Abstract
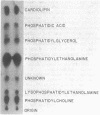
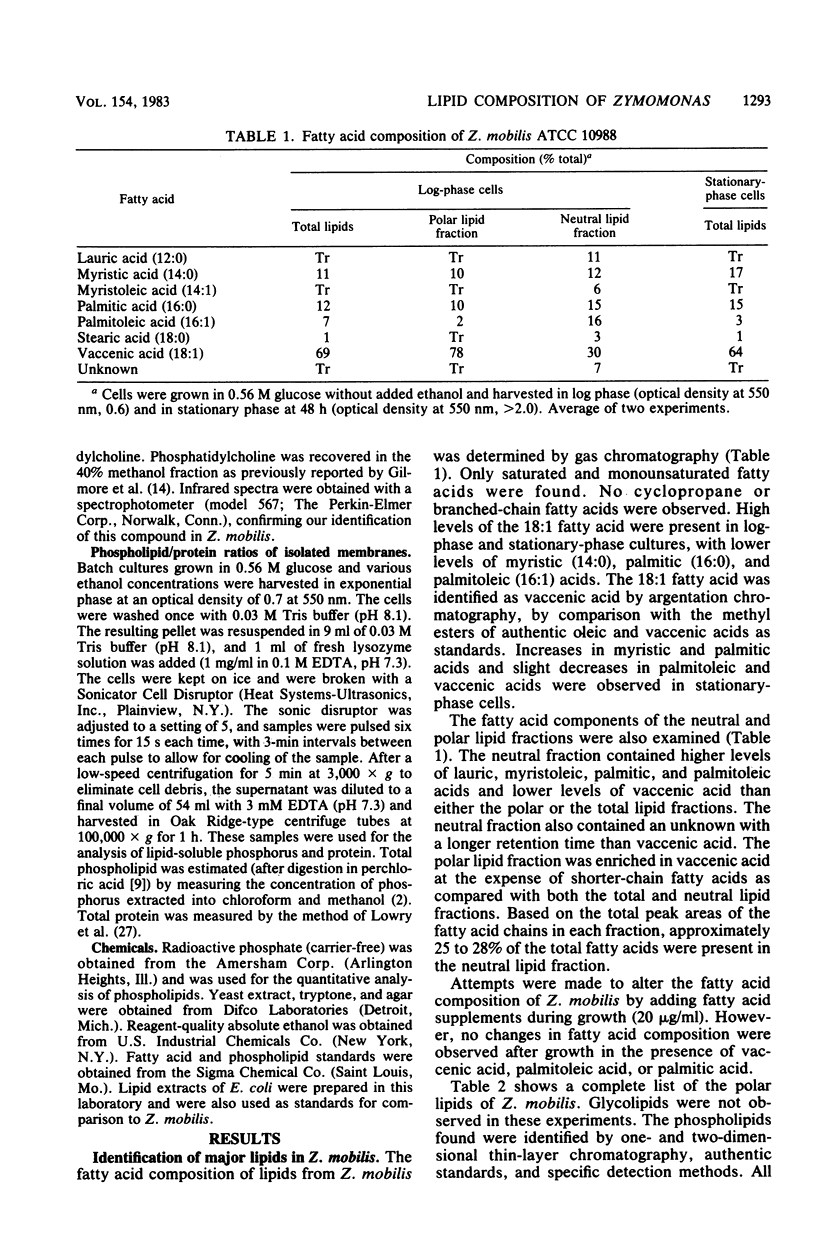
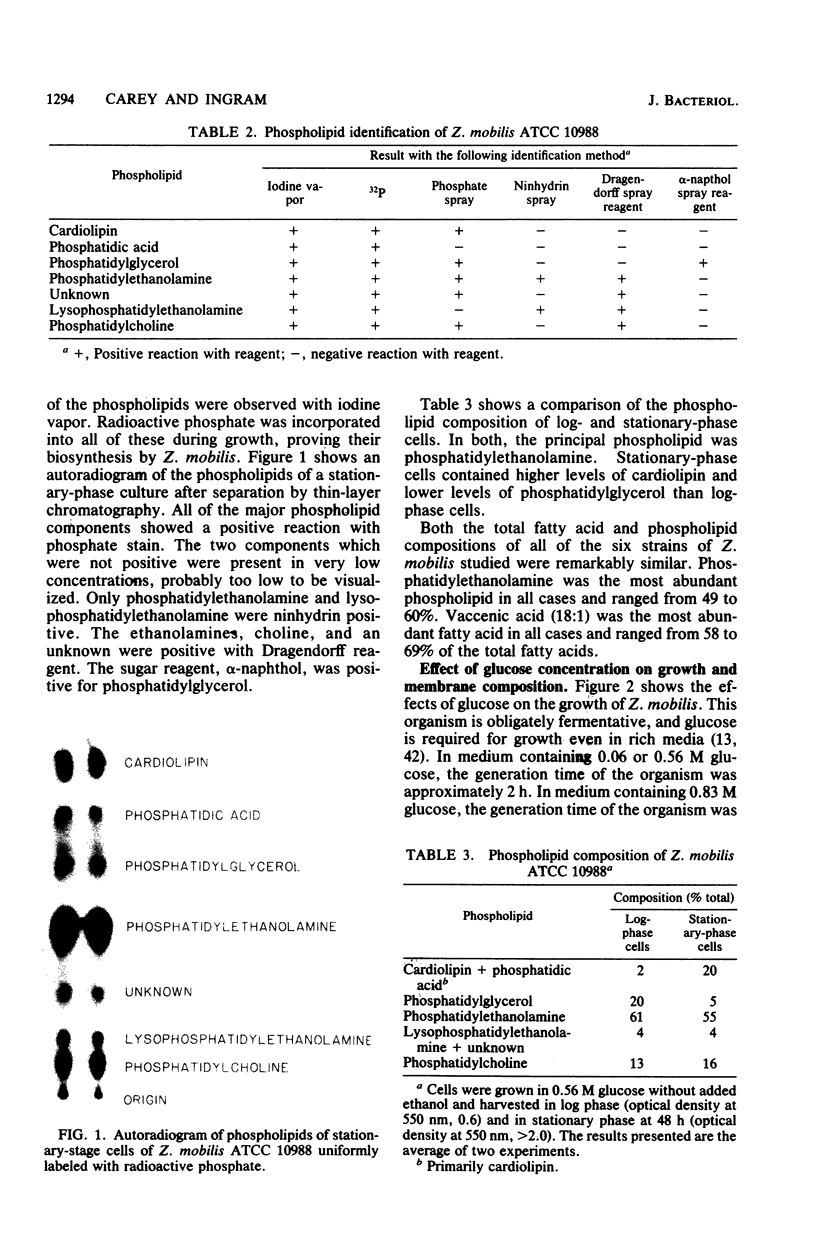
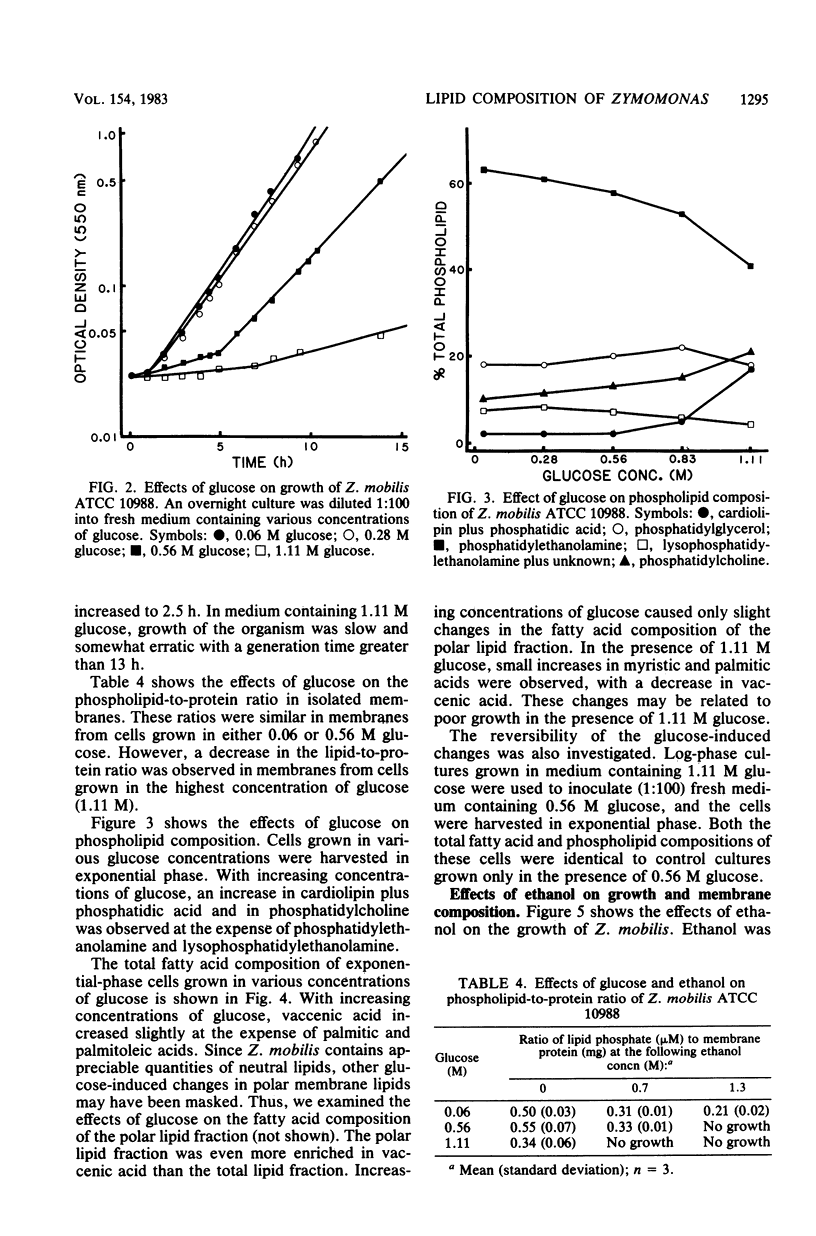
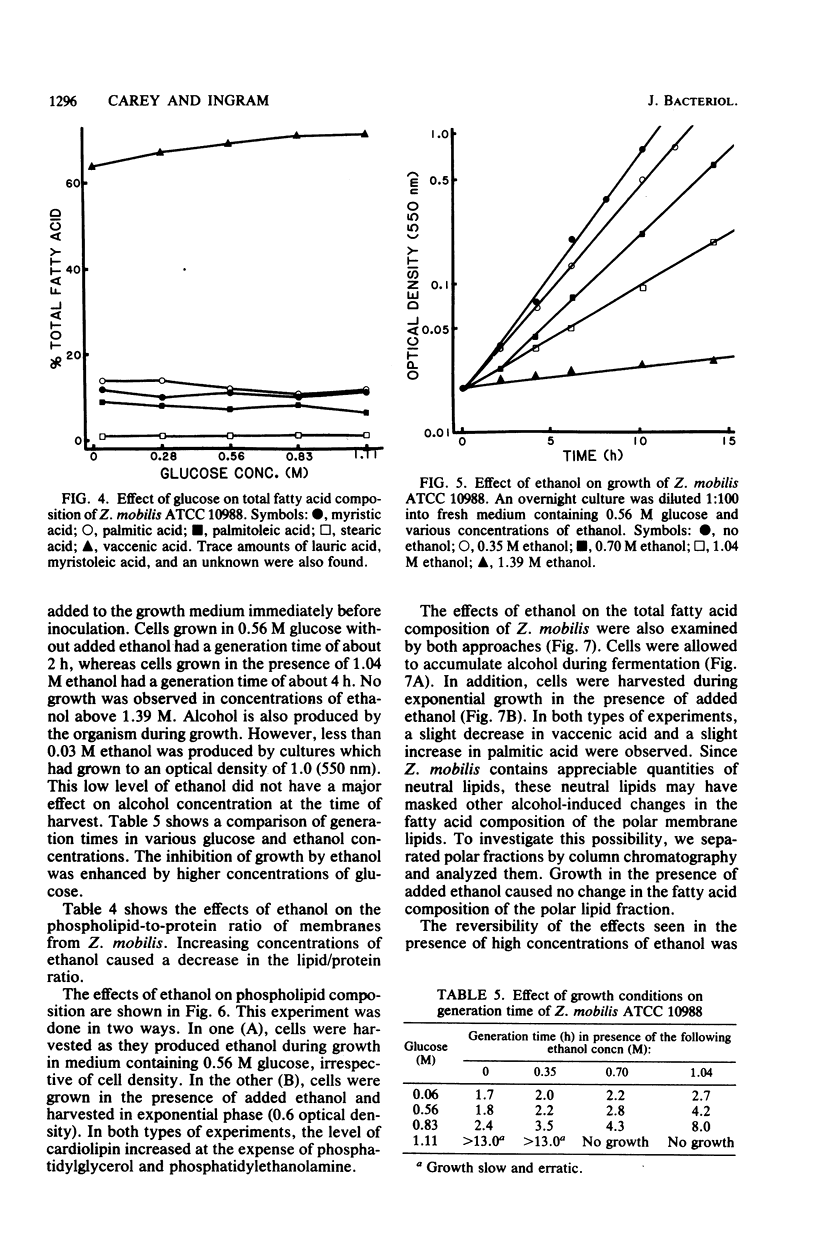
Zymomonas mobilis is an alcohol-tolerant microorganism which is potentially useful for the commercial production of ethanol. This organism was found to contain cardiolipin, phosphatidylethanolamine, phosphatidylglycerol, and phosphatidylcholine as major phospholipids. Vaccenic acid was the most abundant fatty acid, with lesser amounts of myristic, palmitic, and palmitoleic acids. No branched-chain or cyclopropane fatty acids were found. Previous studies in our laboratory have shown that ethanol induces the synthesis of phospholipids enriched in vaccenic acid in Escherichia coli (L. O. Ingram, J. Bacteriol. 125:670-678, 1976). The fatty acid composition of Z. mobilis, an obligately ethanol-producing microorganism, represents an extreme of the trend observed in E. coli. In Z. mobilis, vaccenic acid represents over 75% of the acyl chains in the polar membrane lipids. Glucose and ethanol had no major effect on the fatty acid composition of Z. mobilis. However, both glucose and ethanol caused a decrease in phosphatidylethanolamine and phosphatidylglycerol and an increase in cardiolipin and phosphatidylcholine. Ethanol also caused a dose-dependent reduction in the lipid-to-protein ratios of crude membranes. The lipid composition of Z. mobilis may represent an evolutionary adaptation for survival in the presence of ethanol.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BEISS U. ZUR PAPIERCHROMATOGRAPHISCHEN AUFTRENNUNG VON PFLANZENLIPIDEN. J Chromatogr. 1964 Jan;13:104–110. doi: 10.1016/s0021-9673(01)95079-4. [DOI] [PubMed] [Google Scholar]
- Campbell T. B., Lueking D. R. Long-chain fatty acid assimilation By rhodopseudomonas sphaeroides. J Bacteriol. 1983 Feb;153(2):782–790. doi: 10.1128/jb.153.2.782-790.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M. A hydrolytic procedure for the identification and estimation of individual phospholipids in biological samples. Biochem J. 1960 Apr;75:45–53. doi: 10.1042/bj0750045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes E. A., Ribbons D. W., Large P. J. The route of ethanol formation in Zymomonas mobilis. Biochem J. 1966 Mar;98(3):795–803. doi: 10.1042/bj0980795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMOSS R. D., GIBBS M. Ethanol formation in Pseudomonas lindneri. Arch Biochem Biophys. 1951 Dec;34(2):478–479. doi: 10.1016/0003-9861(51)90028-8. [DOI] [PubMed] [Google Scholar]
- Enequist H. G., Hirst T. R., Harayama S., Hardy S. J., Randall L. L. Energy is required for maturation of exported proteins in Escherichia coli. Eur J Biochem. 1981 May 15;116(2):227–233. doi: 10.1111/j.1432-1033.1981.tb05323.x. [DOI] [PubMed] [Google Scholar]
- GIBBS M., DEMOSS R. D. Anaerobic dissimilation of C14-labeled glucose and fructose by Pseudomonas lindneri. J Biol Chem. 1954 Apr;207(2):689–694. [PubMed] [Google Scholar]
- Gilmore R., Cohn N., Glaser M. Rotational relaxation times of 1,6-diphenyl-1,3,5-hexatriene in phospholipids isolated from LM cell membranes. Effects of phospholipid polar head-group and fatty acid composition. Biochemistry. 1979 Mar 20;18(6):1050–1056. doi: 10.1021/bi00573a018. [DOI] [PubMed] [Google Scholar]
- Ingram L. O. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976 Feb;125(2):670–678. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O., Dickens B. F., Buttke T. M. Reversible effects of ethanol on E. coli. Adv Exp Med Biol. 1980;126:299–337. doi: 10.1007/978-1-4684-3632-7_24. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Eaton L. C., Erdos G. W., Tedder T. F., Vreeland N. L. Unsaturated fatty acid requirement in Escherichia coli: mechanism of palmitate-induced inhibition of growth of strain WN1. J Membr Biol. 1982;65(1-2):31–40. doi: 10.1007/BF01870466. [DOI] [PubMed] [Google Scholar]
- Ingram L. O. Mechanism of lysis of Escherichia coli by ethanol and other chaotropic agents. J Bacteriol. 1981 Apr;146(1):331–336. doi: 10.1128/jb.146.1.331-336.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram L. O. Preferential inhibition of phosphatidyl ethanolamine synthesis in E. coli by alcohols. Can J Microbiol. 1977 Jun;23(6):779–789. doi: 10.1139/m77-115. [DOI] [PubMed] [Google Scholar]
- Ingram L. O., Vreeland N. S., Eaton L. C. Alcohol tolerance in Escherichia coli. Pharmacol Biochem Behav. 1980;13 (Suppl 1):191–195. doi: 10.1016/s0091-3057(80)80030-x. [DOI] [PubMed] [Google Scholar]
- Issenberg P., Hornstein I. Analysis of volatile flavor components of foods. Adv Chromatogr. 1970;9:295–340. [PubMed] [Google Scholar]
- KANFER J., KENNEDY E. P. METABOLISM AND FUNCTION OF BACTERIAL LIPIDS. I. METABOLISM OF PHOSPHOLIPIDS IN ESCHERICHIA COLI B. J Biol Chem. 1963 Sep;238:2919–2922. [PubMed] [Google Scholar]
- KATES M., KUSHNER D. J., JAMES A. T. The lipid composition of Bacillus cereus as influenced by the presence of alcohols in the culture medium. Can J Biochem Physiol. 1962 Jan;40:83–94. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nandini-Kishore S. G., Mattox S. M., Martin C. E., Thompson G. A., Jr Membrane changes during growth of Tetrahymena in the presence of ethanol. Biochim Biophys Acta. 1979 Mar 8;551(2):315–327. doi: 10.1016/0005-2736(89)90009-6. [DOI] [PubMed] [Google Scholar]
- Rigomier D., Bohin J. P., Lubochinsky B. Effects of ethanol and methanol on lipid metabolism in Bacillus subtilis. J Gen Microbiol. 1980 Nov;121(1):139–149. doi: 10.1099/00221287-121-1-139. [DOI] [PubMed] [Google Scholar]
- Rose A. H., Beavan M. J. End-product tolerance and ethanol. Basic Life Sci. 1981;18:513–531. doi: 10.1007/978-1-4684-3980-9_31. [DOI] [PubMed] [Google Scholar]
- Ruiz-Argueso T., Rodriguez-Navarro A. Microbiology of ripening honey. Appl Microbiol. 1975 Dec;30(6):893–896. doi: 10.1128/am.30.6.893-896.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Siakotos A. N. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J Am Oil Chem Soc. 1965 Nov;42(11):913–919. doi: 10.1007/BF02632444. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ladenson R. C., Honegger J. L. The unsaturated fatty acid requirement in Escherichia coli. Temperature dependence and total replacement by branched-chain fatty acids. Biochim Biophys Acta. 1973 Jul 6;311(3):349–361. doi: 10.1016/0005-2736(73)90315-5. [DOI] [PubMed] [Google Scholar]
- Skotnicki M. L., Lee K. J., Tribe D. E., Rogers P. L. Comparison of ethanol production by different zymomonas strains. Appl Environ Microbiol. 1981 Apr;41(4):889–893. doi: 10.1128/aem.41.4.889-893.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swings J., De Ley J. The biology of Zymomonas. Bacteriol Rev. 1977 Mar;41(1):1–46. doi: 10.1128/br.41.1.1-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K. Occurrence of saturated and mono-unsaturated fatty acids with unusually-long-chains (C20-C30) in Lactobacillus heterohiochii, an alcoholophilic bacterium. Biochim Biophys Acta. 1974 Apr 26;348(1):86–93. [PubMed] [Google Scholar]
- Vaskovsky V. E., Kostetsky E. Y., Vasendin I. M. A universal reagent for phospholipid analysis. J Chromatogr. 1975 Nov 12;114(1):129–141. doi: 10.1016/s0021-9673(00)85249-8. [DOI] [PubMed] [Google Scholar]