Kao C. Y. Actions of Ba++ on ionic currents of the guinea-pig taenia coli. J Pharmacol Exp Ther. 1985 Apr;233(1):112–124. [PubMed] [Google Scholar]
- Janero D. R., Burghardt B. Antiperoxidant effects of dihydropyridine calcium antagonists. Biochem Pharmacol. 1989 Dec 1;38(23):4344–4348. doi: 10.1016/0006-2952(89)90537-6. [DOI] [PubMed] [Google Scholar]
- Janero D. R., Burghardt B., Lopez R. Protection of cardiac membrane phospholipid against oxidative injury by calcium antagonists. Biochem Pharmacol. 1988 Nov 1;37(21):4197–4203. doi: 10.1016/0006-2952(88)90116-5. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Reuter H. Cellular calcium and cardiac cell death. Am J Cardiol. 1979 Jul;44(1):188–190. doi: 10.1016/0002-9149(79)90270-4. [DOI] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Nordihydroguaiaretic acid inhibits voltage-activated Ca2+ currents independently of lipoxygenase inhibition. Mol Pharmacol. 1990 Oct;38(4):524–530. [PubMed] [Google Scholar]
- Kreye V. A., Rüegg J. C., Hofmann F. Effect of calcium-antagonist and calmodulin-antagonist drugs on calmodulin-dependent contractions of chemically skinned vascular smooth muscle from rabbit renal arteries. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(2):85–89. doi: 10.1007/BF00634253. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Meisheri K. D., Ruegg J. C. Dependence of cyclic-AMP induced relaxation on Ca2+ and calmodulin in skinned smooth muscle of guinea pig Taenia coli. Pflugers Arch. 1983 Dec;399(4):315–320. doi: 10.1007/BF00652759. [DOI] [PubMed] [Google Scholar]
- Middleton E., Jr, Kandaswami C. Effects of flavonoids on immune and inflammatory cell functions. Biochem Pharmacol. 1992 Mar 17;43(6):1167–1179. doi: 10.1016/0006-2952(92)90489-6. [DOI] [PubMed] [Google Scholar]
- Naqui A., Chance B., Cadenas E. Reactive oxygen intermediates in biochemistry. Annu Rev Biochem. 1986;55:137–166. doi: 10.1146/annurev.bi.55.070186.001033. [DOI] [PubMed] [Google Scholar]
- Nayler W. G. Calcium channels and their involvement in cardiovascular disease. Biochem Pharmacol. 1992 Jan 9;43(1):39–46. doi: 10.1016/0006-2952(92)90658-6. [DOI] [PubMed] [Google Scholar]
- Nayler W. G., Poole-Wilson P. A., Williams A. Hypoxia and calcium. J Mol Cell Cardiol. 1979 Jul;11(7):683–706. doi: 10.1016/0022-2828(79)90381-x. [DOI] [PubMed] [Google Scholar]
- Northover B. J. The effect of drugs on the constriction of isolated depolarized blood vessels in response to calcium or barium. Br J Pharmacol. 1968 Oct;34(2):417–428. doi: 10.1111/j.1476-5381.1968.tb07062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruterbories K. J., Lindstrom T. D. Pharmacokinetics of a novel butylated hydroxytoluene-thiazolidinone CNS antiischemic agent LY256548 in rats, mice, dogs, and monkeys. Drug Metab Dispos. 1990 Sep-Oct;18(5):674–679. [PubMed] [Google Scholar]
- Satoh S., Kubota Y., Itoh T., Kuriyama H. Mechanisms of the Ba2+-induced contraction in smooth muscle cells of the rabbit mesenteric artery. J Gen Physiol. 1987 Feb;89(2):215–237. doi: 10.1085/jgp.89.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M., Thomas G., Towart R., Franckowiak G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature. 1983 Jun 9;303(5917):535–537. doi: 10.1038/303535a0. [DOI] [PubMed] [Google Scholar]
- Sgaragli G., Corte L. D., Rizzotti-Conti M., Giotti A. Effects of monocyclic compounds on biomembranes. Biochem Pharmacol. 1977 Nov 15;26(22):2145–2149. doi: 10.1016/0006-2952(77)90266-0. [DOI] [PubMed] [Google Scholar]
- Shirota H., Chiba K., Ono H., Yamamoto H., Kobayashi S., Terato K., Ikuta H., Yamatsu I., Katayama K. Pharmacological properties of the novel non-steroidal antiinflammatory agent N-methoxy-3-(3,5-di-tert-butyl-4-hydroxybenzylidene)pyrrolidin-2 -one. Arzneimittelforschung. 1987 Aug;37(8):930–936. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Devine C. E., Peters P. D., Hall T. A. Electron microscopy and electron probe analysis of mitochondrial cation accumulation in smooth muscle. J Cell Biol. 1974 Jun;61(3):723–742. doi: 10.1083/jcb.61.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spedding M. Assessment of "Ca2+ -antagonist" effects of drugs in K+ -depolarized smooth muscle. Differentiation of antagonist subgroups. Naunyn Schmiedebergs Arch Pharmacol. 1982 Feb;318(3):234–240. doi: 10.1007/BF00500485. [DOI] [PubMed] [Google Scholar]
- Ubeda A., Tejerina T., Tamargo J., Villar A. Effects of khellin on contractile responses and 45Ca2+ movements in rat isolated aorta. J Pharm Pharmacol. 1991 Jan;43(1):46–48. doi: 10.1111/j.2042-7158.1991.tb05447.x. [DOI] [PubMed] [Google Scholar]
- Valoti M., Sipe H. J., Jr, Sgaragli G., Mason R. P. Free radical intermediates during peroxidase oxidation of 2-t-butyl-4-methoxyphenol, 2,6-di-t-butyl-4-methylphenol, and related phenol compounds. Arch Biochem Biophys. 1989 Mar;269(2):423–432. doi: 10.1016/0003-9861(89)90126-4. [DOI] [PubMed] [Google Scholar]
- Wayner D. D., Burton G. W., Ingold K. U., Barclay L. R., Locke S. J. The relative contributions of vitamin E, urate, ascorbate and proteins to the total peroxyl radical-trapping antioxidant activity of human blood plasma. Biochim Biophys Acta. 1987 Jun 22;924(3):408–419. doi: 10.1016/0304-4165(87)90155-3. [DOI] [PubMed] [Google Scholar]



