Abstract
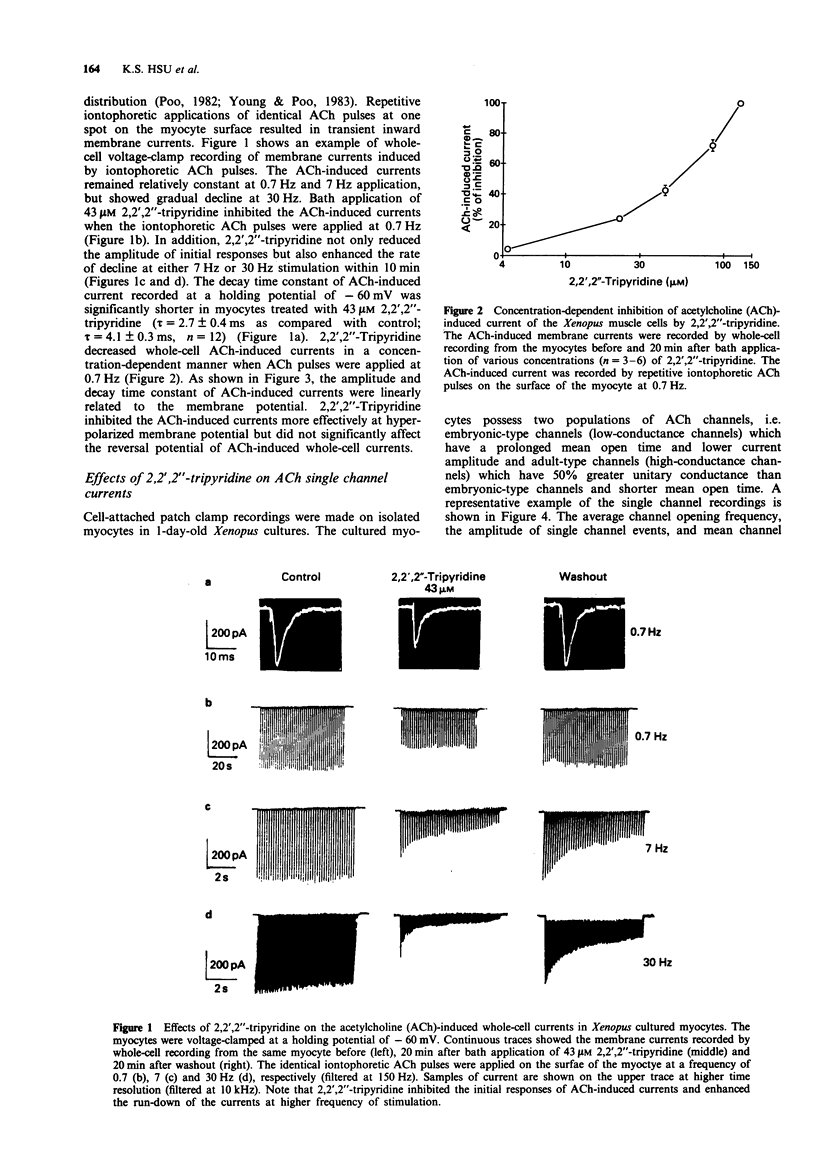
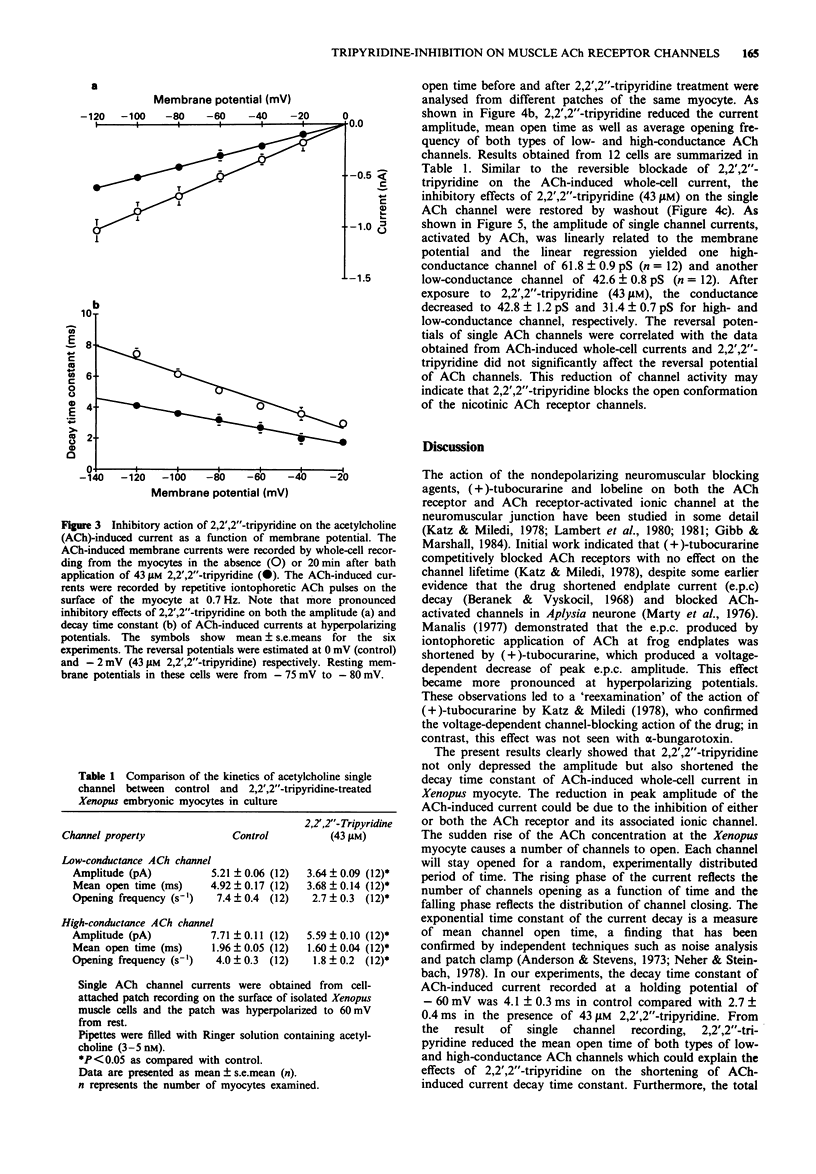
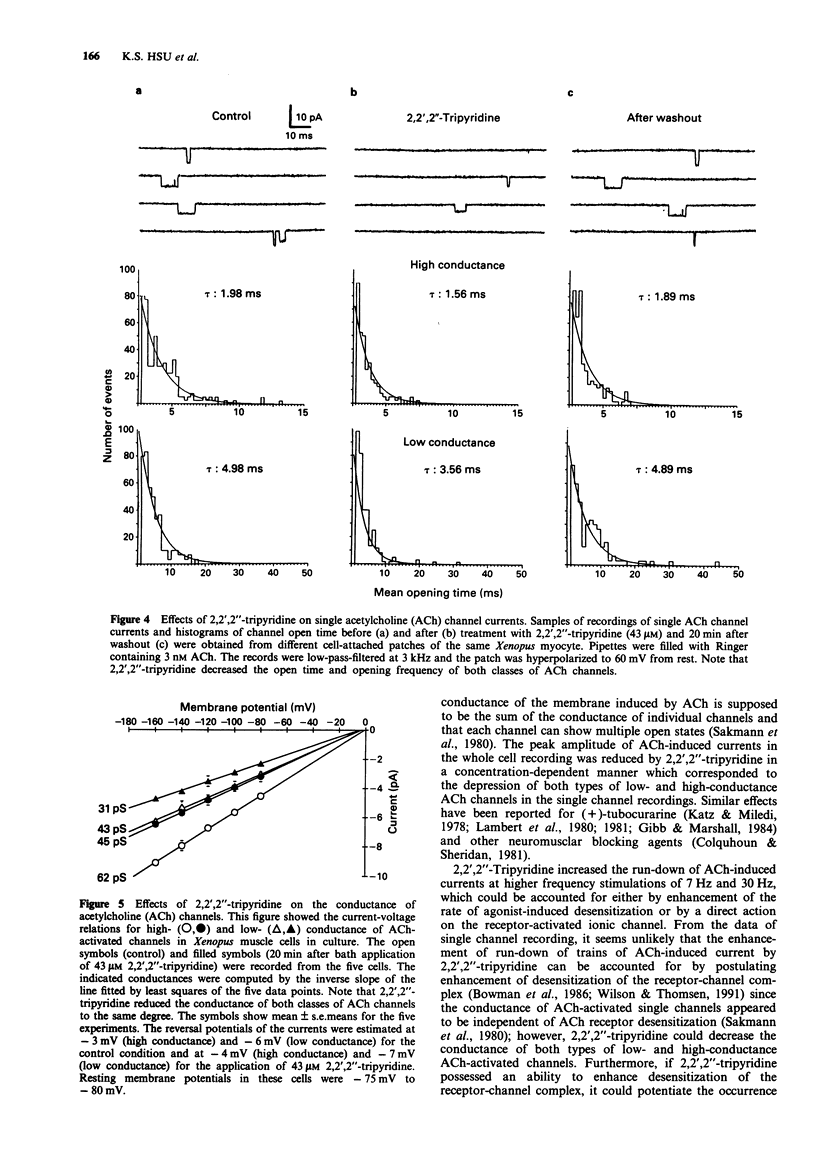
1. The effects of 2,2',2''-tripyridine on the nicotinic acetylcholine (ACh) receptor channels were studied in the cultured myocytes of 1-day-old Xenopus embryos. 2. 2,2',2''-Tripyridine depressed the amplitude of iontophoretic ACh-induced current at a low frequency of 0.7 Hz stimulation and it not only decreased the initial responses but also enhanced the run-down of ACh-induced current at higher frequency stimulation of 7 Hz and 30 Hz. 3. Single ACh channel recordings showed that 2,2',2''-tripyridine decreased the channel conductance, the opening frequency and mean open time of both types of low- and high-conductance channels. 4. These results suggest that the blocking actions of 2,2',2''-tripyridine on ACh receptor channels in the skeletal muscle may contribute to the depression of the nerve-evoked contraction of the mouse diaphragm as reported previously.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo L. G., Witkop B., Albuquerque E. X. Voltage- and time-dependent effects of phencyclidines on the endplate current arise from open and closed channel blockade. Proc Natl Acad Sci U S A. 1986 May;83(10):3523–3527. doi: 10.1073/pnas.83.10.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracava Y., Albuquerque E. X. Meproadifen enhances activation and desensitization of the acetylcholine receptor-ionic channel complex (AChR): single channel studies. FEBS Lett. 1984 Sep 3;174(2):267–274. doi: 10.1016/0014-5793(84)81171-0. [DOI] [PubMed] [Google Scholar]
- Beránek R., Vyskocil F. The effect of atropine on the frog sartorius neuromuscular junction. J Physiol. 1968 Mar;195(2):493–503. doi: 10.1113/jphysiol.1968.sp008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp J. S., Aronstam R. S., Witkop B., Albuquerque E. X. Electrophysiological and biochemical studies on enhancement of desensitization by phenothiazine neuroleptics. Proc Natl Acad Sci U S A. 1983 Jan;80(1):310–314. doi: 10.1073/pnas.80.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sheridan R. E. The modes of action of gallamine. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):181–203. doi: 10.1098/rspb.1981.0002. [DOI] [PubMed] [Google Scholar]
- Gibb A. J., Marshall I. G. Pre-and post-junctional effects of tubocurarine and other nicotinic antagonists during repetitive stimulation in the rat. J Physiol. 1984 Jun;351:275–297. doi: 10.1113/jphysiol.1984.sp015245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M., Nojima H., Muroi M., Kimura I. Mechanism of the blocking action of beta-eudesmol on the nicotinic acetylcholine receptor channel in mouse skeletal muscles. Neuropharmacology. 1991 Aug;30(8):835–841. doi: 10.1016/0028-3908(91)90117-t. [DOI] [PubMed] [Google Scholar]
- Lambert J. J., Durant N. N., Reynolds L. S., Volle R. L., Henderson E. G. Characterization of end-plate conductance in transected frog muscle: modification by drugs. J Pharmacol Exp Ther. 1981 Jan;216(1):62–69. [PubMed] [Google Scholar]
- Lambert J. J., Volle R. L., Henderson E. G. An attempt to distinguish between the actions of neuromuscular blocking drugs on the acetylcholine receptor and on its associated ionic channel. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5003–5007. doi: 10.1073/pnas.77.8.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Shiau S. Y., Hsu K. S., Fu W. M. Studies on curare-like action of 2,2',2''-tripyridine in the mouse phrenic nerve-diaphragm. Br J Pharmacol. 1992 May;106(1):55–60. doi: 10.1111/j.1476-5381.1992.tb14292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neild T., Ascher P. Voltage sensitivity of acetylcholine currents in Aplysia neurones in the presence of curare. Nature. 1976 Jun 10;261(5560):501–503. doi: 10.1038/261501a0. [DOI] [PubMed] [Google Scholar]
- Poo M. Rapid lateral diffusion of functional A Ch receptors in embryonic muscle cell membrane. Nature. 1982 Jan 28;295(5847):332–334. doi: 10.1038/295332a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sanes D. H., Poo M. M. In vitro analysis of position- and lineage-dependent selectivity in the formation of neuromuscular synapses. Neuron. 1989 Mar;2(3):1237–1244. doi: 10.1016/0896-6273(89)90308-5. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Lamborghini J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. D., Li W. E., Hu F. C., Hu K. H. Occupational risk and the development of premalignant skin lesions among paraquat manufacturers. Br J Ind Med. 1987 Mar;44(3):196–200. doi: 10.1136/oem.44.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Thomsen R. H. Nicotinic receptors on the rat phrenic nerve: evidence for negative feedback. Neurosci Lett. 1991 Nov 11;132(2):163–166. doi: 10.1016/0304-3940(91)90292-2. [DOI] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Spontaneous release of transmitter from growth cones of embryonic neurones. Nature. 1983 Oct 13;305(5935):634–637. doi: 10.1038/305634a0. [DOI] [PubMed] [Google Scholar]
- Young S. H., Poo M. M. Topographical rearrangement of acetylcholine receptors alters channel kinetics. Nature. 1983 Jul 14;304(5922):161–163. doi: 10.1038/304161a0. [DOI] [PubMed] [Google Scholar]


