Abstract
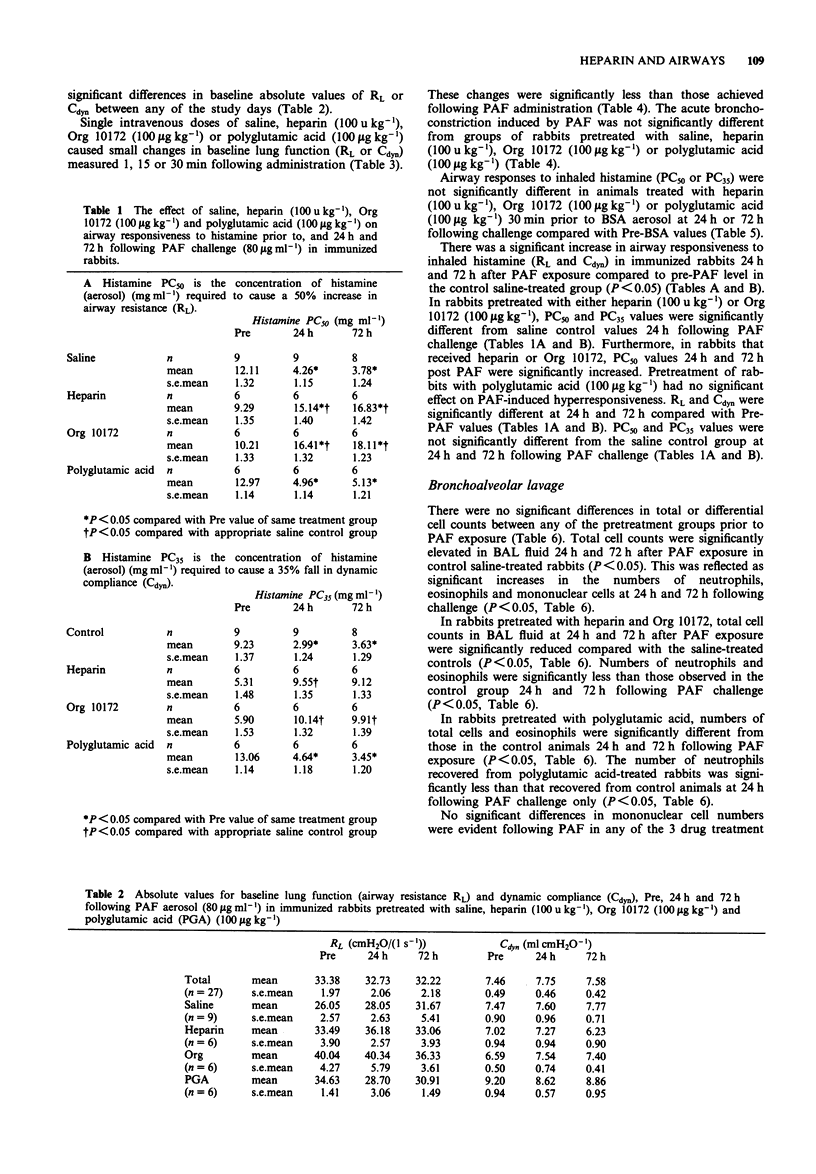
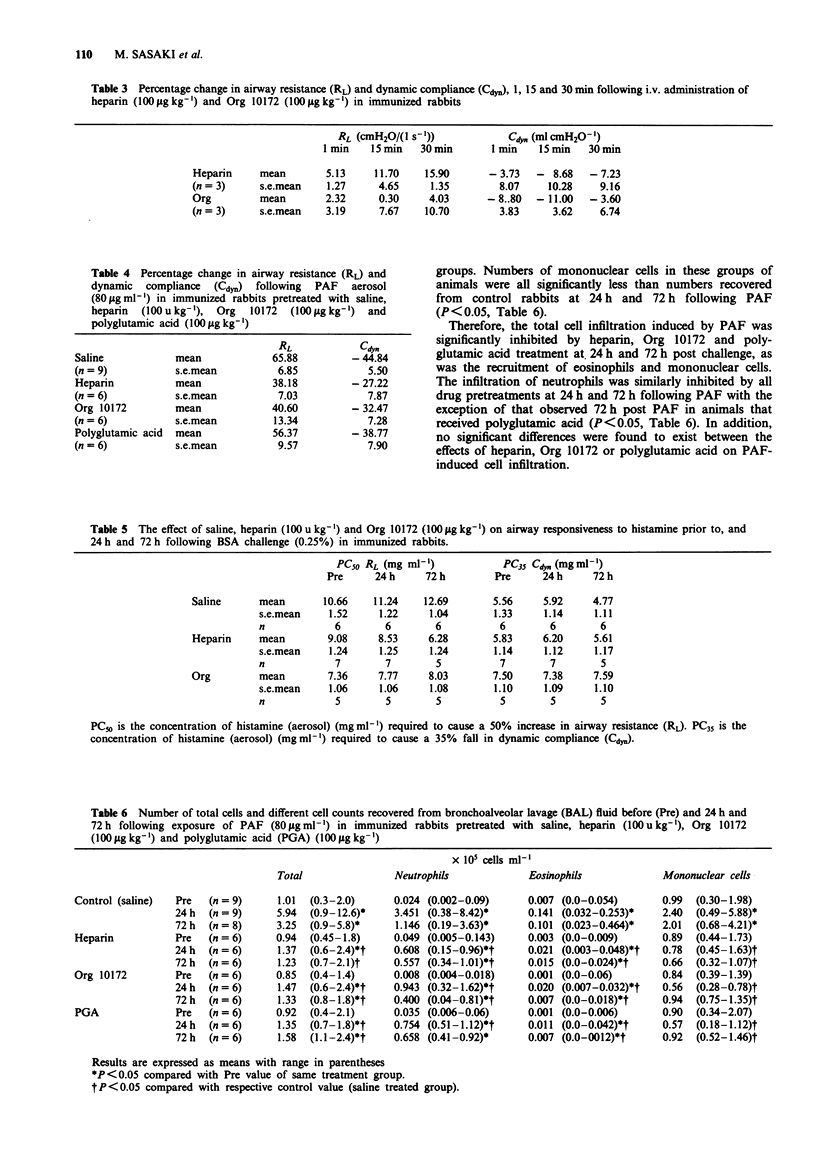
1. We have investigated the effect of an unfractionated heparin preparation, a low-molecular weight heparinoid (Org 10172) and the polyanionic molecule polyglutamic acid against PAF-induced airway hyperresponsiveness and pulmonary cell infiltration in neonatally immunized rabbits in vivo. 2. Exposure of neonatally immunized rabbits to aerosolized platelet activating factor (PAF) (80 micrograms ml-1 for 60 min) elicited an increase in airway responsiveness to inhaled histamine 24 h and 72 h following challenge which was associated with an infiltration of inflammatory cells into the airways, as assessed by bronchoalveolar lavage (BAL). 3. A significant increase in the total numbers of cells recovered from BAL fluid was associated with significantly increased cell numbers of neutrophils, eosinophils and mononuclear cells 24 h following PAF exposure. The numbers of eosinophils and neutrophils in the airways remained elevated 72 h after challenge. 4. The intravenous administration of an unfractionated preparation of heparin (100 units kg-1) or Org 10172 (100 micrograms kg-1) 30 min prior to PAF exposure significantly inhibited the airway hyperresponsiveness induced by PAF, 24 h and 72 h following challenge. PAF-induced hyperresponsiveness was not significantly affected by prior intravenous administration of polyglutamic acid (100 micrograms kg-1). 5. The intravenous administration of unfractionated heparin (100 units kg-1), Org 10172 (100 micrograms kg-1) or polyglutamic acid (100 micrograms kg-1) 30 min prior to PAF exposure significantly inhibited the expected increase in total cell infiltration. 6. This study shows that unfractionated heparin and a low-molecular weight heparinoid, Org 10172, are capable of inhibiting both the airway hyperresponsiveness and pulmonary cell infiltration induced by PAF in the rabbit.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed T., Abraham W. M., D'Brot J. Effects of inhaled heparin on immunologic and nonimmunologic bronchoconstrictor responses in sheep. Am Rev Respir Dis. 1992 Mar;145(3):566–570. doi: 10.1164/ajrccm/145.3.566. [DOI] [PubMed] [Google Scholar]
- Antunes E., Mariano M., Cirino G., Levi S., de Nucci G. Pharmacological characterization of polycation-induced rat hind-paw oedema. Br J Pharmacol. 1990 Dec;101(4):986–990. doi: 10.1111/j.1476-5381.1990.tb14193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averill F. J., Hubbard W. C., Proud D., Gleich G. J., Liu M. C. Platelet activation in the lung after antigen challenge in a model of allergic asthma. Am Rev Respir Dis. 1992 Mar;145(3):571–576. doi: 10.1164/ajrccm/145.3.571. [DOI] [PubMed] [Google Scholar]
- BOYLE J. P., SMART R. H., SHIREY J. K. HEPARIN IN THE TREATMENT OF CHRONIC OBSTRUCTIVE BRONCHOPULMONARY DISEASE. Am J Cardiol. 1964 Jul;14:25–28. doi: 10.1016/0002-9149(64)90100-6. [DOI] [PubMed] [Google Scholar]
- Beresford I. J., Birch P. J., Hagan R. M., Ireland S. J. Investigation into species variants in tachykinin NK1 receptors by use of the non-peptide antagonist, CP-96,345. Br J Pharmacol. 1991 Oct;104(2):292–293. doi: 10.1111/j.1476-5381.1991.tb12423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock P. E., Luscombe M., Marshall S. E., Pepper D. S., Holbrook J. J. The multiple complexes formed by the interaction of platelet factor 4 with heparin. Biochem J. 1980 Dec 1;191(3):769–776. doi: 10.1042/bj1910769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler S. D., Smith S. M., Lavercombe P. S. Heparin inhibits the immediate response to antigen in the skin and lungs of allergic subjects. Am Rev Respir Dis. 1993 Jan;147(1):160–163. doi: 10.1164/ajrccm/147.1.160. [DOI] [PubMed] [Google Scholar]
- Bradbrook I. D., Magnani H. N., Moelker H. C., Morrison P. J., Robinson J., Rogers H. J., Spector R. G., Van Dinther T., Wijnand H. ORG 10172: a low molecular weight heparinoid anticoagulant with a long half-life in man. Br J Clin Pharmacol. 1987 Jun;23(6):667–675. doi: 10.1111/j.1365-2125.1987.tb03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. The anti-inflammatory action of heparin: heparin as an antagonist to histamine, bradykinin and prostaglandin E1. Thromb Res. 1979;16(3-4):507–516. doi: 10.1016/0049-3848(79)90097-5. [DOI] [PubMed] [Google Scholar]
- Corrigan C. J., Hartnell A., Kay A. B. T lymphocyte activation in acute severe asthma. Lancet. 1988 May 21;1(8595):1129–1132. doi: 10.1016/s0140-6736(88)91951-4. [DOI] [PubMed] [Google Scholar]
- Coyle A. J., Spina D., Page C. P. PAF-induced bronchial hyperresponsiveness in the rabbit: contribution of platelets and airway smooth muscle. Br J Pharmacol. 1990 Sep;101(1):31–38. doi: 10.1111/j.1476-5381.1990.tb12084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHL S. [Heparin and "asthma" (A preliminary 1st report)]. Z Tuberk Erkr Thoraxorg. 1962 Jul;118:255–262. [PubMed] [Google Scholar]
- DOLOWITZ D. A., DOUGHERTY T. F. The use of heparin as an anti-inflammatory agent. Laryngoscope. 1960 Jul;70:873–884. [PubMed] [Google Scholar]
- Ekre H. P., Fjellner B., Hägermark O. Inhibition of complement dependent experimental inflammation in human skin by different heparin fractions. Int J Immunopharmacol. 1986;8(3):277–286. doi: 10.1016/0192-0561(86)90109-8. [DOI] [PubMed] [Google Scholar]
- Frieri M., Metcalfe D. D. Analysis of the effect of mast cell granules on lymphocyte blastogenesis in the absence and presence of mitogens: identification of heparin as a granule-associated suppressor factor. J Immunol. 1983 Oct;131(4):1942–1948. [PubMed] [Google Scholar]
- Frigas E., Gleich G. J. The eosinophil and the pathophysiology of asthma. J Allergy Clin Immunol. 1986 Apr;77(4):527–537. doi: 10.1016/0091-6749(86)90341-6. [DOI] [PubMed] [Google Scholar]
- Gibson P. G., Dolovich J., Denburg J., Ramsdale E. H., Hargreave F. E. Chronic cough: eosinophilic bronchitis without asthma. Lancet. 1989 Jun 17;1(8651):1346–1348. doi: 10.1016/s0140-6736(89)92801-8. [DOI] [PubMed] [Google Scholar]
- Herd C. M., Donigi-Gale D., Shoupe T. S., Page C. P. Effect of a 5-lipoxygenase inhibitor and leukotriene antagonist (PF 5901) on PAF-induced airway responses in neonatally immunized rabbits. Br J Pharmacol. 1992 Dec;107(4):1108–1115. doi: 10.1111/j.1476-5381.1992.tb13415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyoshi Y., Dörschner A., Mallet A. I., Christophers E., Schröder J. M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992 Aug 1;176(2):587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B. Lymphocytes in asthma. Respir Med. 1991 Mar;85(2):87–90. doi: 10.1016/s0954-6111(06)80283-0. [DOI] [PubMed] [Google Scholar]
- Kazatchkine M. D., Fearon D. T., Metcalfe D. D., Rosenberg R. D., Austen K. F. Structural determinants of the capacity of heparin to inhibit the formation of the human amplification C3 convertase. J Clin Invest. 1981 Jan;67(1):223–228. doi: 10.1172/JCI110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kings M. A., Chapman I., Kristersson A., Sanjar S., Morley J. Human recombinant lymphokines and cytokines induce pulmonary eosinophilia in the guinea pig which is inhibited by ketotifen and AH 21-132. Int Arch Allergy Appl Immunol. 1990;91(4):354–361. doi: 10.1159/000235141. [DOI] [PubMed] [Google Scholar]
- Knauer K. A., Lichtenstein L. M., Adkinson N. F., Jr, Fish J. E. Platelet activation during antigen-induced airway reactions in asthmatic subjects. N Engl J Med. 1981 Jun 4;304(23):1404–1407. doi: 10.1056/NEJM198106043042307. [DOI] [PubMed] [Google Scholar]
- Lasser E. C., Simon R. A., Lyon S. G., Hamblin A. E., Stein R. Heparin-like anticoagulants in asthma. Allergy. 1987 Nov;42(8):619–625. doi: 10.1111/j.1398-9995.1987.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Lider O., Mekori Y. A., Miller T., Bar-Tana R., Vlodavsky I., Baharav E., Cohen I. R., Naparstek Y. Inhibition of T lymphocyte heparanase by heparin prevents T cell migration and T cell-mediated immunity. Eur J Immunol. 1990 Mar;20(3):493–499. doi: 10.1002/eji.1830200306. [DOI] [PubMed] [Google Scholar]
- Lundgren R., Söderberg M., Hörstedt P., Stenling R. Morphological studies of bronchial mucosal biopsies from asthmatics before and after ten years of treatment with inhaled steroids. Eur Respir J. 1988 Dec;1(10):883–889. [PubMed] [Google Scholar]
- Matsuse T., Thomson R. J., Chen X. R., Salari H., Schellenberg R. R. Capsaicin inhibits airway hyperresponsiveness but not lipoxygenase activity or eosinophilia after repeated aerosolized antigen in guinea pigs. Am Rev Respir Dis. 1991 Aug;144(2):368–372. doi: 10.1164/ajrccm/144.2.368. [DOI] [PubMed] [Google Scholar]
- Matzner Y., Marx G., Drexler R., Eldor A. The inhibitory effect of heparin and related glycosaminoglycans on neutrophil chemotaxis. Thromb Haemost. 1984 Oct 31;52(2):134–137. [PubMed] [Google Scholar]
- McManus L. M., Morley C. A., Levine S. P., Pinckard R. N. Platelet activating factor (PAF) induced release of platelet factor 4 (PF4) in vitro and during IgE anaphylaxis in the rabbit. J Immunol. 1979 Dec;123(6):2835–2841. [PubMed] [Google Scholar]
- Motojima S., Frigas E., Loegering D. A., Gleich G. J. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am Rev Respir Dis. 1989 Mar;139(3):801–805. doi: 10.1164/ajrccm/139.3.801. [DOI] [PubMed] [Google Scholar]
- Needham L., Hellewell P. G., Williams T. J., Gordon J. L. Endothelial functional responses and increased vascular permeability induced by polycations. Lab Invest. 1988 Oct;59(4):538–548. [PubMed] [Google Scholar]
- Page C. P. The role of platelet-activating factor in asthma. J Allergy Clin Immunol. 1988 Jan;81(1):144–152. doi: 10.1016/0091-6749(88)90233-3. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjar S., Aoki S., Kristersson A., Smith D., Morley J. Antigen challenge induces pulmonary airway eosinophil accumulation and airway hyperreactivity in sensitized guinea-pigs: the effect of anti-asthma drugs. Br J Pharmacol. 1990 Apr;99(4):679–686. doi: 10.1111/j.1476-5381.1990.tb12989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spina D., McKenniff M. G., Coyle A. J., Seeds E. A., Tramontana M., Perretti F., Manzini S., Page C. P. Effect of capsaicin on PAF-induced bronchial hyperresponsiveness and pulmonary cell accumulation in the rabbit. Br J Pharmacol. 1991 May;103(1):1268–1274. doi: 10.1111/j.1476-5381.1991.tb12335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Schneeberger E., McCluskey R., Greene M. I., Rosenberg R. D., Benacerraf B. Inhibition of delayed-type hypersensitivity by heparin depleted of anticoagulant activity. Cell Immunol. 1983 Nov;82(1):23–32. doi: 10.1016/0008-8749(83)90137-5. [DOI] [PubMed] [Google Scholar]
- Willenborg D. O., Parish C. R. Inhibition of allergic encephalomyelitis in rats by treatment with sulfated polysaccharides. J Immunol. 1988 May 15;140(10):3401–3405. [PubMed] [Google Scholar]


