Abstract
We explored the ventral part of the premotor cortex (PMV) with intracortical microstimulation (ICMS) while monkeys performed a visual fixation task, to see whether the PMV is involved in oculomotor control. ICMS evoked saccades from a small-restricted region in the PMV, without evoking movements in the limbs, neck, or body. We found the saccade-evoking site in the PMV in a total of three hemispheres in two monkeys. Quantitative analysis of the effects of eye position on saccades evoked by microstimulation of the PMV characterized the evoked saccades as goal directed. The nature of the saccades evoked in the PMV contrasted with the fixed vector nature of saccades evoked by ICMS of the frontal eye field. We also found that neurons in this restricted area of the PMV were active while the animals were performing a saccade task that required them to make saccades toward targets without arm movements. These data provide evidence for the presence of an oculomotor-specific subregion within the PMV. This subregion and the surrounding skeletomotor-representing regions of the PMV seem to coordinate oculomotor and skeletomotor control in performing goal-directed motor tasks.
The ventral portion of the premotor cortex (PMV) of primates has been implicated in the sensorial guidance of arm movements (1–3). It is natural to postulate some involvement of the PMV in oculomotor control, because eye movements usually are coupled with limb movements in visually guided reaching, and oculomotor and skeletomotor performance must be coordinated. In this study, we looked for oculomotor representation in the PMV by applying intracortical microstimulation (ICMS) while monkeys performed oculomotor tasks. Interest in the issue of PMV involvement in oculomotor control has increased after a recent report by Thier and Andersen (4). They found that microstimulation of the rostral part of the intraparietal cortex (intercalated zone) elicited goal-directed saccadic eye movements, whereas microstimulation of the lateral wall of the lateral intraparietal sulcus produced constant-vector saccades. In view of the corticocortical connections between portions of the intraparietal cortex and the PMV (5), it seemed worthwhile to look for microstimulation effects in the PMV. We found a subregion within the PMV that is a low-threshold saccade-evoking area. Furthermore, we showed that neurons in this subregion are selectively active in association with voluntary, visually guided saccades.
METHODS
We trained two monkeys (Macaca fuscata) to perform the three different oculomotor tasks described below. The animals were cared for in accordance with the National Institutes of Health’s Guide for Care and Use of Laboratory Animals, and Guidelines for Institutional Animal Care and Use published by our institute. Eye positions and movements were monitored with an infrared monitoring system with a time resolution of 4 ms. The animal sat in a primate chair with its head and arms restrained, facing a panel equipped with five light-emitting diodes (LEDs). The central LED was directly in front of the animal at 30 cm distance, and the other four LEDs were 20 degrees of visual angle above, below, and to the right and left of the central LED. Initially, the monkeys performed a gaze-fixation task, in which a randomly selected LED was illuminated as the target of fixation for the monkey. If fixation was maintained for 1.5–2.0 s within a 2° window, a reward of fruit juice was delivered. Conventional techniques were used for recording and stimulating the cortex with microelectrodes (Elgiloy with 1–2 MΩ impedance). The recording manipulator was tilted laterally by 25, but, antero-posterioly, it was installed parallel to the vertical axis of Horseley-Clarke’s stereotaxic coordinate. During the fixation period, ICMS (with 10–50 pulses of 0.2-ms duration at 333 Hz) was delivered 300 ms after the onset of fixation. We first identified the frontal eye field (FEF) in the anterior wall of the arcuate sulcus as the area from which saccades were evoked with low thresholds (20–50 μA). We then mapped ICMS-evoked movements and muscle contraction in the forelimb in the ventral part of the primary motor cortex and the PMV. As discussed below, we found a saccade-evoking area within the PMV, surrounded by cortical sites from which either forelimb or facial movements were evoked. We compared the effect of saccades evoked in this area on eye position with that of saccades evoked in the FEF. These effects were analyzed by examining the correlation of two variables, eye position and saccade amplitude, and by calculating the regression coefficient corresponding to the orbital perturbation index defined by Russo and Bruce (6). In a subsequent study, we analyzed neuronal activity in the PMV while the monkeys were performing two different tasks; one involved saccades without arm movement and the other involved reaching toward a target without saccades. Monkeys were required to maintain fixation on an LED for 1.5–2.0 s (fixation period). If the monkey in the saccade task continued to maintain fixation on the original LED, then a second red LED, which was the future target, was illuminated continuously 700–1,000 ms before the end of the fixation period. When the original LED was dimmed, which served as the go signal, the monkey then was required to make a saccade to the target without any limb movement. In the reaching task, at the end of the fixation period, the color of the fixation LED changed from red to green, serving as the go signal. The monkey had to push the original fixation target with its hand, without an accompanying saccade. The arm reaching, therefore, was directed to a target being fixed with the eyes, but was directed to five different points in space. The monkey was rewarded if the correct target was captured appropriately, with either a saccade or an arm movement, within 400 ms of the go signal. We also recorded electromyogram activity in the muscles of the forearm, upper arm, shoulder, neck, face, back, and waist. Cortical stimulation sites and recording sites were verified by histological analysis (7).
RESULTS
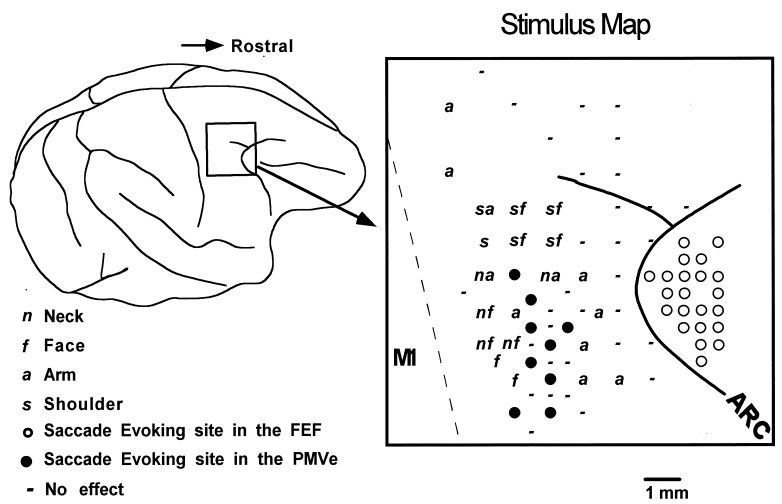
First, we identified cortical landmark structures such as the central sulcus and the arcuate sulcus with its spur, and localized the FEF and primary motor cortex (MI) with ICMS responses (7, 8). Then we mapped the PMV by ICMS in detail while monkeys were performing the fixation tasks. In accordance with previous reports (9, 10), the thresholds evoking movements in the arm, neck, face and back were higher than in the MI and required currents of more than 40 μA or pulse trains of >20 pulses. Unexpectedly, we discovered a subregion from which saccades were evoked with currents of 20–80 μA. The saccade-evoking region was within the PMV and surrounded by cortical sites where ICMS evoked movements in the arm, shoulder, neck, and face (Fig. 1). We found a total of 16 low-threshold saccade-evoking sites in the two monkeys, 11 in one and five in the other. The location of the saccade-evoking areas in the three hemispheres fell on corresponding sites within the PMV, although in one hemisphere the mapping was less complete than others. In these stimulation sites, no body or facial movements, other than saccades, were evoked with currents of <80 μA. We tried to find topographical organization of evoked saccades, but failed to find any topography in the direction of evoked saccades, because the saccade-evoking region was small and the saccade directions evoked at neighboring penetrations often differ greatly. The saccades were evoked when the tips of the electrode were at the depth of 0.5–3.5 mm within the gray matter, but not when they were within the white matter. The velocities of the evoked saccades were comparable to those of voluntary saccades, with a maximum of more than 500°/s (Fig. 2 B and C). We propose that this oculomotor representation area within the PMV be called the PMVe. It is important to note that the PMVe is physically separated from the FEF by an arm-representing area at least 2.5 mm wide, which is intercalated between the PMVe and the superficial and deep parts of the arcuate sulcus.
Figure 1.
(Left) Surface view of the cerebral cortex indicating the location of the frontal cortex penetrated with microelectrodes. (Right) Distribution of stimulus effects mapped on the cortical surface. Solid and open circles denote saccade-evoking sites in the PMV and FEF. Cortical sites evoking movements in the arm, face, neck, and shoulder are labeled a, f, n, and s, respectively. ICMS-negative sites are marked with −. The dashed line denotes the boundary between the primary and premotor cortex. ARC, arcuate sulcus.
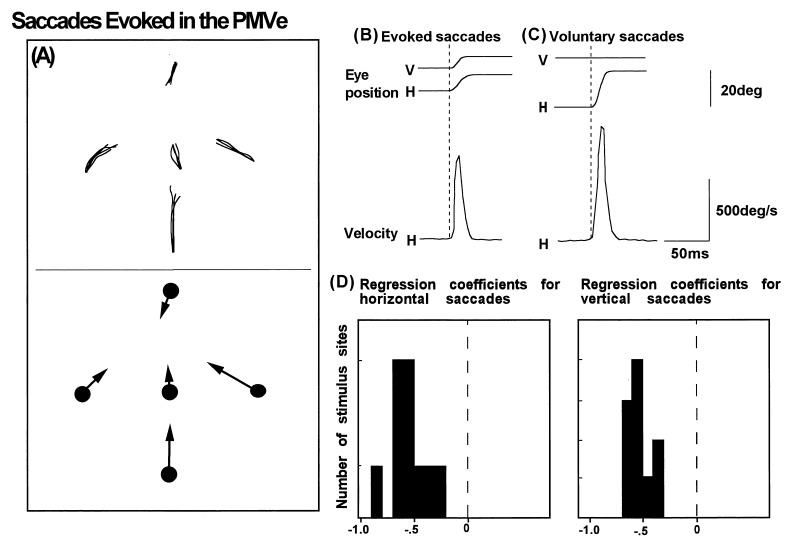
Figure 2.
(A) Trajectories of saccades (Upper) and their mean vectors (Lower) from five different fixation points, evoked by stimulation of the PMVe. (B and C) Averaged traces of eye position (Upper) and velocity (Lower) observed in representative examples of electrically evoked (B) and voluntary (C) saccades. (D) The distribution of regression coefficients calculated from correlation plots of eye position and the amplitudes of horizontal and vertical saccades. The mean horizontal and vertical correlation coefficients and SD are −0.44 ± 0.12 and −0.56 ± 0.17, with a grand mean of −0.47 ± 0.21.
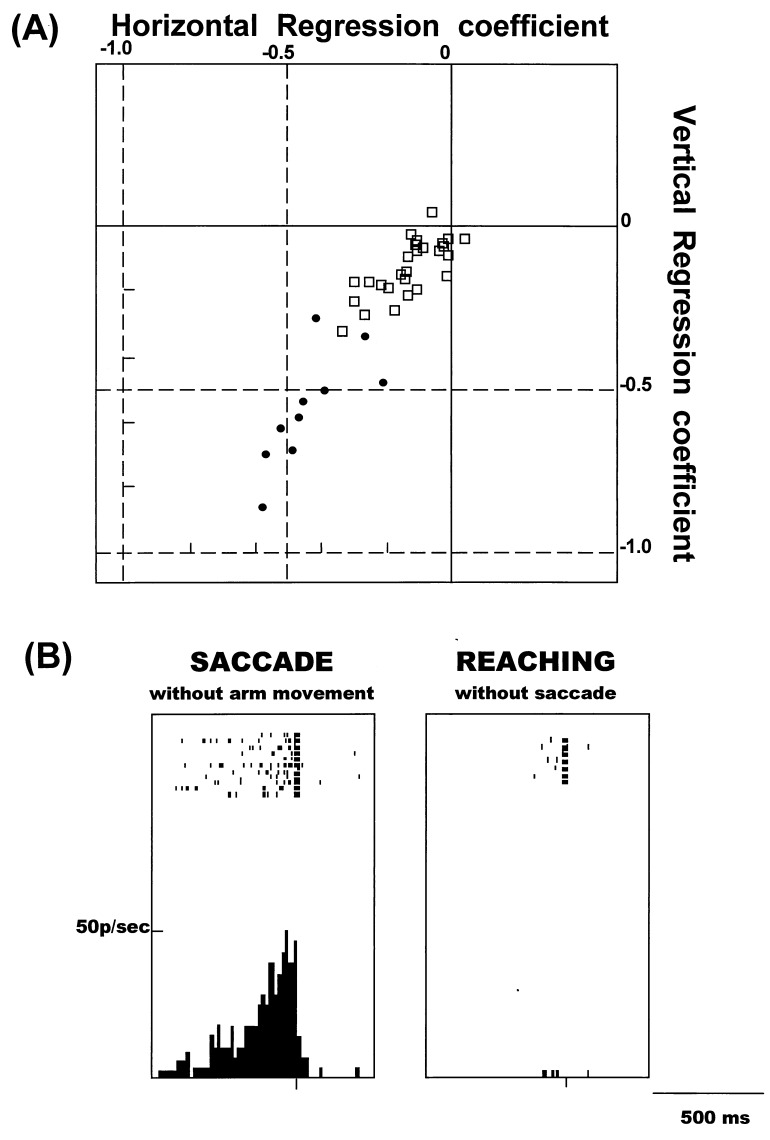
We compared the properties of saccades evoked in the PMVe and FEF and found that they differ according to their dependence on eye position. As shown in Fig. 2A, the vectors of saccades evoked in the PMVe were greatly influenced by eye position, suggesting that these saccades were converging or goal-directed rather than along a fixed vector. To study the effects of eye position on evoked saccades further, we examined the correlation of two variables, eye position and saccade amplitude, and calculated their horizontal and vertical regression coefficients (Fig. 2D). In the PMVe, the regression coefficient was −0.47 ± 0.21 (SD), whereas in the FEF the value was close to zero (−0.16 ± 0.10, Fig. 3A). The scatter plot in Fig. 3A shows the distribution of the vertical and horizontal regression coefficients in the PMVe (•) and FEF (□). The values in the PMVe and FEF were significantly different (P < 0.001), further suggesting that the evoked saccades in the PMVe were more convergent and goal directed than the constant-vector saccades evoked in the FEF. The points of convergence were directed to the direction contralateral to the side of stimulation, though at some sites the saccades were directed to points close to the midline, as shown in Fig. 2.
Figure 3.
(A) Scatter plot of the horizontal and vertical regression coefficients calculated from saccades evoked in the PMVe and FEF. □, Coefficients in the FEF. •, Coefficients in the PMVe. (B) Discharges of a cell in the PMVe while the monkey was performing the saccade task (Left) and arm-reaching task (Right). Discharges, shown as short vertical bars, are aligned with the onset of either saccades or reaching movements.
The discovery of the saccade-evoking region in the PMV led to the question of whether neurons in that area are actively associated with voluntary saccades. To answer this question, we studied neuronal activity in the PMVe while monkeys were performing the tasks involving either saccades or arm-reaching movements. Only a brief summary of the experimental results is presented in this paper, because a detailed description of the neuronal activity in the PMVe is the subject of a separate paper. We found 102 neurons in the PMVe that were active before and during the saccade made to the visual target, as shown in a representative example in Fig. 3B (Left). In this neuron and 35 others, activity was selectively observed with the saccade task and not with the arm-reaching task (Fig. 3B, Right). No limb-muscle activity was detected by electromyogram monitoring when the saccade occurred, and no eye movements occurred during the arm-reaching task. Neurons that were selectively active with saccades were rarely found in other portions of the PMV outside the PMVe. We also observed a second group of neurons that were active during both the saccade and the arm-reaching tasks; details of these findings will be reported elsewhere.
DISCUSSION
Traditionally, the PMV was thought to be primarily involved in the control of limb movement and postural control involving body muscles. However, we found a subregion characterized as a low-threshold saccade-evoking area within the ventral part of the PMV, where ICMS only evokes saccades and no other movements, provided the stimulus currents are less than 80 μA. This oculomotor region is surrounded by portions of the PMV where ICMS evokes movements of the arm, shoulder, neck, and face. The saccade-evoking region appears to be separated from the FEF and from the pursuit-related oculomotor area in the posterior bank of the arcuate sulcus (11). The PMVe was located in the gyral surface and not in the arcuate sulcus. Furthermore, we confirmed that neurons in this area were selectively active before and during voluntarily initiated saccades. We propose that this oculomotor-specific portion of the PMV be referred to as the PMVe.
The analysis of evoked saccades identified a unique property of the PMVe. The saccade vector was greatly influenced by the eye position at the start of ICMS, so that the saccades appeared to be converging or goal directed, rather than along a fixed vector. The goal-directed nature of saccades evoked in this area suggests that the PMVe may exert its control in the craniocentric coordinate rather than the retinotopic coordinate (12). But there were other possible interpretations for these findings. However, the converging property of evoked saccades may suggest other possibilities. For instance, it may mean that the PMVe may be involved in gaze position control, rather than eye position. Although we did not observe neck muscle activation by ICMS within the PMVe, this area still may be involved in gaze control, in collaboration with the surrounding neck region in the PMV. Another possible interpretation for the converging saccades is the involvement of this area in controlling saccades to different points in depth, especially to points close to the animal (13–15). Although we have not observed clear indication for the vengeance of bilateral eyes evoked by ICMS, this remains a possibility. Further studies are necessary to clarify functional significance of the converging saccades. Were there previous reports of converging saccades evoked in other parts of the cortex? Evoked saccades in the FEF (8, 16), supplementary eye field (ref. 17, but see refs. 18 and 19 for a different view), and lateral wall of the intraparietal cortex (LIP) (4) are predominately of the fixed-vector type. Recently, Thier and Andersen (4) discovered an exception (4). They subdivided the oculomotor area of the LIP into two distinct areas, the lateral wall of the intraparietal sulcus (IPS) and a rostrally adjacent intercalated zone that is located in the fundus of the IPS. In contrast to ICMS of the lateral wall of the LIP, ICMS in the intercalated zone revealed apparent eye-position effects so that the saccades appeared to be converging. In their data, the regression coefficient was calculated as −0.435. That value agrees with our result obtained in the PMVe. The similarity between the intercalated zone in the IPS and the PMVe is striking; both areas produce converging evoked saccades and both areas are an oculomotor subregion surrounded by skeletomotor representation areas. It will be interesting to determine whether the two areas are anatomically interconnected.
What is the functional significance of the PMVe? Its location in the middle of the PMV, surrounded by areas representing the face, arm, and axial body parts, suggests that it be involved in coordinating eye movements with the movement of other parts of the body. It may be involved in moving the eyes as a part of a general orienting response toward a point of interest. It also may be possibly involved in the eye-arm coordination required to reach for visual targets. The effect of lesions of the PMV that disturb visually guided arm movements (20) is consistent with this view. The goal-directed nature of saccades evoked in this area suggests that the PMVe may exert its control in the craniocentric coordinate rather than the retinotopic coordinate (12). However, we have to be cautious about the interpretation of the ICMS data. Other possibilities for the PMVe involvement, such as in gaze position control, must be considered. Further studies are necessary to learn how this restricted area collaborates with other portions of the PMV to coordinate spatially oriented movements.
Acknowledgments
We thank M. Kurama and Y. Takahashi for technical assistance. This work was supported by Precursory Research for Embryonic Science and Technology (PRESTO), and the Ministry of Education, Science, and Culture of Japan (09680809, 08279101, 09308032, and 08044236).
ABBREVIATIONS
- PMV
premotor cortex
- ICMS
intracortical microstimulation
- FEF
frontal eye field
- LED
light-emitting diode
References
- 1. Godschalk M, Lemon R N, Kuypers H G J M, van der Steen J. Behav Brain Res. 1985;18:143–158. doi: 10.1016/0166-4328(85)90070-1. [DOI] [PubMed] [Google Scholar]
- 2.Wise S P. Annu Rev Neurosci. 1985;8:1–19. doi: 10.1146/annurev.ne.08.030185.000245. [DOI] [PubMed] [Google Scholar]
- 3.Kurata K. Behav Brain Res. 1994;61:135–142. doi: 10.1016/0166-4328(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 4.Thier P, Andersen R A. Proc Natl Acad Sci USA. 1996;93:4962–4967. doi: 10.1073/pnas.93.10.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson P B, Ferraina S, Caminiti R. Exp Brain Res. 1993;97:361–365. doi: 10.1007/BF00228707. [DOI] [PubMed] [Google Scholar]
- 6.Russo G, Bruce C J. J Neurophysiol. 1993;69:800–817. doi: 10.1152/jn.1993.69.3.800. [DOI] [PubMed] [Google Scholar]
- 7.Mushiake H, Tanatsugu Y, Tanji J. J Neurophysiol. 1997;78:567–571. doi: 10.1152/jn.1997.78.1.567. [DOI] [PubMed] [Google Scholar]
- 8.Fujii N, Mushiake H, Tanji J. J Neurophysiol. 1998;79:2240–2244. doi: 10.1152/jn.1998.79.4.2240. [DOI] [PubMed] [Google Scholar]
- 9.Kurata K, Tanji J. J Neurosci. 1986;6:403–411. doi: 10.1523/JNEUROSCI.06-02-00403.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R, Rizzolatti G. Exp Brain Res. 1988;71:475–490. doi: 10.1007/BF00248741. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb J P, MacAvoy M G, Bruce C J. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- 12.Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M, Rizzolatti G. J Neurophysiol. 1996;76:141–157. doi: 10.1152/jn.1996.76.1.141. [DOI] [PubMed] [Google Scholar]
- 13.Colby C L, Duhamel J R, Goldberg M E. J Neurophysiol. 1993;69:902–914. doi: 10.1152/jn.1993.69.3.902. [DOI] [PubMed] [Google Scholar]
- 14.Duhamel J R, Colby C L, Goldberg M E. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- 15.Rizzolatti G, Scandolara C, Matelli M, Gentilucci M. Behav Brain Res. 1981;2:147–163. doi: 10.1016/0166-4328(81)90053-x. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg M E, Bruce C J. J Neurophysiol. 1990;64:489–508. doi: 10.1152/jn.1990.64.2.489. [DOI] [PubMed] [Google Scholar]
- 17.Fujii N, Mushiake H, Tanji J. NeuroReport. 1995;6:2565–2568. doi: 10.1097/00001756-199512150-00028. [DOI] [PubMed] [Google Scholar]
- 18.Schall J D. J Neurophysiol. 1991;66:530–558. doi: 10.1152/jn.1991.66.2.530. [DOI] [PubMed] [Google Scholar]
- 19.Mann S E, Thau R, Schiller P H. Exp Brain Res. 1988;69:460–468. doi: 10.1007/BF00247300. [DOI] [PubMed] [Google Scholar]
- 20.Kurata K, Hoffman D S. J Neurophysiol. 1994;71:1151–1164. doi: 10.1152/jn.1994.71.3.1151. [DOI] [PubMed] [Google Scholar]