Abstract
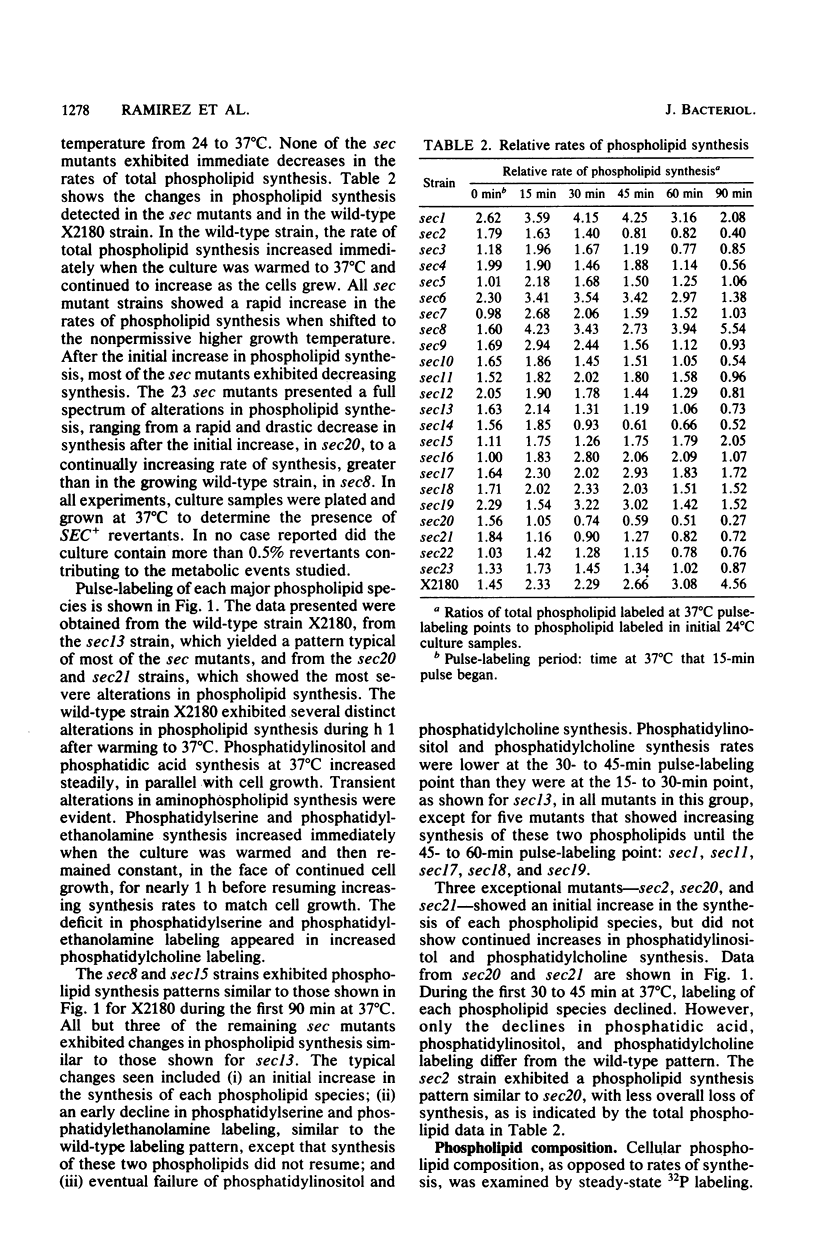
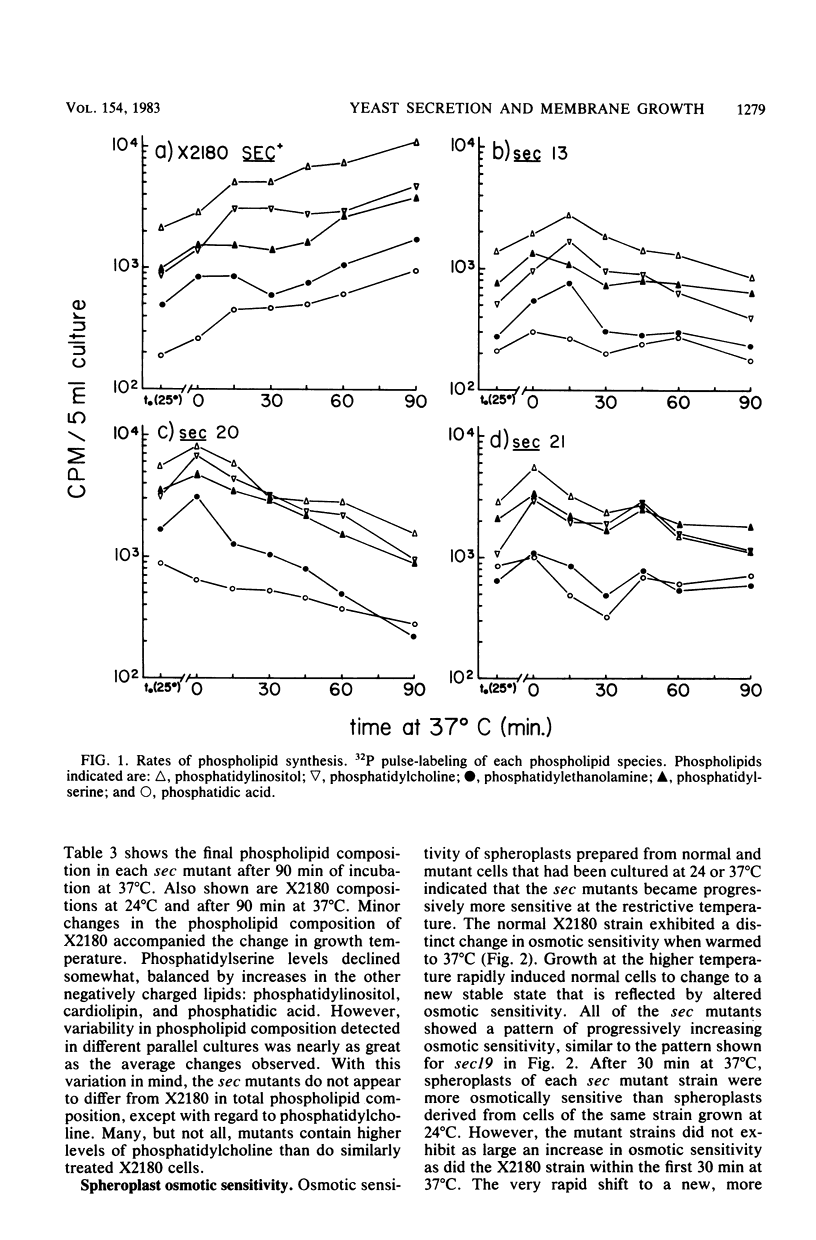
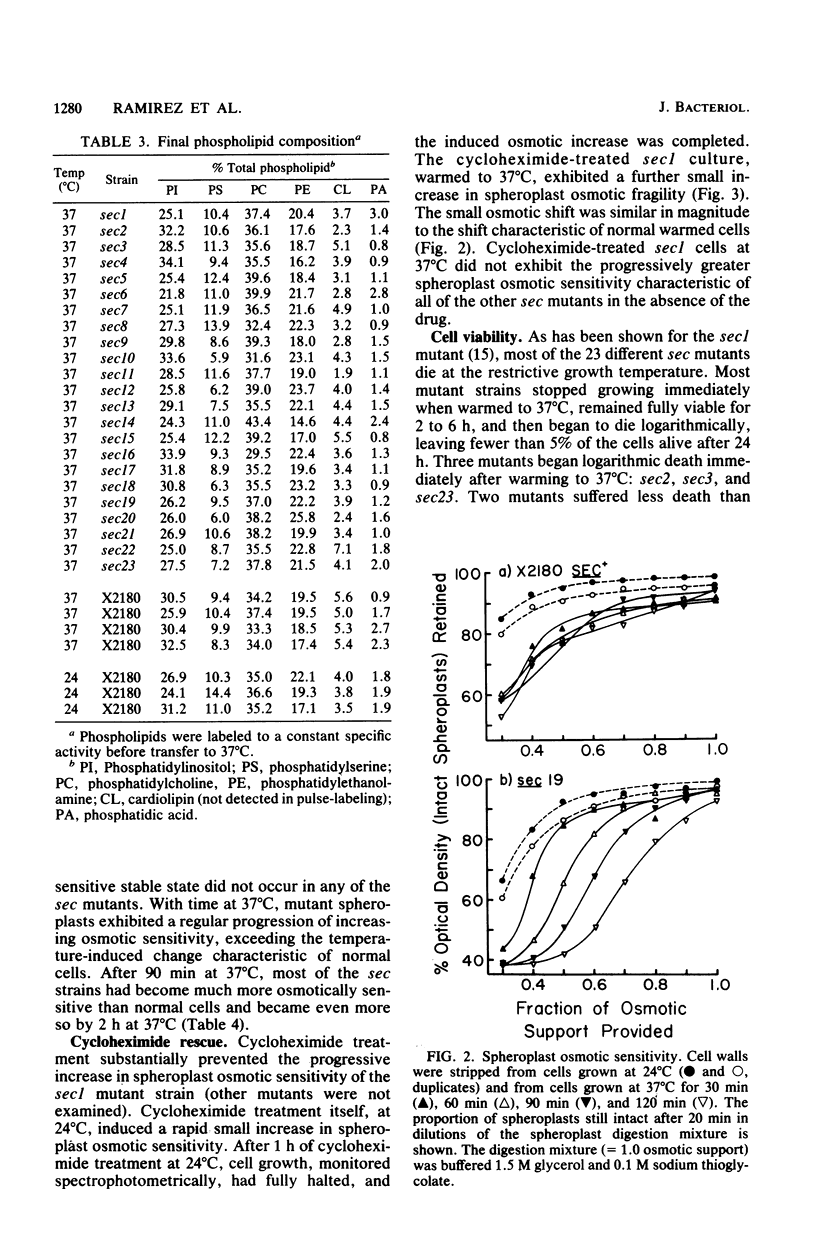
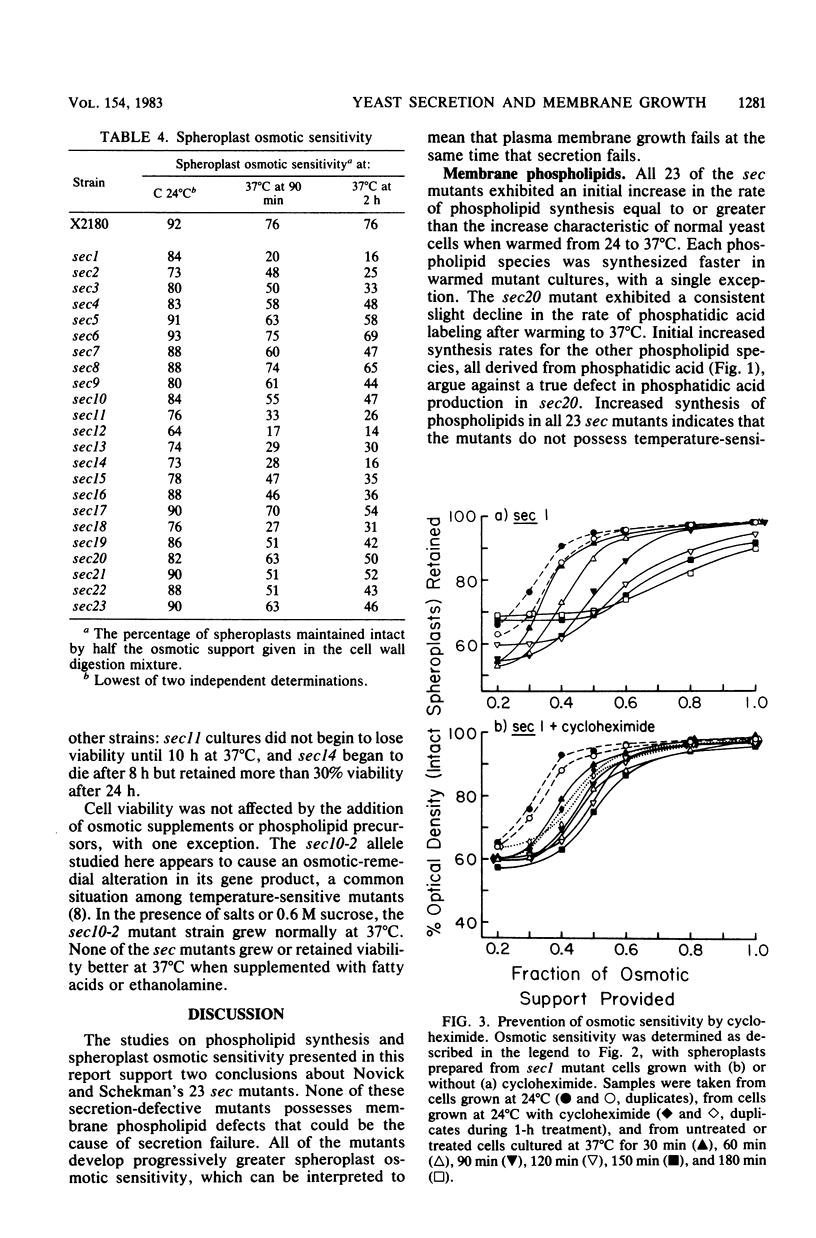
Phospholipid synthesis activity and plasma membrane growth have been studied in the Saccharomyces cerevisiae temperature-sensitive, secretion-defective mutants isolated by Novick and Schekman (Proc. Natl. Acad. Sci. U.S.A. 76:1858-1862, 1979; Novick et al., Cell 21:205-215, 1980). The mutants, sec1 through sec23, do not grow at 37 degrees C and exhibit lower rates of phospholipid synthesis than does the wild-type strain X2180. None of the mutants exhibits a decline in lipid synthesis rapid enough to explain secretion failure. Plasma membrane growth was assessed indirectly by examining the osmotic sensitivity of spheroplasts derived from cultures transferred from 24 to 37 degrees C. Spheroplasts from the normal-growing strain X2180 exhibited a small rapid increase in osmotic sensitivity and stabilized at a more sensitive state. Spheroplasts from the sec mutants exposed to the same temperature shift exhibited progressively increasing osmotic sensitivity. Cycloheximide treatment prevented progressive increases in osmotic fragility. These data are compatible with the hypothesis that plasma membrane expansion is restricted in the sec mutants. During incubation at 37 degrees C, the accumulation of intracellular materials within the no-longer expanding plasma membrane exerts osmotic stress on the membrane, increasing with time. The gene products defective in Novick and Schekman's sec mutants appear to be required for both extracellular protein secretion and plasma membrane growth in yeast cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alterthum F., Rose A. H. Osmotic lysis of sphaeroplasts from Saccharomyces cerevisiae grown anaerobically in media containing different unsaturated fatty acids. J Gen Microbiol. 1973 Aug;77(2):371–382. doi: 10.1099/00221287-77-2-371. [DOI] [PubMed] [Google Scholar]
- Atkinson K. D., Kolat A. I., Henry S. A. Osmotic imbalance in inositol-starved spheroplasts of Saccharomyces cerevisiae. J Bacteriol. 1977 Dec;132(3):806–817. doi: 10.1128/jb.132.3.806-817.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker G. W., Lester R. L. Changes in phospholipids of Saccharomyces cerevisiae associated with inositol-less death. J Biol Chem. 1977 Dec 10;252(23):8684–8691. [PubMed] [Google Scholar]
- Beisson J., Lefort-Tran M., Pouphile M., Rossignol M., Satir B. Genetic analysis of membrane differentiation in Paramecium. Freeze-fracture study of the trichocyst cycle in wild-type and mutant strains. J Cell Biol. 1976 Apr;69(1):126–143. doi: 10.1083/jcb.69.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J., ARMSTRONG W. M. The total intracellular concentration of solutes in yeast and other plant cells and the distensibility of the plant-cell wall. Biochem J. 1961 Dec;81:631–639. doi: 10.1042/bj0810631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. J., Rose A. H. Osmotic properties of spheroplasts from Saccharomyces cerevisiae grown at different temperatures. J Bacteriol. 1970 May;102(2):311–319. doi: 10.1128/jb.102.2.311-319.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HAWTHORNE D. C., FRIIS J. OSMOTIC-REMEDIAL MUTANTS. A NEW CLASSIFICATION FOR NUTRITIONAL MUTANTS IN YEAST. Genetics. 1964 Nov;50:829–839. doi: 10.1093/genetics/50.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Atkinson K. D., Kolat A. I., Culbertson M. R. Growth and metabolism of inositol-starved Saccharomyces cerevisiae. J Bacteriol. 1977 Apr;130(1):472–484. doi: 10.1128/jb.130.1.472-484.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A. Death resulting from fatty acid starvation in yeast. J Bacteriol. 1973 Dec;116(3):1293–1303. doi: 10.1128/jb.116.3.1293-1303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossack J. A., Sharpe V. J., Rose A. H. Stability of the plasma membrane in Saccharomyces cerevisiae enriched with phosphatidylcholine or phosphatidylethanolamine. J Bacteriol. 1977 Feb;129(2):1144–1147. doi: 10.1128/jb.129.2.1144-1147.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts V. A., Dawes I. W. Mutations affecting lipid biosynthesis of Saccharomyces cerevisiae: isolation of ethanolamine auxotrophs [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):976–977. doi: 10.1042/bst0070976. [DOI] [PubMed] [Google Scholar]
- Matt H., Plattner H., Reichel K., Lefort-Tran M., Beisson J. Genetic dissection of the final exocytosis steps in Paramecium tetraurelia cells: trigger analyses. J Cell Sci. 1980 Dec;46:41–60. doi: 10.1242/jcs.46.1.41. [DOI] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Metabolism of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Jan 27;260(1):82–87. doi: 10.1016/0005-2760(72)90076-8. [DOI] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Studies on the diversity of inositol-containing yeast phospholipids: incorporation of 2-deoxyglucose into lipid. J Bacteriol. 1972 Jan;109(1):81–88. doi: 10.1128/jb.109.1.81-88.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



