Abstract
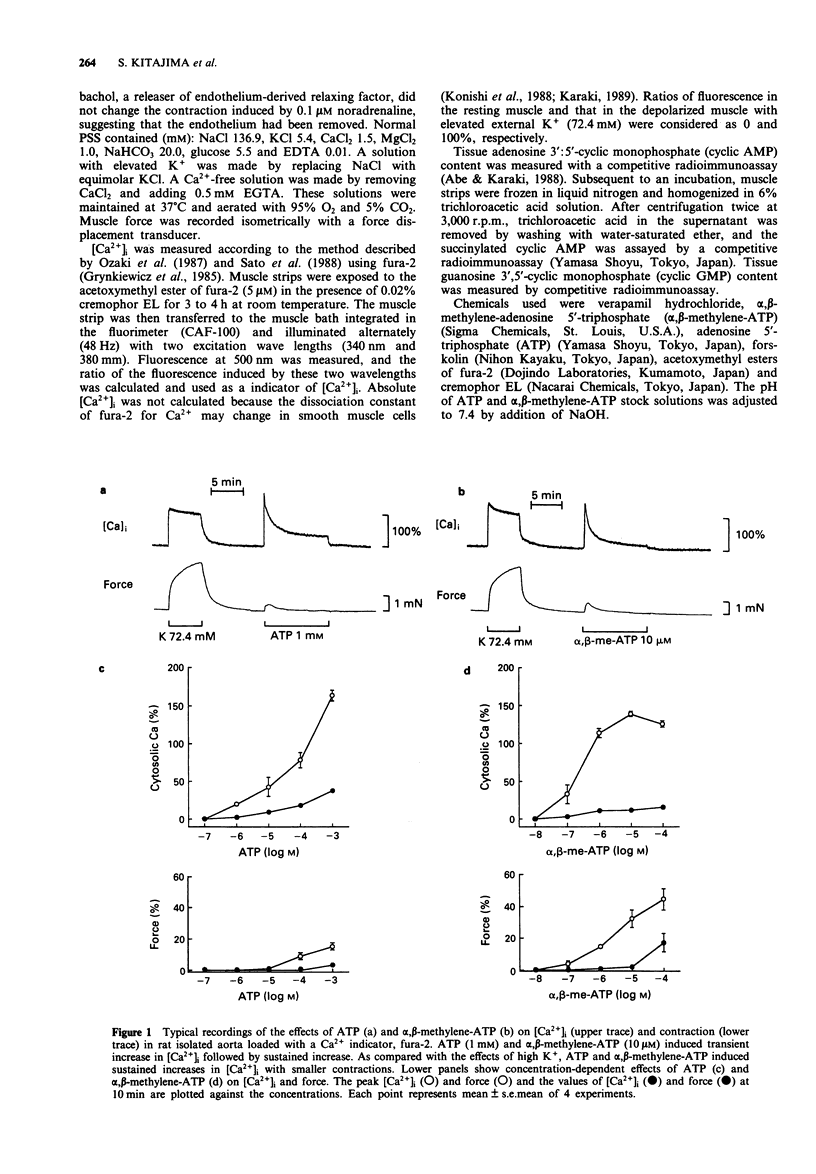
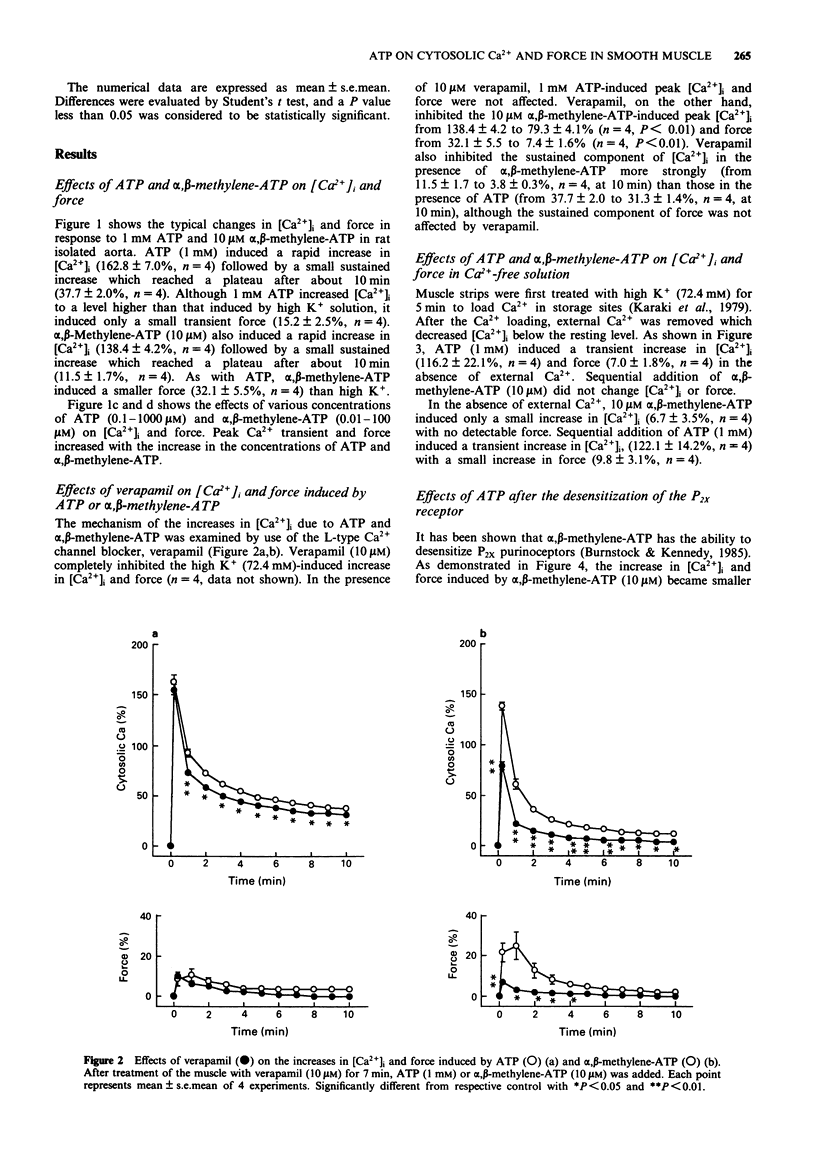
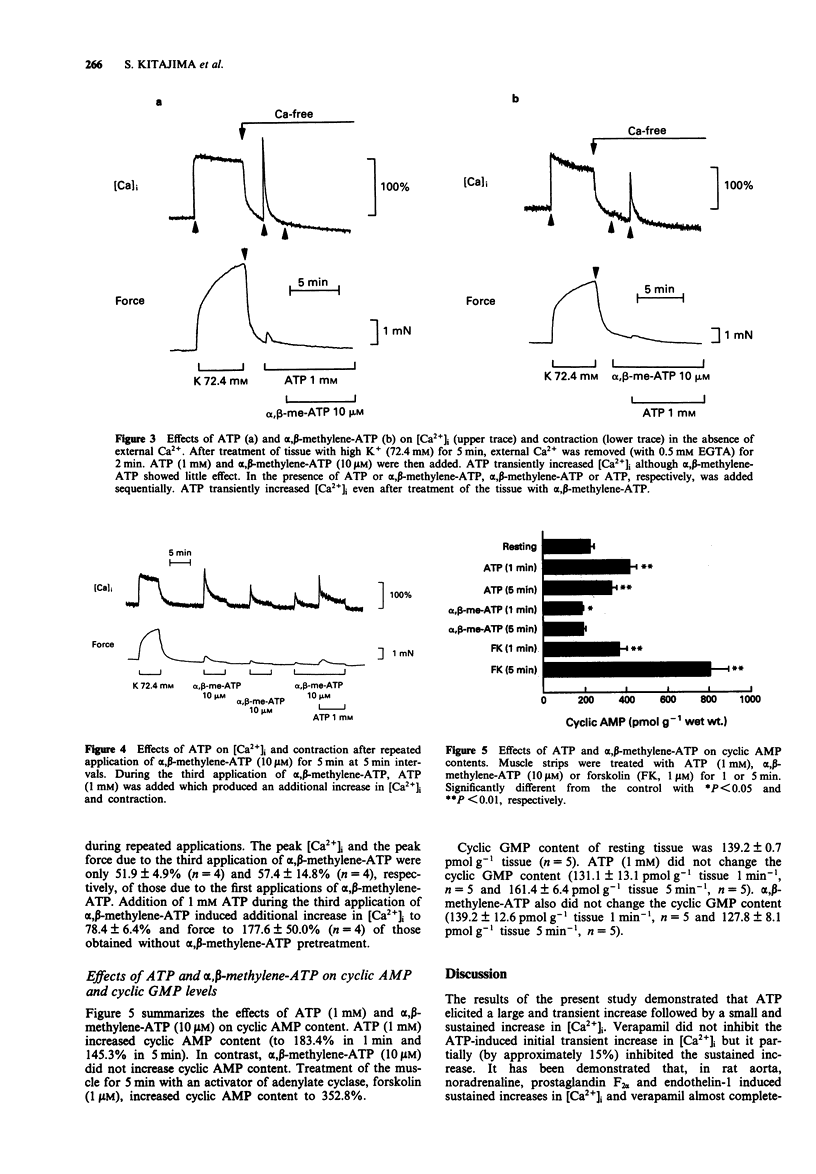
1. The effects of a non-selective P2-receptor agonist ATP and a selective P2x-receptor agonist alpha,beta-methylene-ATP on intracellular free Ca2+ level ([Ca2+]i) and force were examined in rat isolated aorta without endothelium. 2. Both ATP (1-1000 microM) and alpha,beta-methylene-ATP (0.1-100 microM) induced transient increase followed by small sustained increase in [Ca2+]i in a concentration-dependent manner. Compared with the force induced by a high concentration of KCl, the force induced by alpha,beta-methylene-ATP was smaller and that induced by ATP was much smaller at a given [Ca2+]i. 3. An L-type Ca2+ channel blocker, verapamil (10 microM), completely inhibited the high K(+)-stimulated [Ca2+]i and force. Verapamil partially inhibited the transient and sustained increases in [Ca2+]i induced by 10 microM alpha,beta-methylene-ATP and the sustained increase but not the transient increase induced by 1 mM ATP. 4. In the absence of extracellular Ca2+ (with 0.5 mM EGTA) 1 mM ATP caused transient increase in [Ca2+]i while 10 microM alpha,beta-methylene-ATP was ineffective 5. ATP, but not alpha,beta-methylene-ATP, increased the tissue adenosine 3':5'-cyclic monophosphate (cyclic AMP) level. 6. These data suggest that ATP and alpha,beta-methylene-ATP increase [Ca2+]i by an activation of both L-type and non-L-type Ca2+ channels. In addition, ATP, but not alpha,beta-methylene-ATP, increases [Ca2+]i by a release of Ca2+ from an intracellular Ca2+ store. Possible reasons are discussed as to why the increase in [Ca2+]i due to ATP and alpha,beta-methylene-ATP resulted in only a small contraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe A., Karaki H. Inhibitory effects of forskolin on vascular smooth muscle of rabbit aorta. Jpn J Pharmacol. 1988 Mar;46(3):293–301. doi: 10.1254/jjp.46.293. [DOI] [PubMed] [Google Scholar]
- Benham C. D., Tsien R. W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987 Jul 16;328(6127):275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Chinellato A., Ragazzi E., Pandolfo L., Froldi G., Caparrotta L., Fassina G. Pharmacological characterization of a new purinergic receptor site in rabbit aorta. Gen Pharmacol. 1992 Nov;23(6):1067–1071. doi: 10.1016/0306-3623(92)90288-u. [DOI] [PubMed] [Google Scholar]
- Fasolato C., Pizzo P., Pozzan T. Receptor-mediated calcium influx in PC12 cells. ATP and bradykinin activate two independent pathways. J Biol Chem. 1990 Nov 25;265(33):20351–20355. [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Himpens B., De Smedt H., Droogmans G., Casteels R. Differences in regulation between nuclear and cytoplasmic Ca2+ in cultured smooth muscle cells. Am J Physiol. 1992 Jul;263(1 Pt 1):C95–105. doi: 10.1152/ajpcell.1992.263.1.C95. [DOI] [PubMed] [Google Scholar]
- Honoré E., Martin C., Mironneau C., Mironneau J. An ATP-sensitive conductance in cultured smooth muscle cells from pregnant rat myometrium. Am J Physiol. 1989 Aug;257(2 Pt 1):C297–C305. doi: 10.1152/ajpcell.1989.257.2.C297. [DOI] [PubMed] [Google Scholar]
- Hori M., Sato K., Sakata K., Ozaki H., Takano-Ohmuro H., Tsuchiya T., Sugi H., Kato I., Karaki H. Receptor agonists induce myosin phosphorylation-dependent and phosphorylation-independent contractions in vascular smooth muscle. J Pharmacol Exp Ther. 1992 May;261(2):506–512. [PubMed] [Google Scholar]
- Karaki H. Ca2+ localization and sensitivity in vascular smooth muscle. Trends Pharmacol Sci. 1989 Aug;10(8):320–325. doi: 10.1016/0165-6147(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Karaki H., Kubota H., Urakawa N. Mobilization of stored calcium for phasic contraction induced by norepinephrine in rabbit aorta. Eur J Pharmacol. 1979 Jun 15;56(3):237–245. doi: 10.1016/0014-2999(79)90176-6. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Burnstock G. ATP produces vasodilation via P1 purinoceptors and vasoconstriction via P2 purinoceptors in the isolated rabbit central ear artery. Blood Vessels. 1985;22(3):145–155. doi: 10.1159/000158592. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Delbro D., Burnstock G. P2-purinoceptors mediate both vasodilation (via the endothelium) and vasoconstriction of the isolated rat femoral artery. Eur J Pharmacol. 1985 Jan 2;107(2):161–168. doi: 10.1016/0014-2999(85)90055-x. [DOI] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Fujimori K., Takanaka A., Inoue K. Reversible and selective antagonism by suramin of ATP-activated inward current in PC12 phaeochromocytoma cells. Br J Pharmacol. 1990 Sep;101(1):224–226. doi: 10.1111/j.1476-5381.1990.tb12117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor S. E. Recent developments in the classification and functional significance of receptors for ATP and UTP, evidence for nucleotide receptors. Life Sci. 1992;50(22):1657–1664. doi: 10.1016/0024-3205(92)90420-t. [DOI] [PubMed] [Google Scholar]
- Olsson R. A., Pearson J. D. Cardiovascular purinoceptors. Physiol Rev. 1990 Jul;70(3):761–845. doi: 10.1152/physrev.1990.70.3.761. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Sato K., Satoh T., Karaki H. Simultaneous recordings of calcium signals and mechanical activity using fluorescent dye fura 2 in isolated strips of vascular smooth muscle. Jpn J Pharmacol. 1987 Nov;45(3):429–433. doi: 10.1254/jjp.45.429. [DOI] [PubMed] [Google Scholar]
- Ozaki H., Zhang L., Buxton I. L., Sanders K. M., Publicover N. G. Negative-feedback regulation of excitation-contraction coupling in gastric smooth muscle. Am J Physiol. 1992 Dec;263(6 Pt 1):C1160–C1171. doi: 10.1152/ajpcell.1992.263.6.C1160. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Mathie R. T., Alexander B., Burnstock G. Characterization of P2X- and P2Y-purinoceptors in the rabbit hepatic arterial vasculature. Br J Pharmacol. 1991 May;103(1):1108–1113. doi: 10.1111/j.1476-5381.1991.tb12308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembold C. M., Weaver B. A., Linden J. Adenosine triphosphate induces a low [Ca2+]i sensitivity of phosphorylation and an unusual form of receptor desensitization in smooth muscle. J Biol Chem. 1991 Mar 25;266(9):5407–5411. [PubMed] [Google Scholar]
- Sato K., Ozaki H., Karaki H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J Pharmacol Exp Ther. 1988 Jul;246(1):294–300. [PubMed] [Google Scholar]
- Tawada Y., Furukawa K., Shigekawa M. Cyclic AMP enhances inositol trisphosphate-induced mobilization of intracellular Ca2+ in cultured aortic smooth muscle cells. J Biochem. 1988 Nov;104(5):795–800. doi: 10.1093/oxfordjournals.jbchem.a122552. [DOI] [PubMed] [Google Scholar]
- el-Moatassim C., Dornand J., Mani J. C. Extracellular ATP and cell signalling. Biochim Biophys Acta. 1992 Feb 19;1134(1):31–45. doi: 10.1016/0167-4889(92)90025-7. [DOI] [PubMed] [Google Scholar]


