Abstract
Summary: Human infection with Fusobacterium necrophorum usually involves F. necrophorum subsp. funduliforme rather than F. necrophorum subsp. necrophorum, which is a common pathogen in animals. Lemierre's syndrome, or postanginal sepsis, is the most common life-threatening manifestation. Tonsillitis is followed by septic thrombophlebitis of the internal jugular vein and then a septicemia with septic emboli in lungs and other sites. Recent evidence suggests that F. necrophorum can be limited to the throat and cause persistent or recurrent tonsillitis. F. necrophorum is unique among non-spore-forming anaerobes, first for its virulence and association with Lemierre's syndrome as a monomicrobial infection and second because it seems probable that it is an exogenously acquired infection. The source of infection is unclear; suggestions include acquisition from animals or human-to-human transmission. Approximately 10% of published cases are associated with infectious mononucleosis, which may facilitate invasion. Recent work suggests that underlying thrombophilia may predispose to internal jugular vein thrombophlebitis. Lemierre's syndrome was relatively common in the preantibiotic era but seemed to virtually disappear with widespread use of antibiotics for upper respiratory tract infection. In the last 15 years there has been a rise in incidence, possibly related to restriction in antibiotic use for sore throat.
INTRODUCTION
Among the countless thousands of cases of human infection with non-spore-forming anaerobes, Fusobacterium necrophorum constitutes a tiny proportion (fewer than 1% of bacteremias), with only a few hundred case reports in the literature. However, it is arguably unique among the non-spore-forming anaerobes for its very strong association with clinically distinctive, severe septicemic infections variously known as necrobacillosis (12), postanginal sepsis (3, 103), or Lemierre's syndrome (391, 340).
This review focuses principally on the pathogenesis, natural history, epidemiology, clinical features, diagnosis, and management of these infections. The emphasis is on F. necrophorum infection originating from the oropharynx rather than other sites such as the gut or genital tract. A review of published cases is included. The review seeks to answer questions such as the source of infection, whether F. necrophorum is an essential requisite for Lemierre's syndrome, or whether other bacteria can cause the condition in the absence of F. necrophorum. In the face of widely differing inclusion criteria in different studies, a suggested case definition is provided. Recent developments such as use of molecular techniques and the possible role of F. necrophorum in other, non-life-threatening infections are highlighted.
HISTORICAL REVIEW
F. necrophorum is a much more common and important pathogen in animals than in humans. It is therefore not surprising that it was first described in animals. Loeffler (248) was the first to recognize the organism as the cause of an infection, namely, calf diphtheria, in 1884. He identified a thin gram-negative rod with filamentous forms at the border between the sound and necrotic tissues in stained sections of diphtheritic material. On injecting this material into mice, he generated foul-smelling purulent lesions containing similar bacilli. He isolated the organism in a calf serum broth but could not further subculture the bacterium. The organism was named Bacillus necrophorus by Flugge (146). Bang (22) was the first worker to isolate the organism in culture from liver abscess of cattle. Bang also identified the organism in hog cholera (23) and was the first to coin the term necrobacillosis when he referred to “nekrosebazillus”.
The first human infections of reasonable provenance were described by Schmorl in 1891 (332) and involved an animal strain. The patients consisted of Schmorl and one of his assistants. They had been working on rabbits with necrobacillosis, and both developed abscesses on the fingers. Stained smears of the pus showed the characteristic filamentous gram-negative bacilli that Schmorl had previously described.
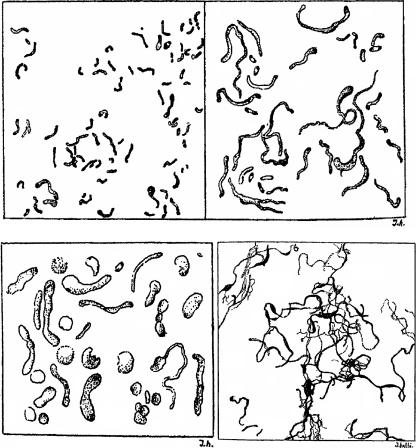
The first description of the isolation from a human of what we now call F. necrophorum subsp. funduliforme was made by Jean Hallé in 1898 as part of a Ph.D. thesis on the bacteriology of the female genital tract (182). He called the organism Bacillus funduliformis as a descriptive term of the Gram stain morphology, from the Latin fundula, because he felt that some of the bacilli resembled boudins (black puddings or blood sausages). His line drawings of the microscopic appearance of the organism under different conditions remain a valuable aid today (Fig. 1). Lemierre et al. (243) credited Veillon and Züber (386) with the first description of human systemic infection with F. necrophorum. This involved a young child with chronic purulent otitis presenting with septic arthritis of the knee, cerebral abscess, and signs of overwhelming systemic infection. The organism isolated from the knee was consistent with F. necrophorum.
FIG. 1.
Line drawings of Bacillus funduliformis from a thesis by Jean Hallé (182). Top left, common short, slightly curved rods seen in pus and tissues; top right, rare giant forms; bottom left, poorly staining vesicular forms sometimes seen in culture; bottom right, bizarre pleomorphic forms commonly seen in culture. Drawings are not on the same scale.
Most reviewers now regard the paper by Courmont and Cade in 1900 (98) as the first description of Lemierre's syndrome, i.e., a human postanginal septicemic infection with F. necrophorum. The patient complained of cough and sore throat, and a few days later, when the throat had settled, had sudden onset of rigors and progressed to an overwhelming sepsis, likened to plague because of the presence of a large abscess in the supraclavicular fossa. The autopsy showed multiple lung abscesses which were thought to be septic embolic infarcts. Courmont and Cade were able to demonstrate bacilli in pus and to isolate anaerobic gram-negative bacilli, with which they subsequently successfully infected animals. The description of the colonies forming in liquid culture and the Gram stain morphology of the bacilli is entirely consistent with F. necrophorum. Among the postmortem findings there is no mention of a detailed examination of the neck, so it is unclear whether the patient had internal jugular vein thrombophlebitis.
According to Abt (3), the first recognized case of postanginal sepsis with internal jugular venous thrombophlebitis was described by Long in 1912 (250). This patient suffered from tonsillitis. On day 4 chills and irregular fever developed. Several days later the patient was blind in the left eye due to vitreous hemorrhage. Long explained this by cavernous sinus thrombosis which had extended from the internal jugular vein through the inferior petrosal sinus. The internal jugular vein and linked affected vessels were dissected out, ligated, and excised. Numerous thrombi containing pus and streptococci were found.
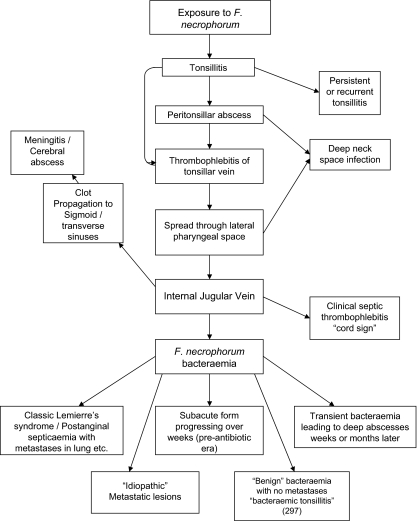
Lemierre (239) credited Schottmüller (333) with highlighting the importance of postanginal sepsis and its association with what he called Bacillus symbiophiles. Both Abt (3) and Lemierre et al. (243) credit Fränkel (149) with recognizing the importance of the progression from thrombophlebitis of the tonsillar veins to internal jugular vein thrombophlebitis with subsequent septicemia due to release of small pieces of infected thrombus. These septic emboli may produce infarcts in the lungs with associated pleurisy and empyema, or they may pass into the general circulation to involve joints, muscles, kidneys, spleen, and liver.
Lemierre 1938 (243) highlighted that septic thrombophlebitis was also a feature of F. necrophorum infection at other sites. They quoted cases reported by many authors, including otogenic infection, iliac vein thrombophlebitis associated with postprostatectomy infection, portal vein thrombophlebitis associated with infection secondary to rectal carcinoma, and mesenteric thrombophlebitis after appendectomy. The ability to stimulate clot formation and multiply in the clot with subsequent embolic spread is clearly a fundamental feature of the pathogenesis of F. necrophorum infection.
Despite these excellent clinicopathological descriptions, there was considerable confusion about the causative organism, partly related to duplication of names for F. necrophorum but also due to misattribution of a causal role to organisms such as streptococci (3, 180, 250), probably because of inadequate anaerobic culture methods. Workers at the Hospital Claude Bernard in Paris came to the fore because of their success in isolating F. necrophorum. Lemierre et al. (243) paid tribute to the expertise of Reilly in relation to anaerobic culture. This was crucial in demonstrating the key role of F. necrophorum in postanginal septicemia. Two papers by Teissier et al. (377, 378) and a thesis by Pham (304), highlighted the preeminent role of Bacillus funduliformis in postanginal septicemia.
Thus, the role of André Lemierre was neither to discover the organism now known as Fusobacterium necrophorum, nor to provide the first descriptions of postanginal septicemia, nor indeed to link the organism to the clinical condition. Indeed, in his first paper on this condition he appears to have misidentified the organism as B. fragilis (241). Lemierre made it clear that, contrary to some previous reports (e.g., reference 250), in which organisms other than anaerobes had been deemed to be the cause, postanginal septicemia was principally due to anaerobic gram-negative bacilli. He also highlighted the confusion of nomenclature, with German authors referring to Bacillus symbiophiles (333) and French workers to Bacillus funduliformis (182), and suggested that these might be a single organism on the basis of the properties of the organisms and the clinical descriptions of the associated cases.
However, Lemierre's greatest contribution was the commendable clarity of his clinical description of the postanginal septicemia associated with what we now know as F. necrophorum and the much-quoted comment on the ease of clinical diagnosis to those who know the condition. “To anyone instructed as to the nature of these septicemias it becomes relatively easy to make a diagnosis on the simple clinical findings. The appearance and repetition several days after the onset of a sore throat (and particularly of a tonsillar abscess) of severe pyrexial attacks with an initial rigor or still more certainly the occurrence of pulmonary infarcts and arthritic manifestations, constitute a syndrome so characteristic that mistake is almost impossible” (239).
According to Brocard and Guibé (54), Lemierre also made important observations on the natural history of F. necrophorum septicemia and in particular the fact that in addition to the acute, fulminant form which he described so graphically (239), other patients had transient postanginal bacteremia but then presented months or weeks later with an abscess such as in the liver or joint. Finally, Lemierre identified a benign form in which a bacteremia could be detected in association with a rigor and spike of fever but which did not lead to any suppurative consequences. According to Justin-Besançon 1956 (214), Lemierre also observed the dramatic improvement in prognosis of postanginal septicemia following the introduction of antimicrobial therapy.
Credit also needs to be given to Alston (11) for collating a remarkable number of cases of F. necrophorum infections under the epithet human necrobacillosis and for further highlighting the importance of this organism in causing postanginal septicemia.
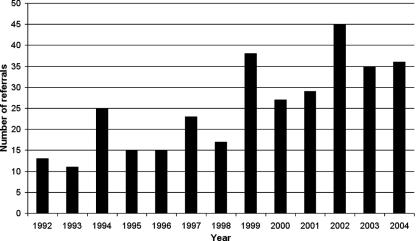
Thus, by the dawn of the antibiotic era, the key elements of postanginal septicemia or Lemierre's syndrome (occurrence after a tonsillitis, progression via thrombophlebitis of tonsillar veins to the internal jugular vein and thence to septicemia and metastatic abscesses, and causally associated with F. necrophorum) had all been established. With the ensuing decline in the condition, this experience was then virtually forgotten for 30 years, to be rediscovered with the realization that this striking condition still existed and indeed was undergoing a resurgence (52, 136, 177, 245, 247, 253, 312).
Biographical Note
André Lemierre (1875 to 1956) (Fig. 2) was a physician and professor of microbiology and infectious diseases at the Hospital Claude Bernard in Paris. It is clear that Lemierre was first and foremost a clinician of limitless energy and enthusiasm, with brilliant clinical abilities to uncover obscure diagnoses at the bedside, simply from careful history taking and clinical examination (214). At the same time he was totally at home in the mortuary, where he would personally conduct autopsies on his own patients, and was equally versed in a wide range of laboratory skills. This holistic approach to medicine, pathology, and laboratory medicine was the basis of a whole series of original observations and discoveries. In the field of microbiology, he had a particular interest in blood cultures and was responsible for demonstrating the diagnostic value of blood cultures in a wide range of conditions from typhoid to Streptobacillus moniliformis and pneumococcal infections. Although the only paper by Lemierre relating to infection with F. necrophorum that is regularly cited in current literature is the address to the Middlesex Hospital Medical School (239), he wrote several other papers, rarely quoted, on this subject (240, 242, 243). Lemierre's status among the French medical hierarchy was indicated by the fact that during World War II, he was chosen to undertake a politically sensitive investigation of outbreaks of louse-borne typhus among French prisoners of war incarcerated in Germany (121). He continued to work and publish throughout his life. Apparently he was still attending ward rounds at the Claude Bernard hospital until his death and would astound colleagues not only by his razor-sharp clinical skills but also by his ability to recall, and describe in minute detail, patients he had seen more than 50 years previously (214). A street in Paris is named in his honor.
FIG. 2.
André Lemierre. (Courtesy of the U.S. National Library of Medicine.)
BACTERIOLOGY
The confusion over the nomenclature of F. necrophorum has already been referred to, and indeed Finegold (143) commented that there were up to 52 names under which the organism has probably been known; it was referred to by early authors by such names as Bacillus necrophorus, Bacillus funduliformis, Bacillus symbiophiles, and Actinomyces necrophorus. Prévot (309) proposed the name Sphaerophorus for the pleomorphic anaerobic gram-negative rods that were not fusiform, distinguishing them from the genus Fusobacterium introduced by Knorr (226) for pointed, non-spore-forming gram-negative bacilli found in the mouth. However, on the basis of a range of studies, including DNA base ratios and physiological studies such as the production of butyric acid as a major end product of metabolism, the species of Sphaerophorus were later transferred into the genus Fusobacterium (194). Subsequent nucleic acid studies have confirmed levels of sequence similarity that are consistent with a single genus (33). The phylogenetic tree of Fusobacterium spp. described by Citron (90) after Conrads et al. (94) shows F. necrophorum to be clearly distinct from the most common human species, F. nucleatum.
Beerens (31) addressed what was clearly a considerable controversy as to whether Sphaerophorus necrophorus (332) and Sphaerophorus funduliformis (182) were distinct species or were members of a single species. He studied 4 strains of animal origin described as S. necrophorus and 14 strains of human origin described as S. funduliformis. On the basis of both differing virulence in rabbits and differences in ability to agglutinate fowl and sheep red cells, he staunchly defended the French view that they were distinct. Fiévez (141) confirmed differences between strains from different sources and divided strains into three biotypes, A, B, and C, with an intermediate group (AB) between A and B. Shinjo et al. (346) proposed that F. necrophorum has two subspecies, F. necrophorum subsp. necrophorum (biotype A) and F necrophorum subsp. funduliforme (biotype B). This was on the basis of clear biochemical, morphological, and DNA homology differences between the two biotypes. Subsequent small-subunit rRNA sequencing (289) supported this subdivision. Recent studies by Narongwanichgarn et al. (285) using randomly amplified polymorphic DNA-PCR have demonstrated that this method can distinguish the two subspecies consistently. These workers also developed a one-step duplex PCR method for the rapid detection and differentiation of the two subspecies.
This separation into subspecies is more than a mere taxonomic nicety. The two subtypes had different origins in that B. necrophorus was originally isolated from an animal source whereas B. funduliformis was a human isolate. More recent studies carried out in collaboration confirmed these differences. Thus, Hall et al. (181) studied 21 human isolates and 17 animal isolates using phenotypic reactions, pyrolysis mass spectrometry, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The results of the three approaches were consistent. All the animal “biotype A group” isolates clearly corresponded to F. necrophorum subsp. necrophorum, while the human isolates were distinct and could not be characterized simply as animal “biotype B isolates.” Brown et al. (70) examined phenotypic characteristics and lipopolysaccharides of human and animal strains and found that human isolates were predominantly coccobacillary, whereas animal isolates were predominantly rod shaped. In addition there were differences in the lipopolysaccharides of the strains from the two sources. Smith and Thornton (353) conducted comparative pathogenicity tests in mice. Fourteen strains, all of animal origin, showed the characteristic behavior of biotype A. Twenty-eight strains, 10 of which were animal and 18 human, were classified as biotype B. Thus, there is a substantial amount of evidence to support the view that F. necrophorum subsp. necrophorum is the principal pathogen in animals and F. necrophorum subsp. funduliforme the principal pathogen in humans. The significance of this observation is considerable, since it renders much of the work on pathogenesis, based as it is on animal models and using F. necrophorum subsp. necrophorum, of very limited relevance to human infection. As pointed out by Hall et al. (181), not all human isolates fit into F. necrophorum subsp. funduliforme, as confirmed by a more recent study of human isolates from throat swabs (30).
ANALYSIS OF PUBLISHED CASES
Methods
An extensive search of French and English literature was undertaken to identify published cases, using multiple search criteria: Lemierre's syndrome, F. necrophorum, necrobacillosis, postanginal sepsis, and septic jugular thrombophlebitis. The search was limited to cases published since 1970 to avoid distortion due to historical effects such as limited imaging modalities and unavailability of antibiotics. Many cases, particularly from series from a single institution, were excluded because of inadequate clinical or microbiological detail about individual patients. The case series was analyzed in two ways. First, a group of patients with necrobacillosis, i.e., systemic infection with F. necrophorum, with presumed origin in the head and neck was identified. Second, using the series, a variety of parameters were evaluated for use as criteria for a case definition of Lemierre's syndrome. Having derived a case definition, cases fitting this were studied. There are a number of limitations of analyses of this type. First, case reports rarely provide details of the criteria by which identification of fusobacteria was undertaken. Second, it is inevitable in a series gathered in this way that a range of selection and publication biases affect the mix of cases. Equally, it is effectively impossible to identify all published cases.
Results
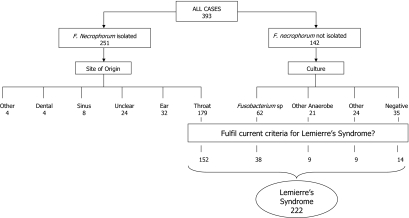
For full details of the cases and the source papers, see the supplemental material. A total of 393 cases were included in the analysis (Fig. 3). This comprised 251 patients with F. necrophorum and 142 other patients reported to have Lemierre's syndrome or postanginal sepsis. Among the F. necrophorum cases, the site of origin was identified as follows: throat, 179; ear, 32; sinusitis, 8; dental, 4; esophageal, 1; posttraumatic/postoperative, 3; and unknown, 24. The postanginal cases involving F. necrophorum included five cases with evidence of local direct spread in the head and neck only (5, 14, 260, 313, 388). Among the remainder, 152 had typical Lemierre's syndrome, i.e., lung and/or other systemic metastases; 20 had no evidence of metastases; and 2 had insufficient information to determine.
FIG. 3.
Summary of cases identified from the literature after 1970 and contained in the database.
CASE DEFINITIONS
Necrobacillosis
Necrobacillosis is essentially a veterinary term first used by Bang (23) in relation to the “nekrosebazillus” he identified in hog cholera. The term was used in relation to human infection with F. necrophorum by Cunningham (102), Shaw and Bigger (343), and Alston (11). Alston applied it to all human infections with F. necrophorum, including localized infection such as caesarean section wound infection, abortion with thrombosis of iliac veins, neck abscess, and peritonsillar and sublingual abscess. Eykyn (136) suggested that the term was best reserved in humans for septicemic illness caused by F. necrophorum. Similarly, Hagelskjaer et al. (177) in their Danish series required isolation of F. necrophorum from blood cultures. Thus, necrobacillosis is defined primarily by microbiology findings; this avoids the difficulties with inclusion criteria that beset Lemierre's syndrome.
Postanginal Sepsis
The term postanginal sepsis was used by many authors in the first 40 years of the 20th century before any consensus had emerged as to the causative agent (3, 41, 317). It is essentially a clinical term to describe the complications following tonsillitis with thrombophlebitis. Most published cases have had evidence of septicemia and metastatic lesions. Cases of this type formed the major element of Lemierre's seminal paper “on certain anaerobic septicemias” (239). However, some reported cases have had local complications such as deep neck space infection and mediastinitis without metastatic lesions (274, 341).
Lemierre's Syndrome
Vogel and Boyer (391) in 1980 were the first to include Lemierre in the title of their case report, and in the paper they refer to a clinical entity known as “postanginal septicemia … summarized in 1936 by Lemierre.” Shannon et al. (340) in 1983 seem to have been the first authors to use the term Lemierre's syndrome, in the title of their paper. However, it was not until the late 1980s that authors regularly referred to Lemierre's syndrome.
Lemierre (239) described a series of cases which was exclusive about neither the organism isolated nor the source of infection. Indeed, he included not just cases of postanginal septicemia but also those arising from jaw infections, ear infections, and from the gut, genital tract, and urinary tract. Unfortunately, authors publishing collected series of five or more cases have used widely differing criteria (Table 1). There is general agreement that Lemierre's syndrome relates to infection arising in the oropharynx rather than F. necrophorum infection arising from another site. However, fundamental differences include whether Lemierre's syndrome should be limited to postanginal cases (80, 210, 245) or should include infections arising in any site in the oropharynx (12, 85, 177, 275, 338, 310, 312, 348).
TABLE 1.
Case definitions of Lemierre's syndrome used in previous seriesa
| Authors (reference) | Type of series | Other | Source | IJV thrombosis | Metastatic lesions | Positive microbiology | No. of cases |
|---|---|---|---|---|---|---|---|
| Seidenfield et al. (338) | Primary | BC, F. necrophorum | O-P | BC, F. necrophorum | 5 | ||
| Sinave et al. (348) | Literature search | O-P | Yes, clinical or radiological or SPE | BC positive | 38 | ||
| Leugers and Clover (245) | Literature search | Pharyngitis | Assumed | Evidence of dissemination | BC, F. necrophorum | 40 | |
| Alvarez and Schreiber (12) | Literature search | Patients <18 yr | O-P | Yes, clinical or radiological | Metastatic infection | BC positive | 14 |
| Hagelskjaer et al. (177) | Primary | O-P | BC, F. necrophorum | 24 | |||
| Chan et al. (80) | Literature search | Pharyngitis | BC, anaerobes | 85 | |||
| Jones et al. (210) | Primary | Pharyngitis | Metastatic infection | 6 | |||
| Moore et al. (275) | Literature search | O-P | Yes, clinical or radiological | At least one metastatic focus | BC positive | 40 | |
| Chirinos et al. (85) | Literature search | IJV or BC not F. necrophorum | O-P | Yes, radiological | BC, F. necrophorum | 105 | |
| Ramirez et al. (312) | Primary | O-P | Yes, clinical or radiological | BC, F. necrophorum | 5 | ||
| Pulcini et al. (310) | Primary | O-P | Metastatic pulmonary lesions | 6 | |||
| Goldenberg et al. (160) | Primary | Pharyngitis or other febrile illness | Yes, radiologically confirmed | Bilateral pulmonary infiltrates or loculated infection in head and neck | Positive BC unless therapy with antibiotics | 9 |
IJV, internal jugular vein thrombophlebitis; O-P, oropharyngeal; BC, blood culture; SPE, septic pulmonary emboli.
Similarly, authors vary as to whether proven thrombophlebitis of the internal jugular vein is a requirement. Some authors omit this as a criterion (80, 177, 210, 310, 338). Some authors require it, although accepting septic pulmonary emboli or lung infarcts as a surrogate marker for this (12, 275, 312, 348). Yet others insist on radiological confirmation of internal jugular vein thrombophlebitis as an inclusion criterion (160). However, many reports have described cases of classical Lemierre's syndrome with metastatic lesions and F. necrophorum isolated from blood cultures but no evidence of internal jugular vein thrombosis (190), even with ultrasound scans (114), computed tomography (CT) scanning (401), or magnetic resonance (MR) scanning (292). Boharas (41) had previously reported that there was no correlation between the extent of phlebitis and the severity of symptoms, i.e., that severe septicemia could occur with slight or marked phlebitis and with or without thrombosis. Similarly, Brocard and Guibé (54) commented from extensive clinical experience that internal jugular vein thrombosis was far from a constant finding and that thrombophlebitis could be confined to the peritonsillar veins. It therefore seems illogical to insist on evidence of internal jugular vein thrombophlebitis in otherwise typical cases. Some authors require the presence of one or more metastatic lesion (12, 210, 245, 275, 310).
There are also wide variations in microbiological criteria, ranging from (i) none (160, 210, 310), to (ii) a positive blood culture with any potential pathogen (12, 275, 348), (iii) a positive blood culture with an anaerobic organism (80), and (iv) isolation of F. necrophorum from blood cultures (85, 177, 245, 312). The overall effect of the variable criteria is to create a wide diversity in what has been included, from very open to very tight definitions.
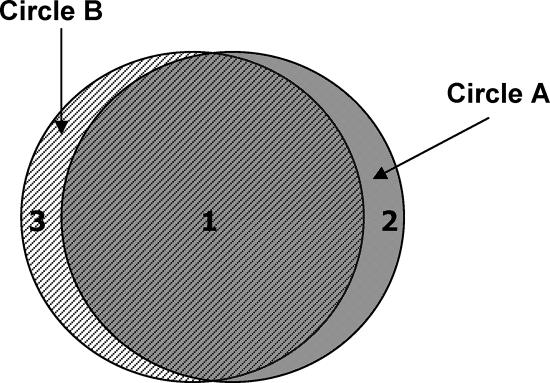
Riordan and Wilson (319) highlighted the overlap of the three terms (Fig. 4) and argued that in relation to Lemierre's syndrome, the criteria should be based on the central features of Lemierre's description (239). Thus, the major emphasis of his paper was on postanginal septicemia, and therefore the term should be restricted to cases of this nature. In many previous series, cases with F. necrophorum arising from ear infection have been included as Lemierre's syndrome (85, 177, 348). As can be seen from Table 2, there is a fundamental difference in the age distribution of otogenic cases compared to postanginal cases. In addition, the features of the condition are very different, with otogenic cases having on the one hand a low incidence of internal jugular vein thrombophlebitis and lung and other systemic lesions and on the other hand a much higher incidence of intracranial complications than those secondary to throat infection. It is therefore proposed that infections arising from sites in the oropharynx other than the throat should be excluded.
FIG. 4.
Patient groups: necrobacillosis and Lemierre's syndrome. Circle A, postanginal sepsis with internal jugular vein thrombosis and metastatic manifestations; circle B, necrobacillosis; 1, classical Lemierre's postanginal sepsis; 2, clinical Lemierre's syndrome but F. necrophorum not detected; 3, necrobacillosis with other source.
TABLE 2.
Comparison of F. necrophorum cases arising from ear and throat
| Parameter | Value for:
|
|
|---|---|---|
| Ear-associated cases | Throat-associated cases | |
| No. of cases | 32 | 179 |
| Age range | 2 mo-51 yr | 8 mo-63 yr |
| Age median | 5 yr | 19 yr |
| % Male | 44 | 57 |
| % of cases with: | ||
| Positive blood culture | 81 | 89 |
| Deep neck complications | 3 | 15 |
| Jugular venous thrombosis | 19 | 48 |
| Lung lesions | 0 | 77 |
| Empyema | 0 | 15 |
| Other metastatic lesions | 9 | 27 |
| Any intracerebral complication | 66 | 6 |
| Meningitis | 41 | 0.6 |
| Sinus thrombosis | 25 | 2 |
| Mortality | 13 | 6 |
Lemierre's paper (239) referred to a septicemic illness with metastatic lesions. Indeed, he commented that pure septicemias were distinctly rare. Therefore, it is surely essential to have clinical evidence of metastatic lesions to qualify as a case. It seems unjustified to include a requirement for positive blood cultures, since the presence of metastatic lesions is proof itself of bacteremia and blood cultures are not infrequently taken after antibiotic therapy which, although unable to cure the infection overall, can eliminate the bacteremia.
The current case series was used to compare the effects of different case definitions for Lemierre's syndrome in terms of the number of cases that are included. Purely microbiological definitions were rejected on the basis that cases without metastatic lesions would be included and also culture-negative cases are excluded. The use of internal jugular vein thrombophlebitis as an essential criterion excluded a high proportion of otherwise typical cases. The best choice seemed to be a hybrid of clinical and microbiological criteria. For the purposes of this study, the following combination was chosen: (i) history of anginal illness in the preceding 4 weeks or compatible clinical findings, (ii) evidence of metastatic lesions in lungs and/or another remote site, and (iii) evidence of internal jugular vein thrombophlebitis or isolation of F. necrophorum or Fusobacterium sp. from blood cultures or a normally sterile site.
Using the current case definition for Lemierre's syndrome, 222 cases were identified in the analysis (Fig. 3). In terms of bacteriology, 68% of samples from these cases grew F. necrophorum, overall 86% grew F. necrophorum or another Fusobacterium sp., and in all 90% grew an anaerobic bacterium of some type. The culture details are summarized in Table 3. Given that the current series includes cases included in previous literature reviews (85, 245, 278, 348), comparisons are not very useful. However, in Table 4 the demographic, culture, and clinical data from the current review are presented alongside those from two previous reviews (136, 177) which did not involve significant overlap of cases.
TABLE 3.
Summary of culture results for 222 cases fulfilling the current case definition for Lemierre's syndrome, with details of organisms other than F. necrophorum or Fusobacterium sp. that were isolated
| Organism(s) isolated | No. (%) of Lemierre's syndrome cases with:
|
|
|---|---|---|
| Fusobacteria isolated | Fusobacteria not isolated | |
| All cases | ||
| Pure F. necrophorum | 133 (60) | |
| Pure Fusobacterium sp. | 31 (14) | |
| F. necrophorum in mixed culture | 19 (9) | |
| Fusobacterium sp. in mixed culture | 7 (3) | |
| Organism other than fusobacteria isolated | 18 (8) | |
| Culture negative | 14 (6) | |
| Cases with bacteria other than F. necrophorum | ||
| Anaerobes | ||
| Bacteroides sp. | 4 | 2 |
| Eubacterium sp. | 1 | |
| Fusobacterium sp. (in addition to F. necrophorum) | 1 | |
| Lactobacillus sp. | 1 | |
| Peptococcus sp. | 1 | |
| Peptostreptococcus sp. | 8 | 1 |
| Prevotella sp. | 2 | 1 |
| Porphyromonas sp. | 2 | |
| Unidentified anaerobea | 4a | |
| Total | 18 | 10 |
| Aerobes | ||
| Arcanobacterium haemolyticum | 2 | |
| Eikenella corrodens | 1 | 2 |
| Enterococcus sp. | 1 | |
| E. coli | 1 | |
| Proteus sp. | 2 | 1 |
| Streptococcus bovis | 1 | |
| Streptococcus group A | 1 | |
| Streptococcus group B | 1 | |
| Streptococcus group C | 4 | 1 |
| Streptococci (oral) | 8 | 5 |
| Total | 20 | 11 |
These may have been fusobacteria but were not identified.
TABLE 4.
Comparison of demographic, culture, and clinical data in the current series and two previous primary case series
| Parameter | Value from:
|
|||||
|---|---|---|---|---|---|---|
| Reference 136
|
Reference 177
|
Current review
|
||||
| Necrobacillosisa | Lemierre's syndrome | Necrobacillosisa | Lemierre's syndrome | Necrobacillosisa | Lemierre's syndrome | |
| No. of cases | 44 | 29 | 24 | 15 | 251 | 222 |
| Age range (yr) | 0.1-65 | 9-32 | 0.6-46 | 14-31 | 0.2-73 | 0.7-70 |
| Median age (yr) | 22.6 (male), 20.4 (female) | 17 | 19 | 18 | 19 | |
| % Male | 67 | 66 | 67 | 67 | 55 | 57 |
| % of cases with: | ||||||
| F. necrophorum | 100b | 100b | 100b | 100b | 100b | 68 |
| Any Fusobacterium sp. | 100 | 100b | 100b | 100b | 100b | 86 |
| F. necrophorum or Fusobacterium sp. in mixed culture | 2 | 3 | 33 | 40-53 | 12 | 12 |
| Other organism without fusobacteria | NAb,c | NAb | NAb | NAb | NAb | 8 |
| IJV thrombosis | 4.5 | 3.4 | NR | NR | 37 | 59 |
| Metastases | 75 | 97 | 63 | 80 | 73 | 100 |
| Lung lesions | 55 | 79 | 54 | 80 | 60 | 92 |
| Empyema | NRd | NR | 17 | 27 | 12 | 17 |
| Septic arthritis | 18 | 21 | 0 | 0 | 12 | 11 |
| Osteomyelitis | 7 | 3.4 | 0 | 0 | 7 | 5.4 |
| Muscle involvement | 0 | 0 | 0 | 0 | 5 | 7.2 |
| Skin involvement | 0 | 0 | 0 | 0 | 2.3 | 3.1 |
| Liver abscess | 2.3 | 3.4 | 4.2 | 0 | 6 | 4.0 |
| Meningitis | 9 | 3.4 | 4.2 | 0 | 8 | 1.4 |
| Cerebral abscess | 2.3 | 3.4 | 0 | 0 | 3.6 | 1.8 |
| Septic shock | NR | NR | NR | NR | NR | 7 |
| Renal failure | 2.3 | 3.4 | NR | NR | NR | 2 |
| Disseminated intravascular coagulation | 7 | 10 | 29 | 47 | NR | 4 |
| Antibiotic duration | NR | Mean, 6 wk | 0-58 days (median, 17 days) | 0-35 days (median, 18 days) | NR | NR |
| % Mortality | 4.5 | 3.4 | 0 | 0 | 7 | 4.9 |
Excludes cases arising outside the head and neck.
Determined by entry criteria used by authors.
NA, not applicable.
NR, not recorded.
NECROBACILLOSIS ARISING OUTSIDE THE HEAD AND NECK
Lemierre (239) reported that infection could arise from the gastrointestinal tract, the female genital tract, or the urinary tract. Alston (11) identified 21 published cases of F. necrophorum in the United Kingdom. Six of these were directly related to tonsillar infection and would be compatible with Lemierre's syndrome. Strikingly, seven infections in the series arose from the female genital tract, including three cases that were postabortion or postpartum. Among a series of 45 cases identified by Eykyn (136), 29 were typical of Lemierre's syndrome and only 4 had not arisen from the head and neck. One of these involved isolation of F. necrophorum from blood of a patient with postpartum pyrexia. The remaining three cases involved osteomyelitis with no preceding history of sore throat or other discernible site of entry. In the series of 49 cases of F. necrophorum septicemia reported by Hagelskjaer et al. (177), half were not related to the oropharynx, including 8 from the gastrointestinal tract, 5 from the genitourinary tract, and, unusually, 7 that were said to involve skin infection. This series highlighted a finding reported by others that necrobacillosis arising outside the oropharynx tends to involve significantly older patients, with a high proportion having underlying disease such as malignancy and alcohol or drug abuse.
IS F. NECROPHORUM A SINE QUA NON FOR LEMIERRE'S SYNDROME?
Brocard and Guibé (54) described the complex microbial interactions that occur in the course of F. necrophorum septicemia. Thus, often the original tonsillar portal has a mixed infection. They stated that “it is easy to conceive since the septicemia originates from a polymicrobial source, that the selection is not always absolute and that other bacteria can sometimes temporally be associated with F. necrophorum.” Teissier et al. (378) commented that due to the episodic nature of the bacteremia with F. necrophorum, culture of material from metastatic abscesses was often more productive in revealing the causative organism. Indeed, they noted that blood cultures could be actively misleading by yielding a “saprophytic organism captured by leukocytes and accidentally introduced into the circulation.” This is certainly supported by data from the current series. Thus, for cases fulfilling the criteria for Lemierre's syndrome and from which a Fusobacterium sp. was isolated, 22/168 blood cultures were mixed whereas all 16 cultures from liver abscess, joint fluid, or bone yielded pure growths.
The evidence is inconclusive as to whether F. necrophorum is the sole cause of Lemierre's syndrome. Clearly, F. necrophorum is not isolated from all reported cases. In the current case review only 86% of patients fitting the case definition grew a Fusobacterium sp. Four grew unidentified anaerobes. Other organisms were found in 58% of cases without fusobacteria, compared to 14% of those with fusobacteria. However, the distributions of organisms are similar, with a range of oral anaerobes and streptococci in both groups (Table 3). A number of recent case reports have focused on cases in which other organisms have been isolated, and there may well be publication biases in the data. In many cases it is difficult to be convinced that the organism isolated, e.g., a Proteus sp. or Streptococcus oralis, could be capable of causing the disease alone, and thus one suspects that F. necrophorum may have been present but undetected. This is supported by the fact that by using molecular methods, F. necrophorum was detected in a typical clinical case which was culture negative (9). Further molecular studies are required, but it remains eminently possible that fusobacteria, and more specifically F. necrophorum subsp. funduliforme, are the underlying cause of all typical cases of Lemierre's syndrome.
F. NECROPHORUM IN THE OROPHARYNX
F. necrophorum as Normal Human Flora in the Oropharynx
Lemierre (239) and other French workers (54, 240, 242, 243, 378) stated confidently that B. funduliformis was a part of the normal human flora, including in the gut, female genital tract, and oropharynx. One of the much-quoted sources that is purported to confirm the presence of Fusobacterium necrophorum as part of the normal human flora is the 1898 Ph.D. thesis of Jean Hallé (who isolated the prototype strain of F. necrophorum subsp. funduliforme) on the bacteriological flora of the female genital tract (182). Hallé stated (my translation) that “this organism has been found in the healthy vagina, in the exudates of patients with retained placentas and in the pus of Bartholin's abscesses”.
However, if one studies the individual cases, it can be seen that he described culturing vaginal samples from four prepubertal children and four women, none of whom had symptoms or signs referable to the genital tract. Curiously, in the description of the culture results for these eight individuals, Bacillus funduliformis (as he called it) was not reported for any of them. It is unclear why he concluded that it was present in the healthy vagina. One has to assume that he found the organism in other, unreported patients, but inevitably it is totally unclear what proportion of healthy females were carrying the organism.
Dack (103) reviewed the available data and concluded that F. necrophorum was probably a normal inhabitant of the mucous membranes of humans and commented that “the fact that B. necrophorum has not been found in the normal colon does not indicate that it is not present here but probably that it is present in insufficient numbers to be detected.” I am unaware of any formal studies from that period which reported on carriage rates in the oropharynx. Nevertheless, it seemed to be generally accepted, and thus Bartlett and Gorbach (27) stated, without references, that “F. necrophorum is another normal cohabitant of the upper airways.” This has been reiterated by many subsequent authors (see, e.g., references 49, 178, 194, and 251). However, as pointed out by Aliyu et al. (10) and Batty et al. (30), it is more difficult to obtain the evidence for this from primary sources.
Many papers refer to isolation of fusobacteria without breaking this down into species (see, e.g., references 359 [mouth], 26 [vagina], 88, 101) [gut], 228 [mouth], and 33 [dental plaque]). Clearly, reports of this kind do not help resolve whether F. necrophorum is present in the normal flora of the oropharynx.
There are relatively few studies which are able to shed any light. Alston (11) said that he had failed to grow F. necrophorum from tonsils at postmortem. Gorbach and Bartlett (165) quoted Rosebury (322) and Loesche (249) and made no mention of F. necrophorum among the principle anaerobic constituents of normal oral flora.
Moore et al. (276), despite very detailed anaerobic bacteriology, found no evidence of F. necrophorum in samples of gingival fluid. Hardie (186), Marsh and Martin (263), and Drasar and Duerden (120), in reviewing normal oral anaerobic flora, all refer to other fusobacteria, particularly F. nucleatum, but make no mention of F. necrophorum. Brazier et al. (51) reported isolating fusobacteria from gingival material from 9/16 healthy subjects. Six isolates were identified as F. naviforme, two as F. nucleatum, and one as F. necrophorum. Uematsu and Hoshino (383), in an exquisitely careful and detailed study of the anaerobic flora of periodontal pockets in seven patients, found 21 strains of Fusobacterium spp., but only one of these was F. necrophorum. Tanaka et al. (375) studied four sites in the oropharynx using swabs taken at anesthesia and did not isolate F. necrophorum. Könönen et al. (228) studied the anaerobic flora in saliva of young children. Although F. nucleatum was found as the most common strict anaerobe by 1 year of age, F. necrophorum was not detected.
Falkler et al. (138) studied oral flora of 30 malnourished children in Nigeria. F. nucleatum was isolated from all 30 children, but only 1 had F. necrophorum. Oh et al. (294) reported that a wide range of pathogenic bacteria have been associated with prepubertal periodontitis (aggressive periodontitis), including F. nucleatum, but they made no mention of F. necrophorum.
In the last 10 years molecular techniques for detection of oral bacteria have been increasingly used to facilitate the detection of fastidious organisms and indeed to detect unculturable bacteria (393). In a detailed study of subgingival plaque, Socransky et al. (357) provided no evidence of detecting F. necrophorum. In a sophisticated study which involved cloning 16S rRNA from gingival dental plaque, Paster et al. (303) found 132 species, including 4 species/subspecies of Fusobacterium spp., but none was F. necrophorum. Aas et al. (1) studied nine different sites and make no reference to detection of F. necrophorum. Tsuneishi et al. (381), using molecular methods, conducted detailed studies on the bacterial flora of tonsilloliths and did not detect F. necrophorum in any of the samples from six patients.
Aliyu et al. (10) failed to detect F. necrophorum from any of 100 swabs from throats of healthy control subjects using real-time PCR. By contrast to all the above studies, Jensen et al. (207), in a recent and very important PCR study, examined throat swabs from healthy controls (female student nurses and male soldiers who had neither suffered from sore throat nor received antibiotic therapy in the preceding 2-week period). F. necrophorum subsp. funduliforme was detected in 21% of 92 throat swabs from these healthy individuals. However, the authors were unable to isolate the organism in culture. Their PCR methodology appears to be robust. The authors suggested that the failure to culture the organism related to the fact that the organism resides in the crypts of the tonsils, where it is difficult to detect by surface swabbing. Bacteriological studies of the cores of surgically removed tonsils have certainly shown higher yields of anaerobes than from surface swabs (68), but they have not shown high detection rates of F. necrophorum. Interestingly, however, these studies have tended to involve children rather than young adults.
Questions can be raised, such as whether the selection of healthy subjects in the study by Jensen et al. was representative. There is an obvious need for this study to be repeated with different age groups and settings and also by other groups. Although the age range of the controls was not stated, one has to assume that they were young adults, i.e., the age group at greatest risk of Lemierre's syndrome. Could it therefore be that acquisition of F. necrophorum subsp. funduliforme occurs at this age but that carriage does not normally become lifelong? The authors concluded that F. necrophorum subsp. funduliforme is part of the “normal flora” of the human tonsils. One can question the use of this term. Even using conventional culture methods, oropharyngeal carriage rates of Neisseria meningitidis can exceed 20% in this age group (76), and yet the term normal flora would not be applied to this organism. Is it reasonable to consider speculatively that there could be parallels between N. meningitidis and F. necrophorum, for example, that septicemic infection tends to follow recent acquisition of a virulent strain?
In the meantime, I am forced to conclude that no convincing culture evidence exists to confirm that F. necrophorum is a part of the normal oral flora. Given that only one molecular study has detected the organism in the oropharynges of healthy individuals, it is also uncertain whether it is present even in a nonculturable form. It seems either that Lemierre et al. were misidentifying other commensal organisms such as F. nucleatum when they reported F. necrophorum to be part of the normal oral flora or that there has been a subsequent shift in the prevalence of the organism in the upper respiratory tract. Whatever the explanation for the discrepancy, one must conclude that currently, unlike virtually all other human infection with non-spore-forming anaerobes, F. necrophorum infection must be exogenously acquired. This is consistent with the fact that F. necrophorum is a rare finding in anaerobic pleuropulmonary infection associated with chronic aspiration, etc. (see below).
F. necrophorum and Sore Throat
Hardingham (187) commented that the occurrence of recurrent penicillin-resistant tonsillitis and beta-hemolytic streptococcal tonsillar carrier states had stimulated several studies of the aerobic and anaerobic bacterial populations of infected tonsils. Nord (290) stated that chronic tonsillitis normally manifested as a recurrent sore throat that was sometimes accompanied by foul breath. There is a major difference between the microbial flora in episodes of acute and chronic tonsillitis, with increased numbers of anaerobes (i.e., in terms of CFU per gram) isolated from patients with chronic infection (63, 66). The most frequently isolated species were Bacteroides spp., Fusobacterium spp., Prevotella spp., and anaerobic cocci. F. necrophorum has not featured prominently; thus, in one study of tonsils excised from children aged 4 to 15 years, F. necrophorum was detected in only 2 of 25 samples (67). Equally, it has been very difficult to establish with certainty whether anaerobes have a causative role; these organisms might simply reflect an environment created by tonsillar scarring that was more favorable for anaerobes.
Coming to the role of F. necrophorum, Lemierre (240) regarded B. funduliformis as a secondary invader, rather than the initiator of throat infection. It has been assumed by others (144) that the tonsillitis which initiates Lemierre's syndrome is caused by F. necrophorum. However, rigorous data to support this or indeed a role for F. necrophorum in uncomplicated tonsillitis have been lacking. Bourbeau (42), in a review of laboratory diagnosis of pharyngitis, made no reference to F. necrophorum or indeed anaerobes in general. Three recent studies are therefore of considerable interest.
Aliyu et al. (10) used a real-time PCR with primers specific to the two subspecies of F. necrophorum. By this technique, 100 throat swabs from patients with sore throat in primary care submitted for routine examination were examined together with throat swabs from 100 healthy controls. Testing with control strains suggested that the PCR was both specific and sensitive. F. necrophorum was detected in 10 cases and in none of the controls. Subsequent testing with the HAEM Light Cycler PCR assay indicated that all the strains were F. necrophorum subsp. funduliforme.
Batty and Wren (29) cultured 248 throat swabs received for clinical investigation for hemolytic streptococci, Corynebacterium diphtheriae, Arcanobacterium spp., and F. necrophorum. Twenty-seven (11%) grew group A streptococci, and 24 (10%) grew F. necrophorum. Four of the F. necrophorum cases also grew group A streptococci. The authors noted that F. necrophorum tended to be isolated in older patients compared to those with group A streptococci and also from patients with tonsillitis or persistent sore throat syndrome. The isolation rate of F. necrophorum from the latter group was 21%.
In a parallel publication, Batty et al. (30) described an extraordinary case of a 41-year-old woman with a 24-year history of chronic relapsing sore throat since suffering from infectious mononucleosis at the age of 17. A throat swab grew group A streptococcus but also F. necrophorum. Treatment with erythromycin and metronidazole led to dramatic improvement, not only in her acute symptoms but also in her general health. In addition, her persistent tonsillar swelling and cervical lymphadenopathy resolved.
Most recently, Jensen et al. (207) used a novel F. necrophorum subspecies-specific real-time PCR assay to study tonsillitis patients aged 18 to 32 years and healthy controls. Fifty-one percent of 105 tonsillitis patients were positive for F. necrophorum subsp. funduliforme, compared to 21% of controls. F. necrophorum was present in higher numbers in the recurrent nonstreptococcal tonsillitis cases. The authors reported that since conducting this study, the use of culture for F. necrophorum using specific selective media has been introduced into the routine examination of throat swabs in their hospital, with a detection rate of over 15% among tonsillitis patients aged 18 to 32 years.
This recent evidence is strongly suggestive that F. necrophorum subsp. funduliforme has a causative role in nonstreptococcal tonsillitis, particularly recurrent infection. However, particularly in view of the fact that other anaerobes are also found in increased numbers in recurrent tonsillitis (66), caution is required and further studies are merited before unqualified acceptance of this.
Peritonsillar Abscess
In his classic paper, Lemierre (239) commented that peritonsillar abscess is the normal precursor to postanginal septicemia, and therefore it is not surprising that there are elements that overlap with Lemierre's syndrome. Peritonsillar abscess was a common condition in the preantibiotic era. Spires et al. (363) quote that there were an estimated 226 deaths in England from peritonsillar abscess in 1875. In addition, the age range of patients is very similar, with a median age of around 25 (271, 363). Reuben (317) commented that peritonsillar abscess was rarely seen in young children compared to the frequency of retropharyngeal abscess. Peritonsillar abscess also shows a male predominance (87) which parallels that of Lemierre's syndrome. Some workers have noted a marked seasonal variation, with 70% of the cases described by Spires et al. (363) occurring between October and February. This again matches the seasonal incidence for severe F. necrophorum infection reported by Brazier et al. (52).
Lemierre et al. (243) stressed that it was in tonsillitis and peritonsillar abscess that B. funduliformis was most apparent. They stated that Reilly had repeatedly isolated B. funduliformis from peritonsillar abscess. More recent studies, such as those of Jousimies-Somer et al. (212) and Mitchelmore et al. (271), show a high proportion of cases yielding anaerobes. Broadly speaking, two subgroups can be identified, the first having group A streptococci and the second with a predominantly anaerobic flora which, although mixed, has a striking occurrence of F. necrophorum which is totally out of proportion with the virtual absence of culturable F. necrophorum in the oropharynges of healthy individuals. Thus, Jousimies-Somer et al. (212) reported that among 15 cases with purely anaerobic etiology, 14 (93.3%) yielded F. necrophorum. In the current review, 7% of cases fulfilling the case definition for Lemierre's syndrome had peritonsillar abscesses.
Given the historical significance of peritonsillar abscess as a precursor to Lemierre's syndrome, it can provide some interesting insights. Handler and Warren (184) described a case of peritonsillar abscess arising after infectious mononucleosis. At subsequent tonsillectomy, the tonsils were noted to be fibrotic, scarred, and tightly adherent to the tonsillar fossa. Histological examination of the tonsils revealed evidence of chronic inflammation and fibrosis. Hardingham (187) commented that over half of all cases of quinsy have a history of previous tonsillar infection. He suggested that the development of fibrosis in the affected tonsils or adjacent tissues favored the growth of anaerobic organisms and enhanced the pathogenicity of mixed bacterial growths in the tonsils. This accords well with the observations of Jousimies-Somer et al. (212) that in their series, patients with F. necrophorum had a significantly higher (P < 0.01) rate of previous tonsillar/peritonsillar infections than those infected with group A streptococci. Recurrences and or related tonsillectomies were also more common among patients infected with F. necrophorum than among those infected with group A streptococci (P < 0.0001). There is thus an impression that F. necrophorum-associated peritonsillar abscess tends to arise in patients with tonsils affected by recurrent infection.
Deep Neck Space Infection
Deep space infection has been reviewed in depth by several authors (40, 87), and the potential directions of spread have been detailed. Blomquist and Bayer (40) highlighted the fact that the lateral pharyngeal space occupies a pivotal position in the neck, communicating as it does with all the major fascial spaces. Infections spreading posteriorly through the neck inevitably pass through this 2.5-cm-long space. Infections that originate here may in turn spread to a variety of other spaces in the neck, such as the retropharyngeal space, or involve the structures of the carotid sheath. If the retropharyngeal space is involved, this can allow infection to track either up or down to cause dangerous complications such as meningitis or mediastinitis (40, 58).
The bacteria responsible for deep neck infections are usually normal components of the oral flora that become virulent and invasive when mucosal barriers are broken, for example, during pharyngitis, odontogenic infections, and direct trauma, allowing them a portal of entry into the deeper neck spaces. Deep neck infections are mixed aerobic-anaerobic processes with anaerobes predominating. Bartlett and Gorbach (27) commented that in series in which careful anaerobic techniques are applied, three groups of anaerobic organisms are repeatedly isolated: Peptostreptococcus spp., Fusobacterium spp. (predominantly F. nucleatum), and Bacteroides spp. (predominantly B. melaninogenicus). According to Brook (58), the predominant organisms recovered from deep facial infections are Staphylococcus aureus, group A streptococci, and anaerobic bacteria of oral origin. These include pigmented Prevotella spp. and Porphyromonas spp. as well as Fusobacterium spp.
It is apparent from these reports that F. necrophorum does not play a prominent role in deep space infections such as parapharyngeal or retropharyngeal abscess. Brocard and Guibé (54) were very clear that even in typical cases of Lemierre's syndrome with F. necrophorum isolated from blood as the single organism, the infection was typically polymicrobial in the tonsil and the lateral pharyngeal space and the critical event was the formation of thrombophlebitis in the draining veins when F. necrophorum emerged as the key, or indeed the only, organism. Among the cases identified that fulfill the current case definition for Lemierre's syndrome, 10% had parapharyngeal abscesses and one had a retropharyngeal abscess.
Mediastinitis
Although the majority of cases of mediastinitis arise as a result of either esophageal rupture or cardiothoracic surgery, there is a distinctive entity referred to as descending necrotizing mediastinitis that occurs after oropharyngeal infections, particularly dental abscess but also occasionally peritonsillar abscess (134). Infection is typically polymicrobial with anaerobes playing a major role. Thus, in a series of seven cases, Brook and Frazier (62) detected 5 aerobic/facultative organisms and 13 anaerobes, 3 of which were F. nucleatum, but there were no isolates of F. necrophorum.
Cases of descending necrotizing mediastinitis have been reported as Lemierre's syndrome (220). However, the pathogenesis is different in that the method of spread to the chest is not via septic emboli but by direct invasion via the retropharyngeal space. Patients with this presentation will develop empyema in the absence of underlying lung lesions. In the current review, 4/222 fitting the case definition for Lemierre's syndrome had mediastinitis (191, 266, 274, 280), 3 of which involved F. necrophorum (191, 266, 280). Three other cases of F. necrophorum-associated mediastinitis were identified. One was in a 7-year-old child who presented with a mediastinal mass and did not have features of Lemierre's syndrome (257). The other two were in teenage girls with infectious mononucleosis complicated by mediastinitis and bilateral empyemas which yielded F. necrophorum, among a mixture of organisms (14, 260).
Otogenic Infection
Otitis media.
In uncomplicated acute otitis media, anaerobes play a limited role. Thus, Brook et al. (69) showed that 15% of samples yielded anaerobes alone. Brook and Burke (64) highlighted that anaerobes were isolated in only 27% of samples and that the majority of these were anaerobic cocci. It is therefore particularly striking to see the rare cases of very severe acute otitis media with copious foul-smelling purulent otorrhoea that have progressed to sinus venous thrombosis and even meningitis (100, 318). Not surprisingly, these cases tend to involve F. necrophorum.
Brook and Finegold (65) undertook detailed anaerobic culture of 50 samples of pus from chronic otitis media. Anaerobes were recovered from 56% samples. Most patients had a mixture of aerobes and anaerobes. A total of 48 anaerobic isolates were obtained, half of which were gram-positive cocci. Among the gram-negative anaerobes, B. melaninogenicus was most common, and there were only two isolates of Fusobacterium sp., identified as F. nucleatum. Jokipii et al. (209), in a study of 70 cases of chronic otitis media, obtained similar results. F. necrophorum seems to play little or no role in chronic otitis media.
Mastoiditis.
Several studies (61, 157, 221) have highlighted the fact that aerobes such as Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, and Haemophilus influenzae are the most common organisms recovered in acute mastoiditis. By contrast, Brook (55) reported on specimens from 24 children undergoing mastoidectomy with a diagnosis of chronic mastoiditis (duration of over 3 months). Bacteria were grown from all samples, with 29 aerobic isolates and 61 anaerobic isolates. There were 37 isolates of gram-positive anaerobes and 24 of gram-negative anaerobes, including two patients with F. nucleatum. F. necrophorum was not isolated from any patients.
Go et al. (158) described the intracranial complications identified in eight children in a series of 118 cases of acute mastoiditis. Intraoperative specimens from the eight cases were negative in four, and none had anaerobes isolated. Migirov et al. (270) described 28 patients with intracranial complications of ear infection. Meningitis was the most common complication (46.4%), followed by brain abscess, epidural abscess, sigmoid sinus thrombosis, subdural empyema, perisinus abscess, and transverse and cavernous sinus thrombosis. In the current review, 69% of patients with F. necrophorum infections arising from the ear were shown to have mastoiditis, and as can be seen from Table 2, 66% of otogenic cases of F. necrophorum had intracerebral complications. Thus, although rare, F. necrophorum mastoiditis, when established, appears to behave aggressively.
Odontogenic Infection
In the last 50 years a massive amount of research has been undertaken on the complex role of oral bacteria in conditions such as necrotizing ulcerative gingivitis, periodontitis, infected root canals, and dental abscess. While it is clear that various anaerobes are key pathogens in these conditions, most studies show a minimal role for F. necrophorum (21, 59, 88, 358). On the other hand, a small number of cases of necrobacillosis do originate from dental infection (8, 133, 338, 361).
Noma represents an extreme form of necrotizing ulcerative gingivitis, the triggering factors for which include malnutrition and viral infection. In a final invasive stage, the lesion spreads rapidly, probably owing to the presence of necrotizing toxins or tissue-destroying enzymes and inflammatory mediators. Studies point to the disease being polymicrobial with a range of oral anaerobic organisms, including Prevotella intermedia. However, a study by Falkler et al. (137) examined eight children with noma and isolated F. necrophorum from seven and Prevotella intermedia from six. This pair of organisms has been described by Enwonwu et al. as “the key players in a consortium of micro-organisms” (129). Enwonwu (128) also highlighted pathological similarities between noma and necrobacillosis in wallabies.
Sinusitis
There are few descriptions of F. necrophorum being associated with sinusitis, either acute or chronic. In the current analysis 8/251 cases of F. necrophorum arose from sinus infection. Three cases involved purely local spread to the orbit, causing orbital abscesses (132, 306, 313), with isolation of F. necrophorum from sinus pus. The remaining four cases (4, 34, 53, 153) resulted in intracranial complications, including meningitis (4), cavernous sinus thrombosis (53, 153), and cerebral infarction (53). All four of theses cases had positive blood cultures but no systemic metastatic lesions. One patient developed endocarditis (4).
INTRACRANIAL COMPLICATIONS
According to Lemierre (243), Veillon and Zuber (386) provided the first description of intracranial infection by F. necrophorum, in a child presenting with chronic otorrhoea, cerebral abscess, septic arthritis, and severe sepsis. In the current literature review, 37 cases of F. necrophorum infection with confirmed intracranial complications were identified, as detailed below. The incidence of intracranial complications is significantly higher in otogenic F. necrophorum infections than in postanginal cases. Thus, in the current literature review 6.2% of postanginal cases of F. necrophorum developed intracranial complications, versus 65.5% of otogenic cases. Five patients with intracranial infection had no clear site of origin. One of these (44) also had lung lesions. The remaining four did not have systemic metastases.
Sinus Thrombosis
Sinovenous thrombosis has a wide range of causes. De Veber et al. (112), in a multicenter pediatric study, identified 91 cases outside the neonatal period. Bacterial sepsis (etiology unspecified) was identified as a risk factor in 4% of these. Sinovenous thrombosis is a recognized complication of F. necrophorum infection (20, 211, 370). In the current literature review, 15/251 patients with F. necrophorum infection had venous sinus thrombophlebitis; these patients ranged in age from 11 months to 38 years, with a median age of 15 years. Among those with an identified source, 10 cases were otogenic in origin and 4 were secondary to throat infection. All of the postanginal cases had confirmed internal jugular vein thrombophlebitis.
Cerebral Abscess
Anaerobes are an important cause of cerebral abscess, and fusobacteria are among the most common isolates (56, 145, 244). However, F. necrophorum tends either to be a minority isolate (56, 145) or not to be found at all (244), with F. nucleatum being a much more common isolate. The current literature search identified 9/251 F. necrophorum patients with cerebral abscess, ranging from 5 to 45 years in age and with a median age of 17 (9, 109, 183, 203, 204, 259, 269, 313, 392). Among the cases with a clear source, two were otogenic (109, 204) and four followed throat infections (9, 269, 392, 303).
Meningitis
Anaerobes are a rare cause of meningitis. Tärnvik (376) reviewed anaerobic meningitis in children and identified 20 cases in the literature, including 11 due to Fusobacterium spp., of which 8 were identified as F. necrophorum. The age range for the latter cases was 6 months to 16 years, with a median of 4 years. Six of the eight F. necrophorum cases followed ear infection. The current review identified 19/251 F. necrophorum patients who had meningitis, with an age range of 9 months to 51 years and a median age of 6 years; 68% were otogenic in origin, 2 were secondary to sinusitis (4, 34), and only 1 (211) followed throat infection. Three clinical cases of Lemierre's syndrome had meningitis, but none involved F necrophorum. In one the organism was reported to be F. naviforme (394), and in another (175) the culture grew a Bacteroides sp. and an anaerobic streptococcus. The third case had cerebrospinal fluid (CSF) suggestive of meningitis, but no organism was isolated (18). Thus, meningitis is a rare complication of Lemierre's syndrome (1.3%) but a relatively common complication (13/32; 41%) of otogenic F. necrophorum infection. The meningitis associated with F. necrophorum seems to be an aggressive kind, often with frankly purulent CSF (the white cell count ranged from 160 to 36,540/μl, with a median of 1,100/μl) and a high incidence of complications, including sinus thrombosis in five cases (20, 34, 122, 206, 211), cortical ischemia or frank infarction in four (34, 233, 387), and cortical thrombophlebitis in one (318). Two cases also had carotid artery thrombosis or stenosis (211, 387). Not surprisingly, the prognosis seems to be particularly poor, and 6 of the 19 patients died; a further 6 patients recovered but with residual cranial nerve palsies or hemiparesis.
SYSTEMIC MANIFESTATIONS OF F. NECROPHORUM
Anaerobic Bacteremia
Bourgault et al. (43) reviewed the experience with fusobacterial bacteremia at two Canadian teaching hospitals over a 10-year period. Among a total of 40 cases, 8 (20%) were identified as F. necrophorum. Three of these had gastrointestinal sources, three originated from the lower respiratory tract in older patients (age range, 55 to 75 years) with significant underlying disease, and only two cases arose in young adults from oropharyngeal sources consistent with Lemierre's syndrome.
Epaulard et al. (130) reported that among 6,274 bacteremias over a 5-year period at one institution, 26 (0.4%) were adult cases of Fusobacterium sp. bacteremia. Strikingly, 77% of these patients had serious underlying conditions, including cancer, surgery, and chronic organ dysfunction. Six cases involved F. necrophorum, with the remainder being due to F. nucleatum. Four of the F. necrophorum cases were clearly not Lemierre's syndrome. Among the six previously fit patients, two young adults had F. necrophorum bacteremia arising from the oropharynx and another with F. nucleatum bacteremia presented with clinical Lemierre's syndrome. Thus, F. necrophorum cases consistent with Lemierre's syndrome have been reported as occasional cases in recent series of anaerobic bacteremia.
Natural History and Forms of F. necrophorum Septicemia
Classical cases.
Brocard and Guibé (54) described the natural history of postanginal septicemia with F. necrophorum and in particular the various forms that it can present as and evolve into. Classical cases involve the acute fulminant infection so well characterized by Lemierre (239), which led to death within 12 days in the preantibiotic era. In the current literature review these form the great majority of cases (Fig. 3).
Subacute cases.
Subacute cases involved recurrent septicemic episodes with multiple suppurations of a gross form, which inevitably led to a distressing decline to death after 40 to 60 days (54). This is no longer seen with availability of antibiotic therapy.
Late metastatic manifestations.
Brocard and Guibé (54) also described patients who appeared to have a single shower of bacteremia associated with a marked spike of fever following a throat infection. This episode could go uninvestigated and the patient present weeks or months later with a lung abscess, septic arthritis, or liver abscess. It was only by minutely detailed history taking that the original episode could be identified.
In reviewing cases of F. necrophorum in the last 35 years, four cases of this type have been identified. Harrington and Bleicher (188) described a 2-year-old child with sickle cell disease who developed a liver abscess due to F. necrophorum 2 months after being treated for an upper respiratory tract infection. Hagelskjaer and Pedersen (176) described a previously fit 27-year-old man who presented with F. necrophorum liver abscess 2 months after tonsillitis. Trapp et al. (380) reported a 4-year-old child who presented with septic arthritis due to F. necrophorum 1 month after dental work. Sonsale et al. (361) reported a case of septic arthritis in an 8-year-old boy 3 weeks after a dental abscess.
Bacteremia without metastases.
Lemierre et al. (243) described two cases in which the septicemia was of short duration and was followed by rapid recovery without any metastatic lesions. This was described by Brocard and Guibé (54) as an ephemeral bacteremia.
Clearly, with the availability of antibiotics no patient with F. necrophorum bacteremia is left untreated, so the prevalence of ephemeral bacteremia in the antibiotic era cannot be defined. However, in the current review, a total of 21 patients were found to have F. necrophorum bacteremia following a throat infection but with no intracranial or metastatic infection. Three of these patients had deep neck infection; one had a peritonsillar abscess (313), and two had parapharyngeal abscesses (130, 227). Six cases had proven internal jugular vein thrombophlebitis.
There was a considerable spectrum of severity. Thus, one patient died of overwhelming disseminated intravascular coagulation (308), and four other patients had significant complications, i.e., bleeding and peripheral gangrene secondary to disseminated intravascular coagulation (299), hemolytic uremic syndrome (81), septic shock (174), and unilateral deafness (339). On the other hand, in several cases the positive blood culture seemed to come as a surprise (297). Equally, some patients made a rapid recovery and were discharged within a few days (185). Oteo et al. (297) used the term “bacteremic tonsillitis” to describe the relatively benign condition that they had observed, without local or systemic complications or metastases. Six of the 21 patients would fit this description (15, 43, 162, 185, 297).
F. necrophorum cases with systemic metastatic lesions with no clear source.
In her series of 45 cases of necrobacillosis, Eykyn (136) reported that three patients presented with bone or joint infection without an antecedent sore throat or an obvious source. In the current series a total of 18 cases fell into this category. In marked contrast to the F. necrophorum cases fulfilling the current case definition for Lemierre's syndrome, only one (296) had proven internal jugular vein thrombophlebitis and 89% involved metastases to sites other than lung. This included six liver abscesses, five cases of septic arthritis, four cases of osteomyelitis, two cases of endocarditis, two splenic abscesses, one pyomyositis, and one case with skin lesions. These cases seem to fit with Brocard and Guibet's description of patients with a single shower of bacteremia with localization to a site which manifests as an abscess only some time later (54). Eleven of the 18 patients had positive blood cultures, and in 12 patients the organism was isolated from the metastatic lesion. Interestingly, in all 18 patients F. necrophorum was isolated in pure culture. Patients ranged from 3 to 57 years, with a median of 17 years. Two patients had a history of upper respiratory tract infection months before (176, 188). Among the patients with bone or joint infection, four gave a history of preceding trauma or overuse of the affected area. None of the patients died.
Manifestations of sepsis.
Considering the overall severity of the septicemic illness, adult respiratory distress syndrome occurs in a relatively small proportion of cases, and fewer than 10% of cases reported in cited literature since 1990 have required mechanical ventilation. Pham (304) commented from his experience with 12 confirmed cases that neither hypotension nor cardiac failure was a major feature. This is supported by the incidence of septic shock reported in more recent series (Table 4). In the current analysis, 7% of patients with Lemierre's syndrome developed septic shock. One case of bilateral forefoot gangrene secondary to sepsis combined with effects of vasoconstrictor therapy was reported (356).
Although mild renal impairment is quite frequent, significant renal complications are remarkably uncommon for an infection associated with such severe sepsis. Acute renal failure requiring renal replacement therapy occurs in fewer than 5% of cases (136, 245). In the current case review, 2% of Lemierre's syndrome patients developed established renal failure; two of these (155, 390) died, and two (118, 139) required dialysis but eventually recovered. Case reports have described renal abscess, (392), glomerulonephritis, (74), and hemolytic uremic syndrome (81).
Mild thrombocytopenia is not unusual (124), and laboratory evidence of incipient disseminated intravascular coagulation is also relatively common, (177) and was reported in 4% of cases of Lemierre's syndrome in the current review (110, 115, 124, 172, 190, 279, 347, 390, 399). Clinically significant disseminated intravascular coagulation with substantial bleeding is much less common but was seen in one case of Lemierre's syndrome (390) and two other cases of F. necrophorum infection (299, 308).
Metastatic complications. (i) Pleuropulmonary infection.
Pleuropulmonary lesions are the most common metastatic manifestation of Lemierre's syndrome, occurring in 92% of cases in the current case review (Table 4). However, in overall terms, F. necrophorum is a rare cause of anaerobic pleuropulmonary infection such as lung abscess or empyema. Studies (28, 91) show that infection arises in older patients (typically 50 to 60 years) with underlying diseases such as periodontitis and conditions predisposing to chronic aspiration such as alcoholism. In these studies F. necrophorum was rarely reported, with no cases described that were consistent with Lemierre's syndrome. Interestingly, a more recent, large study by Boyanova et al. (45) reported isolation of F. necrophorum from 4% of empyemas.
Septic pulmonary emboli are the archetypal radiologically identifiable lesions of Lemierre's syndrome. However, they can also occur as a manifestation of right-sided endocarditis or septic thrombophlebitis due to other causes. In two series (205, 237) involving a total of 38 cases, infection tended to be associated with intravenous drug use or congenital heart disease, and none were due to F. necrophorum. In another series of 14 cases, Cook et al. (96) reported that four patients had Lemierre's syndrome and all four had fusobacteria (unidentified to the species level) isolated. It is clear that overall F. necrophorum accounts for only a small minority of cases of septic pulmonary emboli. Empyema developed in 27% of postanginal cases of F. necrophorum in the series reported by Hagelskjaer et al. series (177) and was found in 17% of cases fulfilling the Lemierre's syndrome case definition in the current review. A further 33% cases had pleural effusions.
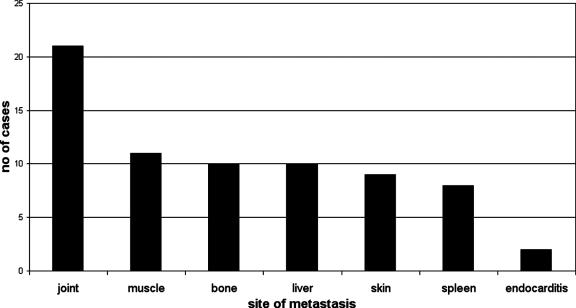
The relative frequency of extrapulmonary lesions is shown in Fig. 5.
FIG. 5.
Distribution of extrapulmonary metastatic lesions in 59/222 cases fitting the current case definition of Lemierre's syndrome cases.
(ii) Bone and joint manifestations.
Culture-positive septic arthritis occurs in around 13 to 27% of cases (Table 4) in published series and in 11% cases fitting the current Lemierre's syndrome case definition. In a number of instances infection has spread beyond the margins of the joint capsule (156, 227, 234). The pus may possess “a peculiarly foul odour” (136). The hip is the most commonly infected joint (eight cases), followed by the knee (six cases) and shoulder (three cases). Other joints reported to be involved include sacroiliac, elbow, ankle, and (in one case) spinal facet joints (402).
Osteomyelitis is reported in fewer than 3% of patients (Table 4) in most series, but 12 cases were identified among the patients fitting the current case definition for Lemierre's syndrome. Bones affected include the humerus (131), femur (148), fibula (268, 364), iliac bone (392), pubic symphysis (326), and cervical vertebra (136). In some cases the process is detected at an early stage, but in others CT scans have revealed intraosseous gas with rapid destruction of bone (148, 392).
(iii) Pyomyositis and abscesses in muscle.
Several reports have described abscesses developing in muscle, including gluteal muscles (2, 127, 227, 236, 239, 305), leg and foot muscles (19, 156, 356, 394), Psoas (84, 127, 301), and the abdominal wall (196, 264). Two cases included pyomyositis of the infraspinatus muscle (398) and leg (8). In the current analysis, 7.2% of Lemierre's syndrome cases had muscle involvement.
(iv) Skin and soft tissue lesions.
Cutaneous pustules and subcutaneous abscesses have been reported in 0 to 3% of cases (Table 4). Seven cases were identified among the 222 cases fulfilling the current case definition of Lemierre's syndrome. Three cases had pustular lesions, (104, 175, 200), three had subcutaneous abscesses (130, 288), and two had necrotic or embolic skin lesions (269, 312). A Fusobacterium sp. was isolated from skin lesion in three patients (104, 288, 312).
(v) Intra-abdominal sepsis.
Abnormal liver function was detected in 49% of patients by Hagelskjaer et al. (177), and patients may be frankly jaundiced (162, 327, 389, 400). Liver abscesses, commonly multiple (37, 92, 125, 218, 227, 279, 286, 365, 400), were identified in 4% of patients in the current series of Lemierre's syndrome and splenic abscesses (9, 106, 219, 222, 236, 327, 392) in 3% patients. Liver abscesses have been reported in other necrobacillosis cases not fulfilling the criteria for Lemierre's syndrome (8, 176, 188, 199, 296, 379). Although abdominal pain is a common presenting symptom (8 out of 15 patients in the series described by Hagelskjaer et al. [177]), peritonitis is a rare complication. Hagelskjaer et al. suggested that the pain might be caused by abdominal microabscesses (177).
(vi) Endocarditis/pericarditis.
Nastro and Finegold (287), in a review of endocarditis due to anaerobic gram-negative bacilli in 1973, identified 37 cases in the literature at that time; 7 were reported to be F. necrophorum, and 2 of these seem likely to be Lemierre's syndrome. However, six of the seven cases were described before 1952. The experience in the antibiotic era has been that endocarditis is a rare complication, with only three case reports (4, 131, 372) since 1970. There was no clear-cut preceding tonsillitis in any of the cases, although one patient had suffered from infectious mononucleosis 5 weeks previously. Two patients (131, 372) had associated lung lesions. Two of the cases (131, 372) required valve replacement.
Golledge et al. (162) described a fatal case of F. necrophorum in which microabscesses were found in the myocardium at autopsy. Four patients with clinical Lemierre's syndrome and a Fusobacterium sp. isolated from blood cultures were reported to have pericarditis or pericardial effusion (252, 274, 367). One patient (266) developed cardiac tamponade.
EPIDEMIOLOGY
Incidence
It is indisputable that Lemierre's syndrome is rare. Collections of case reports can give no information about incidence, but some studies have had population denominators. Thus, Hagelskjaer et al. (177) estimated the incidence in Denmark between 1990 and 1995 to be 0.8 and 1.5 per million persons per year, respectively. Jones et al. (210) reported an incidence of Lemierre's syndrome in southwest England of 0.9 per million persons per year between 1994 and 1999.
Age Incidence
Pham (304) analyzed 12 cases and commented that 10/12 patients were aged between 18 and 29 years. This has been a consistent observation in all later series (Table 4). In the current case series, of 222 cases fitting the Lemierre's syndrome case definition, the median age was 19 years and 89% of patients were aged 10 to 35 years.
Sex Ratio
Although Lemierre (239) stated that both sexes were equally affected, most sizable series show a considerable male preponderance. According to Abt (3), the huge series described by Uffenforde (384) included 87 males and 50 females. Similarly, 19/29 (65.5%) of Eykyn's series (136) were male, 67% of the postanginal cases of F. necrophorum described by Hagelskjaer et al. (177) were male, and 143/208 (68%) of the patients described by Brazier et al. were male (52). By contrast, the difference is less marked in the current series in that 55% of all F. necrophorum cases were male and 57% of clinical Lemierre's syndrome cases were male. There seems to be no adequate explanation for the male preponderance.
Time Trends
Although no rigorous epidemiological data are available, the numbers of cases reported by individual centers suggest a substantial incidence in the first half of the 20th century (11).
Reports of postanginal septicemia or Lemierre's syndrome seemed to dry up between 1955 and the 1980s. In a review in 1976, Bartlett and Gorbach (27) highlighted Gunn's data (171) on anaerobic gram-negative bacteremia prior to 1956, in which 56 of 173 (31%) cases had an oropharyngeal portal of entry, the most common underlying condition being thrombophlebitis of the internal jugular vein. This was in marked contrast to compiled data from surveys in the 1970s, where the portal of entry for anaerobic septicemia was the oropharynx in only 5 of 309 cases (1.5%), none of which involved preceding tonsillar or pharyngeal infection. They concluded that the best explanation for the apparent decrease was the extensive use of antimicrobial drugs in the early stages of pharyngeal infections. This is strongly supported by Brocard and Guibé (54), who in a 1957 review of F. necrophorum septicemia described how, with the advent of antibiotic therapy, the condition had become much rarer.
Sinave et al. (348) reported being able to find only 36 cases in the world literature between 1974 and 1989. Leugers and Clover 1995 (245) were able to identify only 40 blood culture-confirmed cases of necrobacillosis/Lemierre's syndrome between 1950 and 1995. All but two of these had occurred in the 1980s and 1990s. Eykyn (136), in discussing the report she had published with coworkers (277), commented that in the mid-1980s they had been unable to find any doctor who had ever heard of necrobacillosis. There is an overriding impression of a genuine fall in incidence, the explanation of which will be discussed below.
Numerous studies have suggested that the incidence rose in the late 1980s and 1990s. Hagelskjaer et al. (177) reported that there was a significant rise in incidence in Denmark during the course of their study from 1990 to 1995. Ramirez et al. (312) identified 15 cases of F. necrophorum infection at a single institution between 1996 and 2002, including 5 cases of Lemierre's syndrome. Interestingly, all the cases of Lemierre's syndrome occurred in the final 2 years of the study. They discounted any factors such as changes in anaerobic bacteriology techniques or changes in key personnel, e.g., infectious disease physicians or radiologists, as accounting for the rise. Liu and Argent (247) reported seeing four cases in one institution during an 11-month period and suggested that the condition had shown a resurgence.
Although clearly subject to many biases and therefore of questionable provenance as a reflection of incidence, there has been a dramatic rise in published cases of F. necrophorum over the last 30 years. Brazier (50) described trends based on isolates of F. necrophorum from blood cultures referred to a national reference laboratory and showed a sustained rise in the number of cases between 1995 and 2004 (Fig. 6).
FIG. 6.
Referrals of Fusobacterium necrophorum to the United Kingdom Anaerobe Reference Laboratory in 1992 to 2004. (Reprinted from reference 50 with permission from Elsevier.)
Potential Factors Causing Rise in Incidence
Restriction of antibiotic use for sore throat.
In the series reported by Hagelskjaer et al. (177), 30% of Lemierre's syndrome patients had received antibiotics in the early part of their illness. Similarly, in the current review, 29% of Lemierre's syndrome patients were reported to have received an antibiotic. Macrolides seem to feature prominently; 6 of 14 patients in the series reported by Jones et al. (210) had received erythromycin, and macrolides accounted for 50% of prehospital antibiotics in the present review.
Erythromycin resistance is common in F. necrophorum (50), and one could therefore infer that other agents such as penicillin may have some efficacy in aborting progression to Lemierre's syndrome.
Concern about rising levels of antibiotic resistance in many human pathogens has led to the issuing of expert guidelines advocating judicious use of antibiotics in many countries (38, 335). These guidelines have led to changes in practice. Halasa et al. (179) showed a 41% reduction in antibiotic prescriptions for children less than 5 years of age between 1993 and 1999. Smith et al. (350), showed a reduction in antibiotic prescriptions for sore throat in United Kingdom primary care from 80.6/1,000 patient years in 1995 to 42.1/1,000 patient years between 1999 and 2001.
Since the introduction of widespread antibiotic use was associated with the decline in Lemierre's syndrome in the 1950s and 1960s, various authors (210, 245, 253, 312) have speculated that the reduction in antibiotic usage might have contributed to the resurgence in the 1990s. The rise in cases seems to broadly match the timing of changes in antibiotic usage (50). Guidelines which effectively require demonstration of group A streptococcus before prescribing an antibiotic (38) seem the most likely to have an impact on F. necrophorum infection.
Although data show that antibiotic usage can reduce the incidence of significant complications such as peritonsillar abscess (108), the number of patients with sore throat who need to receive an antibiotic to prevent a significant complication is large. In the United Kingdom, Sharland et al. (342) showed that a marked reduction of antibiotic use in children between 1993 and 2003 was not associated with an increase in hospital admissions for peritonsillar abscess. The incidence of Lemierre's syndrome is still extremely low, so this condition in itself cannot dictate overall policy making or a return to excessive use of antibiotics. However, it is a reminder that exclusion of hemolytic streptococcal infection, whether on clinical grounds or based on laboratory testing, does not in itself exclude further assessment for the need for antibiotics. Jones et al. (210) argued that in patients with high fevers and sore throat or tonsillitis, laboratory testing such as that for C-reactive protein could be used to differentiate between those patients likely to have a bacterial infection which would respond to antibiotics and those with viral infections. If an antibiotic is to be used for tonsillitis not associated with streptococcal infection, then erythromycin is not a good choice in view of the rate of resistance in F. necrophorum.
Tonsillectomy, tonsillar architecture, etc.
Although not explicitly stated, it is clear that the vast majority of cases of Lemierre's syndrome occur in individuals who have retained their tonsils (239). In recent decades the indications for tonsillectomy have been greatly restricted, with a concomitant fall in rates of operation from around 60% (267, 395) to 7.5% in the late 1990s (385).
As described in “Peritonsillar Abscess” above, available data suggest that F. necrophorum-associated peritonsillar abscess tends to arise in patients with tonsils affected by recurrent infection. It is therefore tempting to suggest that the presence of scarred tonsils might be a risk factor for Lemierre's syndrome as well. However, Dixon and Helwig (116), in describing six fatal cases, documented the fact that in four of their patients this was the first significant sore throat that the patient had suffered. Similarly, among more recent reports, a history of recurrent throat infections is rarely given (110, 274).
Seasonal Incidence
Although some workers (178) have commented on a lack of obvious seasonal incidence, Brazier et al. (52) reported that the first quarter of the year provided approximately 25% more cases than the other quarters. This is consistent with a similar seasonality seen with peritonsillar abscess (363).
Clusters and Epidemics
Given that Lemierre's syndrome appears to be an exogenously acquired infection, the question arises as to whether epidemiologically linked cases occur. In this regard, Lemierre (239) stated that “Claus [93] and Kissling [223] have observed that small epidemics occur, a fact which I can confirm.” However, no subsequent authors have reported epidemiologically linked clusters.
Ethnic and Geographical Factors
The vast majority of reports are from Europe and North America. However, cases have been reported from Asia and South America. It is impossible to infer from the available data whether there are real differences in incidence by ethnic group or geographical area.
Antibiotic Resistance
In relation to penicillin sensitivity, Busch et al. (72) reported on results of testing 13 respiratory tract isolates of fusobacteria collected in the early 1970s and reported that more than 90% were highly sensitive to penicillin G. Nord et al. (291) reported on β-lactamase-producing anaerobes that they had detected in their own laboratory in Sweden, and no resistant isolates of F. necrophorum were included. By contrast, Applebaum et al. (16), in a much-quoted paper, studied 129 fusobacteria, including 22 strains of F. necrophorum, isolated from normally sterile sites in 28 U.S. centers chosen for their wide geographic spread. The isolates were from recent clinical episodes, and there is no evidence of any selection bias towards more resistant isolates. Five of the F. necrophorum isolates (22.7%) were found to be β-lactamase positive using nitrocefin disks.
Brook (57), in a study without a denominator, reported identifying two children with β-lactamase-producing F. necrophorum strains over a 13-year period. Both originated from perirectal abscesses rather than oropharyngeal sepsis or Lemierre's syndrome. Brazier et al. (52) reported that 2% of 100 referred United Kingdom isolates of F. necrophorum from blood cultures were resistant to penicillin. Ramirez et al. (312) reported that none of their 15 isolates of F. necrophorum was β-lactamase positive. In the current review, no cases of penicillin-resistant F. necrophorum have been identified. Mitre and Rotheram (272) described a typical clinical case of Lemierre's syndrome in a 19-year-old male with an otherwise unidentified anaerobic gram-negative rod which was resistant in vitro to penicillin, chloramphenicol, clindamycin, and tetracycline. The infection did not respond until metronidazole was introduced.
Studies consistently find that 100% of F. necrophorum strains are sensitive to metronidazole, ticarcillin-clavulanate, cefoxitin, co-amoxiclav, and imipenem (16, 52). By contrast, erythromycin resistance is common. Goldhagen et al. (161) documented erythromycin resistance in 22% of cases. Among the isolates studied by Brazier et al. (52), 15% showed either resistance or reduced sensitivity to erythromycin. Similarly, Baquero and Reig (24) reported that more than 66% of fusobacteria isolated in Spain were resistant to erythromycin.
Mortality
In his original series of 20 cases (239), Lemierre reported that only two patients survived. Similarly, Alston, describing 21 cases of necrobacillosis in the United Kingdom (11), most of which arose in the preantibiotic era, reported that only 8/21 survived, and of those with illnesses consistent with Lemierre's syndrome, the survival rate was 3/11. In the current literature review the mortality rate was 6.7% in patients with F. necrophorum and 4.9% among those fulfilling the case definition for Lemierre's syndrome. Possible predisposing factors and complications contributing to death are listed in Table 5. Severity of infection seemed to be a factor; for example, the three cases with bone or joint infection had gross production of gas in the tissues, and four of the six cases with meningitis had frankly purulent CSF with >15 × 109 white blood cells/liter. Delayed presentation or delayed initiation of therapy was identified in six cases. Mediastinitis or retrosternal abscess was present in four cases and in two of these was identified only at autopsy.
TABLE 5.
Fatal cases of F. necrophorum and Lemierre's syndromea
| Author(s) (reference) | Patient age (yr) | Culture result | Site of entry | Lemierre's syndrome | Deep neck abscess or extension | Intracerebral complication(s) | Lung lesions | Other systemic metastases | Day of death (from admission) | Possible contributory factor(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dhawan et al. (115) | 4 | F. necrophorum | Throat | Yes | Yes | Yes | 24 | DP, DIC | ||
| Garimella et al. (154) | 51 | F. necrophorum | Ear | Meningitis | 9 | Alcohol, MGT, BSI, ICP | ||||
| Garnham and Longstaff (155) | 30 | F. necrophorum | Throat | Yes | Yes | 2 | DRx, PI, SS | |||
| Golledge et al. (162) | 17 | F. necrophorum | Throat | Yes | Yes | 5 | DRx, DIC, MA | |||
| Goyal et al. (167) | 8 | F. necrophorum | Throat | Yes | Yes | ? | ||||
| Hirschel et al. (190) | 18 | F. necrophorum | Throat | Yes | Yes | 1 | SS, DIC | |||
| Ieven et al. (200) | 65 | F. necrophorum | Unclear | 5 | PI | |||||
| Islam and Shneerson (203) | 13 | F. necrophorum | Unclear | Meningitis, abscess | 1 | MGT, CI, BH | ||||
| Jacobs et al. (204) | 5 | F. necrophorum | Ear | Meningitis, abscess | 5 | MGT, ICP | ||||
| Larsen et al. (233) | 0.75 | F. necrophorum | Unclear | Meningitis, cerebral infarcts | 2 | MGT, CI, ICP | ||||
| Marangos et al. (261) | 20 | F. necrophorum | Throat | Yes | LPA | Cerebral edema with tonsillar herniation | Yes | 4 | ARDS, CI, ICP, BH | |
| Møller and Dreijer (274) | 43 | F. necrophorum | Throat | Mediastinitis | Yes | 8 | MS, SS | |||
| Møller and Dreijer (274) | 33 | Fusobacterium sp. | Throat | PTrA and RSA | Yes | 27 | DP, SS, RF, RSA | |||
| Mukau (280) | 63 | F. necrophorum | Throat | Yes | RPA and | Yes | 3 | PI, MS, PC | ||
| Potter et al. (308) | 23 | F. necrophorum | Throat | mediastinitis | <1 | SS, DIC | ||||
| Pulcini et al. (310) | 18 | Fusobacterium sp. | Throat | Yes | Yes | <1 | SRx, SS, RF | |||
| Seidenfield et al. (338) | 18 | F. necrophorum | Throat | Yes | PTA | Yes | 9 | OP | ||
| Veldhoen et al. (387) | 4 | F. necrophorum | Ear | Meningitis, ventriculitis, cerebral infarct | 2 | MGT, CI, DP | ||||
| Veldhoen et al. (387) | 5 | F. necrophorum | Ear | Meningitis, cerebral | Yes | 2 | MGT, ICP, CI | |||
| Venkatasawaran and Sze (390) | 36 | Fusobacterium sp. | Throat | Yes | infarcts | Yes | 8 | DP, DIC, RF, SS | ||
| Vohra et al. (392) | 21 | F. necrophorum | Throat | Yes | Cerebral abscess | Yes | Yes | 16 | DRx, ARDS |
Abbreviations: ARDS, adult respiratory distress syndrome; BH, brain herniation; BSI, brain stem infarct; CI, cerebral infarct; DIC, disseminated intravascular coagulation; DP, delayed presentation; DRx, delayed initiation of appropriate therapy; ICP, raised intracranial pressure; LPA, lateral pharyngeal abscess; MS, mediastinitis; MGT, meningitis, MA, myocardial abscess; OP, operative complication; PC, pericarditis; PI, previous illness; PTA, peritonsillar abscess; PTrA, paratracheal abscess; RF, acute renal failure; RPA, retropharyngeal abscess; RSA, retrosternal abscess; SRx, steroid therapy; SS, septic shock.
PATHOGENESIS
Source of Infection
If F. necrophorum is not a part of the normal human oral flora, then a source of infection must be put forward. Pham (304), although quoting the conventional view that Bacillus funduliformis was part of the normal human flora, commented that rather than arising as an endogenous infection, the data from his 12 cases fitted better with an exogenous infection. He postulated that a newly acquired highly pathogenic strain entered across the mucous membranes and caused infection. He commented that human-to-human transmission must be possible and incidentally noted that three of the patients had been found to have used the same swimming pool. He conducted some studies to investigate whether this could be a vehicle of transmission.
Alston (11) suggested that “it is possible that the organism may enter the alimentary tract from animal sources and remain a saprophyte until a circumstance such as tonsillectomy, operation on the intestine or parturition, offers a chance of invasion of the tissue.” Certainly there are data (355) to support the fact that F. necrophorum subsp. funduliforme is present in the guts of herbivores such as cattle, pigs, and dogs, so human infection could arise from this source. Anecdotally, Bhattacharya et al. (36) described a case of Lemierre's syndrome due to F. necrophorum in a veterinary nurse who admitted to frequently kissing her dogs. In relation to F. necrophorum and noma, Falkler et al. (138) postulated that F. necrophorum could enter children's mouths through water and food contaminated with animal feces.
By contrast, Finegold (144) stated that there may be transmission from person to person through intimate contact, such as kissing or even sharing towels or toothbrushes. No evidence for this was quoted. Batty et al. (30) commented that “It is possible that the bacterium is acquired at the same time as infectious mononucleosis by close contact between carriers and susceptible individuals.” For periodontitis there are good data showing intrafamilial transmission of the associated bacteria (238).
The frequency of cases by age of onset peaks in late teens and early 20s. Hentges (189) stated that although the majority of the adult oral flora is acquired by the age of 5 years, there are a few nutritionally fastidious organisms, e.g., Prevotella melaninogenicus and spirochetes, that appear at puberty when presumably the necessary nutritional factors are available from the host. There are no data to support this as a factor for F. necrophorum. Irigoyen et al. (202) suggested that another factor in allowing entry of F. necrophorum into the tonsil may be the atrophy of tonsillar tissue in late childhood and adolescence. They suggested that the deep crypts that form with regression of tonsillar tissue could act as a fertile soil in which infection could be established. There appears to be no firm evidence to support this.
Another factor might be that acquisition of F. necrophorum could be linked to onset of close contact with members of the opposite sex, as with infectious mononucleosis, which is unquestionably linked to onset of Lemierre's syndrome in some cases (see below). It is currently unclear whether acquisition of F. necrophorum is rapidly followed by invasive infection or whether certain individuals, possibly those with abnormal tonsils, become carriers and that invasive infection is then triggered by another event such as a viral infection, as postulated for noma (129).
Spread from Tonsil to Internal Jugular Vein
The sequence of events leading from tonsillitis to full-blown Lemierre's syndrome or postanginal septicemia are complex, with several steps as illustrated in Fig. 7. A key step in developing postanginal septicemia is spread of the organism from the tonsil to the internal jugular vein. Authors have differed on how this occurs. Fränkel (149) was firmly of the view of this being hematogenous via the tonsillar vein. Uffenforde (384), quoted by Abt (3), believed that lymphangitis and lymphadenitis were the primary processes and that the infection extended secondarily to the veins, causing a periphlebitis and endophlebitis with associated thrombosis. Claus (93), on the basis of histological observation, thought that the majority of cases of postanginal sepsis originated in abscesses which were found in the proximity of the tonsil and that these pus collections spread deeper into the loose connective tissue of the pharynx and attach themselves to the walls of the veins, producing purulent periphlebitis and endophlebitis. Boharas (41) cited autopsy evidence in stating that in most cases of postanginal sepsis there was cellulitis or an abscess in the parapharyngeal space. Hematogenous spread via thrombophlebitis seems most likely, although this cannot be demonstrated in all cases.
FIG. 7.
Putative sequence of events after throat infection with Fusobacterium necrophorum.
Host Factors
Factors favoring entry of F. necrophorum.
Pham (304) emphasized the relatively low virulence of B. funduliformis in the throat. He suggested that a break in the mucosa was required to allow entry. In this regard, in a small proportion of cases, infection seems to have become established following some form of trauma to the tonsils or oropharynx. Liu and Argent et al. (247) described a case in which a labial frenectomy had been undertaken shortly before onset. In two reports (32, 323), infection occurred after tonsillectomy. Similarly, Hagelskjaer et al. reported that two of their cases of F. necrophorum bacteremia occurred after tonsillectomy (177). Paaske et al. (298) described a fatal case involving pneumonia and adult respiratory distress syndrome that occurred 5 days after uvulo-palato-pharyngoplasty. The organism was not identified beyond Fusobacterium sp. Dellamonica and Bernard (107) described a woman who admitted to having receptive oral sex with a new partner 2 days preceding onset of illness. Palmer et al. (300) describe a case which arose in a young child following oropharyngeal trauma with a ballpoint pen. Sastre et al. (328) reported a case of splenic abscess due to F. necrophorum in which the bacteremia was attributed to the patient's habit of sucking the needle before injecting.
Golpe et al. (163) quoted a study by Sayers et al. (330) that nicotine may enhance the potency of toxins of oral pathogens. However, few reports of Lemierre's syndrome mention smoking in the patient's history.
Role of EBV.
The first report of a possible link between F. necrophorum and Epstein-Barr virus (EBV) was a case reported in 1983 by Adams et al. (4). The patient was a 16-year-old girl who was diagnosed as having infectious mononucleosis confirmed by a positive heterophile antibody test 5 weeks prior to an illness starting with sinusitis and involving septicemia, endocarditis, and meningitis. Since then, 23 cases have been reported in which infectious mononucleosis was linked to Lemierre's syndrome or invasive F. necrophorum infection (Table 6). In 5 cases the basis for the diagnosis was solely a positive Monospot test of uncertain provenance; however, in 11 others formal EBV serology was undertaken and showed evidence of recent infection. While the level of importance of infectious mononucleosis may be exaggerated by publication bias, this seems to be a genuine association. At the same time, as can be seen from Table 6, false-positive Monospot tests have been reported in patients with F. necrophorum infection or Lemierre's syndrome.
TABLE 6.
Cases of F. necrophorum/Lemierre's syndrome associated with positive tests for infectious mononucleosis or false-positive Monospot testsa
| Author(s) (reference) | Lemierre's syndrome | Age (yr) | Sex | EBV results | Timing of EBV | Steroids | Clinical feature(s) | Bacteriology results |
|---|---|---|---|---|---|---|---|---|
| Adams et al. (4) | No | 16 | F | HAB | 35 days | No | Meningitis, endocarditis | BC, F. necrophorum |
| Alvarez and Schreiber (12) | Yes | 14 | F | EBV serology, “recent not acute” | Admission | No | IJV thrombosis, pulmonary lesions | BC, Fusobacterium sp. |
| Andrianakis et al. (14) | No | 17 | F | EBV serology, recent infection | 8 days | No | Empyema | Pleural fluid, F. necrophorum, Pseudomonas aeruginosa, Proteus mirabilis |
| Bartell (25) | Yes | 19 | M | Monospot | 28 days | Yes | PTA, SPE | BC, F. necrophorum, Prevotella sp |
| Boz et al. (46) | Yes | 28 | M | EBV serology, recent infection | Admission | No | IJV thrombosis, pulmonary infiltrates | Bone marrow, F. necrophorum |
| Clarke et al. (92) | Yes | 19 | F | HAB | Admission | No | Liver abscess | BC and pus, F. necrophorum |
| Dagan and Powell (104) | Yes | 24 | F | Monospot | 8 days | Yes | SPE | BC, Fusobacterium sp., Streptococcus sp, Eubacterium sp. |
| Dagan and Powell (104) | Yes | 23 | M | Atypical mononuclear cells and Monospot | 6 days | No | SPE, skin lesions, pericardial effusion | BC, F. necrophorum |
| Dagan and Powell (104) | No | 18 | M | Monospot | 9 days | Yes | Pericardial effusion | BC, F. necrophorum |
| Dalamaga et al. (105) | Yes | 20 | M | EBV serology, recent infection | 25 days | No | Lung consolidation | BC, F. necrophorum |
| Gold et al. (159) | Yes | 17 | F | Monospot | 14 days | Yes | IJV thrombosis, SPE | BC, F. varium |
| Haasper et al. (174) | No | 18 | F | Monospot | 6 days | Yes | Septicemia, no metastases | BC, F. necrophorum |
| Koay et al. (227) | No | 32 | M | EBV serology, recent infection | Admission | No | IJV thrombosis, no metastatic lesions | BC and neck pus, F. necrophorum |
| Mann (260) | No | 14 | F | “Positive for infectious mononucleosis” | 7 days | No | Retropharyngeal abscess, empyema, mediastinitis | Pleural fluid, F. necrophorum, Prevotella sp., Peptostreptococcus sp., Eikenella corrodens |
| Martin and Wright (264) | Yes | 17 | M | EBV serology, recent infection | 28 days | No | Abdominal wall abscess | BC, F. necrophorum |
| Matten and Grecu (265) | Yes | 19 | F | Atypical mononuclear cells and HAB | 4 days | Yes | Empyema | Pus, F. necrophorum |
| Møller and Dreijer (274) | Yes | 25 | M | EBV serology, recent infection | Postadmission | No | Empyema | BC, Fusobacterium sp., Streptococcus sp., peptostreptococci, lactobacilli |
| Møller and Dreijer (274) | Yes | 33 | F | EBV serology, recent infection | 21 days | No | Empyema | Pus, Fusobacterium sp., Bacteroides gracilis, streptococci (2), Candida sp. |
| Ramirez et al. (312) | No | 14 | F | EBV serology, recent infection | ? | ? | Septicemia only | BC, F. necrophorum |
| Ramirez et al. (312) | Yes | 16 | M | EBV serology, recent infection | ? | ? | SPE, osteomyelitis | BC, F. necrophorum |
| Rathore et al. (313) | No | 16 | F | EBV serology, recent infection | Admission | Yes | PTA, no metastatic lesions | BC: F. necrophorum |
| Shek et al. (344) | Yes | 15 | F | Atypical mononuclear cells and raised EBV titers | Admission | No | SPE | BC, F. necroformans |
| Woywodt et al. (399) | Yes | 18 | F | EBV serology, recent infection | Admission | No | IJV thrombosis, pulmonary lesions | BC, F. nucleatum |
| Koay et al. (227) | Yes | 17 | F | False-positive Monospot | Admission | No | Multiple liver abscesses | BC, F. necrophorum |
| Møller and Dreijer (274) | Yes | 15 | M | False-positive Monospot | Admission | No | Pulmonary infiltrates | BC, F. necrophorum |
| Pulcini et al. (310) | Yes | 18 | F | False-positive Monospot | ? | Yes | SPE | BC, F. necrophorum |
| Stahlman et al. (364) | Yes | 13 | M | False-positive Monospot | 7 days | Yes | IJV thrombosis, osteomyelitis | Bone aspirate, F. necrophorum |
Abbreviations: BC, blood culture; F, female; HAB, positive heterophile antibody test; M, male; PTA, peritonsillar abscess; SPE, septic pulmonary emboli.
There is also evidence of a link between recent EBV infection and peritonsillar abscess. Parulekar et al. (302) described two cases of clear-cut infectious mononucleosis that were complicated by peritonsillar abscess; one of these patients probably had F. necrophorum in the tonsillar pus. This patient had a history of recurrent tonsillitis. Portman et al. (307) described 30 patients with peritonsillar abscess, of whom 19 were considered possibly to have infectious mononucleosis and Monospot testing was undertaken. Six of 19 were positive. Each of the positive patients had a consistent clinical illness. Peritonsillar abscess accompanied by dysphagia or trismus occurred 10 to 21 days after onset of fever or sore throat. The 13 patients with peritonsillar abscess but no evidence of infectious mononucleosis had a much shorter duration of illness, averaging 5 days. None of these patients had an exudative tonsillitis. Portman et al. (307) quoted that 1% of patients with severe infectious mononucleosis requiring hospitalization develop peritonsillar abscess.
In a very recently published study, Jensen et al. (207), using a PCR for detection of F. necrophorum subsp funduliforme in throat swabs, found the highest detection rate in four patients with infectious mononucleosis. Although the numbers were small, this further supports the link between EBV infection and F. necrophorum infection.
Brook (60) reviewed data on bacterial infection in infectious mononucleosis and concluded not only that there was increased recovery of anaerobic organisms from the tonsillar surfaces during acute infection but also that several studies had demonstrated that antimicrobials such as metronidazole were beneficial, particularly in those with anginose infection.
Several mechanisms whereby infectious mononucleosis might predispose to invasive infection with F. necrophorum have been put forward. Potential factors include lymphatic obstruction, impaired migration of peripheral blood leukocytes, and impaired immunoglobulin production due to T-cell suppression of B cells. In this regard, Stenfors and Raisanen (369) presented data on local antibody production showing that in infectious mononucleosis, attachment of secretory immunoglobulin A (IgA) and IgG to bacteria on the tonsillar surface was greatly reduced compared to controls and this was associated with massive bacterial colonization on the tonsils. Marklund et al. (262) had previously noted that low concentrations of secretory IgA were found in infectious mononucleosis compared to in healthy controls. Stenfors et al. (368) showed an increase in bacterial penetration of the tonsillar epithelium in infectious mononucleosis. Preceding viral infection is recognized to be a risk for other invasive bacterial infections such as meningococcal disease (75) and staphylococcal infection (256) and indeed is believed to trigger the onset of noma (129).
Acquired immunity.
If not only severe invasive disease due to F. necrophorum but also exposure to and acquisition of this organism occur principally in young adults, then one could reasonably postulate that acquired immunity might play a role in determining the age-related decline in disease incidence. There appear to be no human data to support or refute this. However, extensive animal studies have been conducted with a view to developing vaccines. These have shown that antibody generated to leukotoxin affords some protection against experimental infection (325). The relevance of this work to humans is very debatable, but, particularly with the growing information about the role of F. necrophorum subsp. funduliforme in recurrent tonsillitis (see above), this seems to be a fertile field for study.
Steroids.
Steroid therapy in infectious mononucleosis has been associated temporally with peritonsillar abscess (184, 302) and postanginal sepsis (25, 104, 159, 174, 265, 302, 313). In addition, some cases have occurred after steroid use for tonsillitis with no laboratory evidence of infectious mononucleosis (110). Among the 23 cases of Lemierre's syndrome or F. necrophorum infection associated with infectious mononucleosis, 7 patients (30%) were reported to have received steroids (Table 6).
Hypercoagulability, etc.
In recent years there has been growing interest in the importance of thrombophilia, whether inherited or acquired, as a risk factor for thrombo-embolic disease, including in children and young adults. Thus, de Veber et al. (113) showed that among 92 children with sinovenous thrombosis or arterial ischemic stroke, 35 (37%) had one or more coagulation abnormalities. In a subsequent series, de Veber et al. (112) showed that 39 of 123 children with sinovenous thrombosis tested for hyperprothrombotic disorders had abnormal results. Given that thrombophlebitis of the internal jugular vein is an integral component of Lemierre's syndrome and that mastoid infection with F. necrophorum is often complicated by thrombophlebitis of the venous sinuses, it is not unreasonable to question whether thrombophilia might be a risk factor for development of these severe manifestations. The evidence is incomplete as yet, but there are some tantalizing suggestions from recent papers.
Hope et al. (197) described a case of Lemierre's syndrome with extensive thrombosis with reduced anti-thrombin 3. Klinge et al. (225) presented a case with extensive thrombosis of internal jugular vein and sigmoid sinus, in which clotting analysis revealed an activated protein C resistance with a factor V Leiden mutation. Oestereicher-Kedem et al. (293) presented a series of seven children, aged 7 months to 7 years, with acute otitis media complicated by mastoiditis and sinus thrombosis. Five of seven had prothrombotic disorders. Four children had elevated levels of lipoprotein apolipoprotein lp a, which competes with plasminogen for binding to endothelium. Four had antibodies to beta2 glycoprotein and cardiolipin, which are markers of antiphospholipid syndrome. One child was heterozygous for factor V Leiden. Schmid et al. (331) presented a clinical case of Lemierre's syndrome with confirmed internal jugular vein thrombosis and pulmonary lesions. Screening for hypercoagulability revealed two known risk factors: a mutation in the prothrombin gene and elevated lipoprotein a.
Cho et al. (86) described a 34-year-old man with Lemierre's syndrome. Coagulation studies identified an antiphospholipid syndrome. Constantin et al. (95) reported a patient who had a combination of one prothrombogenic and one antifibrinolytic variation. The latter was the 4G-4G homozygous genotype of the PAI-1 gene, which encodes a primary antifibrinolytic molecule. This genotype is responsible for an increase in plasma concentrations of PAI-1. Moreover, this mutation alone, or in combination with other prothrombotic genetic anomalies, is a risk factor for myocardial infarction and venous thromboembolic phenomena. The authors also commented that Westendorp et al. (396) had shown that the 4G-4G genotype, although having no effect on the risk of meningococcal disease, greatly increased the risk of septic shock in those who did develop meningococcal infection.
In a series of nine pediatric cases of septic jugular venous thrombosis secondary to oropharyngeal infection reported by Goldenberg et al. (160), all seven children tested were found to have thrombophilia. This included three with antiphospholipid antibodies, four with raised factor VIII levels, and factor V Leiden in one. Repeat testing for ALPA and factor VIII after 6 months showed that the factor VIII level returned to normal in all cases and that the ALPA was no longer present in two of three patients. The conclusion was reached that these abnormalities had been a manifestation of the acute inflammatory prothrombotic state rather than intrinsic thrombophilia. The cases included in this study seemed atypical in the sense that only one of the nine cases included grew a Fusobacterium sp.
Thus, thrombophilia may be a factor in some cases of Lemierre's syndrome and related invasive infections with F. necrophorum, but the true prevalence of these disorders in this situation needs to be more fully elucidated. In the meantime, the potential practical significance is such that it would be reasonable to investigate all such patients for evidence of thrombophilia.
Variations in the innate immune response.
Constantin et al. (95) described a typical case of Lemierre's syndrome in a 17-year-old female and studied the patient's DNA for single-nucleotide polymorphisms in Toll-like receptors and coagulation genes. They identified functional variations in the Toll-like receptor 5 gene and speculated that this could be associated with an increased risk of infection with F. necrophorum.
Bacterial Virulence Factors
Because of the incidence and economic significance of necrobacillosis in ruminants, including cattle and sheep and also exotic zoological specimens such as wallabies, a considerable amount of effort has gone into the study of the factors that determine virulence in F. necrophorum. Unfortunately, the relevance of the work to human necrobacillosis is limited, partly because of the questionable relevance of animal models and also because of the fact that most attention has logically focused on F. necrophorum subsp. necrophorum, the cause of animal infection, when the available evidence suggests that it is F. necrophorum subsp. funduliforme which is the major cause of human disease. There are currently few data to suggest that more virulent strains are associated with invasive disease. Pham (304) studied 12 human isolates in animals (mainly involving rabbits infected by intravenous inoculation of broth cultures) and reported that there was an organ tropism which correlated with how virulently the strain had behaved in the human case. He reported that virulent strains possessed an exclusive affinity for liver and lung in the animal model whereas the less virulent strains affected bones, joints, and muscles. Batty et al. (30) compared strains from cases of Lemierre's syndrome with isolates from sore throats by using enterogenic repetitive intergenic consensus-PCR and found similar strains in both groups.
Animal models.
The most widely studied model is the ability to generate necrotic lesions by subcutaneous inoculation in mice. Smith and Turner (351) showed that 0.1 ml of an 18-h broth culture of an animal isolate of F. necrophorum subsp. necrophorum invariably led to a fatal infection. By contrast, Smith and Thornton (352) showed that in this model, human isolates from blood cultures and pus produced, at the most, mild local lesions that rapidly healed. In addition, unlike animal strains of F. necrophorum subsp. necrophorum, these human strains showed no enhancement of infectivity when suspended in sublethal concentrations of Staphylococcus aureus broth. Although intravenous inoculation of the human isolates into mice produced purulent lesions in the liver and elsewhere, they did not cause the rapidly fatal infection characteristic of isolates of F. necrophorum subsp. necrophorum.
Leukotoxin.
Roberts (321) was first to document a leukotoxin activity in F. necrophorum. Since then the nature and action of the toxin have been fully characterized (282, 284, 374). Several studies have shown a correlation between virulence and leukotoxin production (99, 126). In addition, studies have shown that immunization with purified or recombinant leukotoxin can generate protective antibody in cattle and mice (283, 325). F. necrophorum subsp. funduliforme has been shown to produce smaller amounts of leukotoxin than F. necrophorum subsp. necrophorum. In a very recent study, Zhang et al. (403) showed significant differences in the nucleotide sequences of the leukotoxins from the two subspecies. It is generally assumed that leukotoxin plays some role in human infection and could contribute to the characteristic necrotic abscesses.
Endotoxin.
Lipopolysaccharide is an important virulence factor in many gram-negative organisms, and F. necrophorum appears to be no exception. Garcia et al. (152) showed that purified lipopolysaccharide from F. necrophorum was virulent in various animal models, behaving like a classical endotoxin. According to Tan (373), Inoue et al. (201) showed that the quantity and composition differed between F. necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme, with a higher lipopolysaccharide content in F. necrophorum subsp. necrophorum. Finally, lipopolysaccharide of F. necrophorum subsp. necrophorum was more potent in causing chicken embryo mortality, hemorrhagic necrosis of solid tumors, a pyrogenic response, and a local Shwartzman reaction than that from F. necrophorum subsp. funduliforme. Garcia et al. (152) undertook chemical analysis of the endotoxins from the two subspecies. The endotoxins isolated were devoid of heptose and 3-deoxy-d-manno-octulosonate. F. necrophorum subsp. necrophorum contained much higher levels of lipid A, whereas higher levels of protein, total phosphorus, and hexose were detected in F. necrophorum subsp. funduliforme (151).
Hemolysin.
There is good evidence that iron availability plays a central role in bacterial pathogenesis of bacterial infection (71). According to Tan et al. (373), Kanoe (215) showed that in bovine hepatic abscesses due to F. necrophorum, the hemolysin appeared to be contributing to abscess formation. Amoako et al. (13) showed that F. necrophorum subsp. necrophorum was more hemolytic than F. necrophorum subsp. funduliforme.
Hemagglutinin.
It has long been known that F. necrophorum produces a hemagglutinin, and the significant differences between the two subspecies were apparent from an early stage (31). Kanoe et al. (216) presented work on guinea pig mesenteric microcirculation, observed in vivo on a microscope stage. They demonstrated that both F. necrophorum and cell-free hemagglutinin caused thrombus formation, initially in venules and then in arterioles. They concluded that thrombosis is an early step in pathogenesis of necrosis. Kanoe et al. (217) provided data that suggested that hemagglutinin is one of the components of the cell surfaces of F. necrophorum subsp. necrophorum. Although thrombophlebitis and necrosis are key parts of the development of Lemierre's syndrome, the relevance of hemagglutinin to pathogenesis of human infection is uncertain.
Adhesins.
Attachment to the host cell is a key first step in pathogenesis. According to Tan et al. (373), Miyazato et al. (273) had demonstrated the presence of fimbriae in both subspecies of F. necrophorum and Shinjo and Kiyoyama (345) had shown that strains without fimbriae did not cause severe infections in mice. The receptor for F. necrophorum adherence is not certain, but there is some evidence that it may contain a protein moiety. Okada et al. (295) studied the adherence of F. necrophorum subsp. necrophorum to the surfaces of animal cells. Pretreatment of the bacterial cells with hemagglutinin antiserum reduced the degree of attachment to murine and rabbit cells. Scanning electron microscopy revealed that the adherent fusobacteria often penetrated into murine and rabbit cell membranes. These observations indicate that bacterial attachment contributes to the establishment of the infection in mice and rabbits.
Platelet aggregation.
Given the importance of septic thrombophlebitis to the pathogenesis of Lemierre's syndrome, the ability of F. necrophorum to cause platelet aggregation (147) seems highly relevant. Forrester et al. (147) showed that although most strains of F. necrophorum subsp. necrophorum were active in this regard, strains of F. necrophorum subsp. funduliforme were not. Thus, yet again the relevance of animal data to human infection is in doubt.
Synergy.
A considerable amount of work has been done on possible synergy between F. necrophorum and other bacteria in animal infection. Thus, Smith et al. (354), using a strain of F. necrophorum from a wallaby, presumably F. necrophorum subsp. necrophorum, in a mouse subcutaneous abscess model, showed that the infective dose of F. necrophorum was drastically reduced by coinfection with Escherichia coli. Alpha-hemolytic streptococci, Corynebacterium pyogenes, Pseudomonas aeruginosa, Bacteroides fragilis, and Fusobacterium nucleatum produced a similar effect. Tan et al. (373) suggested that facultative bacteria utilize oxygen and lower redox potential to create an anaerobic environment which protects F. necrophorum, which is otherwise vulnerable due to lack of superoxide dismutase, and that reciprocally, the leukotoxin of F. necrophorum protects other bacteria from phagocytosis.
The relevance of these demonstrations of synergy to human infection is unclear. Brocard and Guibé (54) commented that even in typical cases of Lemierre's syndrome with F. necrophorum isolated from blood as the single organism, the infection was typically polymicrobial in the tonsil and the lateral pharyngeal space, and synergy of the type described in animals might occur. Jensen et al. (207), in a PCR-based study, showed that in patients with tonsillitis there was a significant association between detection of F. necrophorum subsp. funduliforme and group C streptococci. They postulated that there might be synergy between the two organisms. In the current series, four cases fulfilling the criteria for Lemierre's syndrome had polymicrobial infection involving fusobacteria and group C streptococci.
Conclusion: Overall Pathogenic Mechanism
The virulence and pathogenesis of F. necrophorum are complex and unusual. Although many virulence factors have been identified, it is clear that strains of F. necrophorum subsp. funduliforme tend to either be negative or produce smaller amounts of toxins or less active toxins than F. necrophorum subsp. necrophorum. The relative importance of the different putative virulence factors and the relevance of synergy with other organisms remain in considerable doubt. One of the most striking aspects of F. necrophorum is the tightly clustered age distribution in late teens and early 20s. This applies not just to Lemierre's syndrome but, in parallel, to tonsillitis (207) and peritonsillar abscess (212). The very recent data on molecular detection of F. necrophorum from throat swabs of healthy individuals in exactly this age group (207) suggest the possibility that peak exposure and acquisition might be at that age. The consequences of acquisition and carriage might then depend on a range of factors, including strain virulence, structural aspects of the tonsil, presence or absence of immunity, and concomitant viral infection such as EBV. Host factors such as a predisposition to development of tonsillar and internal jugular vein thrombophlebitis as a result of thrombophilia and genetic factors determining the innate immune response to bacteria such as single-nucleotide polymorphisms in Toll-like receptors (95) may similarly affect the likelihood of development of metastatic lesions and severe sepsis. The putative routes of entry, spread, etc., are shown in Fig. 7.
DIAGNOSIS
Clinical Diagnosis
For a syndrome that is so characteristic, it is remarkable how often the diagnosis is missed until an anaerobic gram-negative rod is isolated from blood culture or another sterile site. There are several contributory factors. First, many clinicians and even medical microbiologists have never seen a case and indeed are unaware of the condition (136). Second, the protean manifestations of the septic emboli can distract clinicians from the initial oropharyngeal sepsis, and indeed in many cases the original sore throat has resolved when the patient presents with septicemia. In addition, the cases can present to a wide variety of specialties, including general medicine, otorhinolaryngology, orthopedics, general surgery, and neurosurgery.
Onset.
Both Abt (3) and Lemierre (239) highlighted the great importance of the onset of chills or rigors, especially if they were observed after the angina had subsided. This typically occurs at around 4 to 5 days after the onset of the sore throat, but the interval may be up to 12 days. Pham (304) highlighted the fact that rigors are a very unusual feature of tonsillitis. The occurrence of chills/rigors indicates that the organisms are gaining access to the circulation. Rash is strikingly unusual in patients with Lemierre's syndrome or necrobacillosis.
Oropharyngeal/cervical lesions.
The initial sore throat varies in severity and may indeed have started to improve when the onset of the septicemic illness occurs. When the patient presents to hospital, the appearance of the throat can vary from a normal appearance through mild tonsillar and/or pharyngeal inflammation to a severe exudative tonsillitis with peritonsillar abscess. Hall (180) issued the following dark warning: “Be not deceived by a comparatively innocent appearing pharynx as the veins of the tonsil may be carrying the death sentence of your patient”.
Patients often complain of neck pain and sometimes stiffness. Cervical lymphadenopathy may be present either unilaterally or bilaterally, often in the anterior triangle. More importantly, there may be a tender (normally unilateral) swelling at the angle of the jaw or anterior to, and parallel with, the sternomastoid muscle (the cord sign), reflecting the development of internal jugular venous thrombophlebitis (195, 255, 338). This has been detected in 26 to 45% of cases (245, 278, 348). Unfortunately, it is often mistakenly thought to be due to tender cervical nodes. Increasing use of imaging has probably improved the ascertainment of this feature, and internal jugular venous thrombophlebitis was recorded in 59% of cases in the current series of Lemierre's syndrome cases. Ipsilateral Horner's syndrome or otherwise unexplained palsies of 9th to 12th cranial nerves are additional premonitory signs of carotid sheath involvement.
Differential Diagnosis
Many case reports describe how even at the stage of hospital admission, viral pharyngitis was the clinical diagnosis (162). The high level of C-reactive protein present in Lemierre's syndrome (178) should readily eliminate uncomplicated viral infection as a possible diagnosis.
Infectious mononucleosis is often considered as the initial diagnosis, and confusion can result from two factors. First, serologically confirmed EBV infection may precede Lemierre's syndrome (104, 274). In addition, false-positive heterophile antibody tests have been reported in patients with confirmed Lemierre's syndrome (104, 274, 364). Distinguishing features will include the presence of generalized rather than purely cervical lymphadenopathy and atypical mononucleosis on blood film with infectious mononucleosis, and unilateral signs of internal jugular venous thrombosis with Lemierre's syndrome, together with metastatic septic lesions and a markedly raised C reactive protein (178).
Lemierre (239) described how lung lesions manifest early (sometimes on the first day of the septicemia) and characteristically cause intense pleuritic-type pain with dyspnea and often hemoptysis. Not infrequently patients are thought to have acute bacterial pneumonia, Legionnaires’ disease (178), or aspiration pneumonia (278). The key distinguishing features are the fact that lung involvement in Lemierre's syndrome is preceded by sore throat and may be accompanied by internal jugular vein thrombosis and that initially lung lesions consist of multiple diffusely disseminated nodular lesions which rapidly cavitate. Not infrequently patients present with hemoptysis (79, 166, 230, 327), which can cause further diagnostic confusion. Jensen et al. (208) highlighted the fact that F. necrophorum can cause false-positive results in Mycoplasma pneumoniae 16S rRNAPCR tests.
The presence of rapidly developing cavitating lung lesions is often mistaken for staphylococcal pneumonia or right-sided staphylococcal endocarditis. Leugers and Clover (245) reported a patient with cavitating lung lesions in whom Staphylococcus aureus was isolated from sputum before blood cultures grew F. necrophorum. Among the series of 11 cases described by Moreno et al., 6 were initially treated with antistaphylococcal therapy for presumed right-sided endocarditis (278). Similarly, four of five of the cases described by Seidenfield et al. were treated initially for presumed staphylococcal infection (338). The history of preceding sore throat will be a clue, together with clinical or radiological evidence of internal jugular vein thrombophlebitis. Patients may rapidly develop pleural effusions, and empyema occurs in 10 to 15% of cases.
The fact that patients frequently present with abdominal pain and have liver function abnormalities can often mislead clinicians into suspecting intra-abdominal sepsis (193). This can be compounded if an initial isolation of an anaerobic gram-negative rod is assumed to be Bacteroides sp. (178). Confusion may arise because the initial chest X ray is often within normal limits (82, 329, 337).
As so graphically described by Lemierre himself (239), once one is familiar with the condition, the pattern of illness is remarkably characteristic, with onset of high fever and rigors in a patient with a history of sore throat and with associated jugular venous thrombosis and metastatic lesions.
In patients in whom drainage of an abscess or empyema is undertaken before the diagnosis has been made, the smell of the pus may be a crucial clue. Rather than the foul odor of mixed anaerobic infection, “the diagnosis of this infection may be suggested by the peculiar odour—like Limburger or overripe Camembert cheese—of pus produced by it” (11).
Radiology
Chest X-ray films may show diffuse ill-defined infiltrates that appear as round opacities in the lung (168), but in many cases there is no definable abnormality on initial films (82, 337). Even when present, the findings are frequently nonspecific. Cavitation visible on chest X ray is a late finding. CT scanning has consistently been shown to be more sensitive than plain X rays and also will detect the lesions earlier (231). The features of septic pulmonary emboli that can be confirmed on CT are multiple, predominantly peripheral lesions that show peripheral enhancement with central areas of reduced attenuation on administration of contrast medium. Other features include wedge-shaped lesions abutting the pleura, air bronchograms within nodules, and extension into the pleural space (231). The opacities are classically related to a branching vessel apparently leading into the center of the nodule, the so-called feeding vessel, which has been considered highly suggestive of septic embolism, with a prevalence ranging from 67 to 100% in various series. However, a recent study by Dodd et al. (117) using high-resolution CT and multiplanar reconstructions has shown that most of these vessels course around the nodule and that the others are pulmonary veins.
Because internal jugular vein thrombophlebitis is frequently unapparent clinically, there is considerable dependence on imaging techniques to detect this important feature. In some patients this may be the first hard evidence to suggest the diagnosis of Lemierre's syndrome (337). In other patients, documentation of extensive thrombophlebitis may be an indication for anticoagulation or even internal jugular vein ligation. The standard method of diagnosis of deep venous thrombosis is catheter venography. However, this is invasive and carries the risk of propagating a septic process (47), and it is now rarely performed.
Ultrasound scanning has been advocated for imaging jugular venous thrombosis. The technique is rapid, low cost, and noninvasive. It also has the advantage that it can be performed at the patient bedside. Color Doppler ultrasound can show an absence of flow inside the vein. However, ultrasound cannot image beneath the clavicle or the mandible. The presence of gas bubbles in vessels may cause pitfalls in examination (288). In addition, recent thrombus has little inherent echogenicity, and so sensitivity is impaired (47). Several studies have demonstrated failure of ultrasonography to demonstrate internal jugular vein thrombosis that was readily demonstrable by contrast CT (12, 196). It is, however, of particular value for serial scanning after initial imaging (7).
The use of CT following intravenous contrast has been advocated by some authors (389). CT has the ability to show distended veins with enhancement of the walls, intramural filling defects, and swelling of adjacent soft tissues (196). However, as pointed out by Braun et al. (47) this contains several sources of error, which include erroneous identification of the thrombosed vein with its internal lucency and peripheral enhancement for necrotic lymph nodes or abscesses. Recent thrombus, by virtue of its hyperdensity, may not be apparent on contrast-enhanced CT scans. On the other hand, CT has the advantage of displaying the complex anatomy of the parapharyngeal space, etc., with exceptional clarity and permits definition of the extension of a collection or abscess. Inevitably CT involves exposure to ionizing radiation, and in addition, contrast CT requires the injection of iodine-containing contrast medium.
Braun et al. (47) demonstrated in three patients that MR imaging was effective at yielding high-quality images of jugular venous thrombosis with bright intraluminal signal intensity from the thrombus. In addition, MR imaging does not have the safety drawbacks of CT in terms of contrast injection and exposure to ionizing radiation. Carpenter et al. (73) compared contrast venography to MR venography for detection of deep venous thrombosis and reported a sensitivity of 100% for MR venography and a specificity of 96%. Nakamura et al. (281) considered that MR imaging was the most accurate and reliable method of detecting the presence and extent of deep venous thrombosis. However, owing to its high cost, it is probably not realistic as the first-line investigation. Mañé et al. (258) used radionucelotide venography to demonstrate delayed blood flow in the jugular vessels, but this is not usually necessary.
Laboratory Diagnosis
There are many case reports in which the laboratory isolated a gram-negative bacillus from blood cultures but several days then ensued before the identification was available. In reality, F. necrophorum has a characteristic pleomorphic morphology, with filaments, short rods, and coccoid elements, that differs from that of other Bacteroidaceae, including other fusobacteria such as F. nucleatum. Several authors (see, e.g., references 54 and 213) have reinforced the observations of Hallé (Fig. 1) on the contrast between the morphology in tissue and pus (predominantly coccobacillary and often showing bipolar staining) and that in culture (remarkable polymorphism with irregularly stained rods, filamentous forms, and vesicular forms). Teissier et al. (378) commented that Rist (320) and Guillemot et al. (170) had described the organism as Bacteroides thetoides because of the resemblance to the Greek letter θ (theta) due to the stain being taken up only at the center and the extremities of the bacillus. Although it is a rare blood culture isolate, as Hagelskjaer Kristensen and Prag (178) pointed out, “This characteristic morphology should be known to all microbiologists and should immediately suggest the diagnosis of necrobacillosis and guide the treatment.” The only other rapid laboratory procedure at this stage in the blood culture would be analysis for the presence of volatile fatty acid end products by gas-liquid chromatography. A volatile fatty acid profile containing a single major peak of butyric acid (with minor peaks of acetic and propionic acid) is highly indicative of a member of the genus Fusobacterium (49). Unfortunately, these days it is rare for a routine diagnostic laboratory to have this capability (47).
Attention to detail is essential to achieve good results in isolating F. necrophorum from pus and tissue samples. De Rautlin de la Roy et al. (109) described how isolation of F. necrophorum from cerebral abscess pus was successful only for the portion of sample inoculated immediately into semisolid medium which had been gassed out.
Basic blood agar media usually support only a very feeble growth, and much larger colonies result if the medium is supplemented with vitamin K, hemin, menadione, and a reducing agent such as cysteine hydrochloride (49). In Brazier's experience (49), fastidious anaerobe agar (Lab M Ltd.) produces the best growth of all media for Fusobacterium spp.
According to Brazier (50), once it is cultured on a suitable medium, the colonial characteristics of F. necrophorum are so typical as to make it easily recognizable by the experienced eye. The colonies are cream-yellow in color, smooth, round, and entire, with an odor redolent of cabbages. Hemolysis may vary between strains, but on horse blood agar most will have a narrow zone of complete hemolysis surrounding the colonies. Under long-wave UV light, colonies fluoresce with a vivid greenish yellow color (48), although this property is medium dependent and is best seen on fastidious anaerobe agar. F. necrophorum also produces indole, a metabolite that can be readily detected directly from colonies on an agar plate by using the spot indole reagent p-dimethylcinnamaldehyde. Another readily detectable feature of F. necrophorum is the production of lipase on egg yolk agar, although F. necrophorum subsp. funduliforme is less strongly positive than F. necrophorum subsp. necrophorum.
Most routine laboratories will depend on commercial kits for full identification. These include API rapid ID 32A (Biomérieux), AN-IDENT identification system (Bio-Mérieux Vitek Inc.), Vitek ANI card (Biomérieux), and RapID-ANA II (Innovative Diagnostic Systems, Inc., Atlanta, GA). Hagelskjaer Kristensen and Prag (178) commented that in the API rapid ID 32A system, the only positive tests are indole and alkaline phosphatase and that in their experience the latter test was not consistently positive, which led to erroneous identification as F. nucleatum. Unfortunately, formal evaluations of commercial systems tend to involve panels of strains dominated by Bacteroides spp., with few if any isolates of F. necrophorum. Schreckenberger et al. (334) undertook an evaluation of Vitek ANI cards, but unfortunately this included only 11 strains of Fusobacterium spp., of which only 1 was F. necrophorum. The system had reasonable success at identification to genus but only limited success to species level (27.3% of strains of Fusobacterium sp. were identified). Similarly, in an evaluation of the ATB 32A system (now known as API rapid ID 32A), Kitch and Applebaum (224) tested only 10 strains of fusobacteria, of which 3 were incorrectly identified. Celig and Schreckenberger (77) evaluated the RapID-ANA II system but did not include any strains of F. necrophorum in their panel of isolates. Quentin et al. (311) evaluated the AN-Ident system but similarly included only three isolates of Fusobacterium spp., all of which were F. nucleatum.
One has to conclude that for reliable identification to species level, additional tests are required. F. necrophorum is the only Fusobacterium sp. that ferments lactate to propionate, a fact that that is used for confirmation of F. necrophorum by gas-liquid chromatography. However, for most routine laboratories, confirmation would probably be best achieved by referral of significant isolates, such as from blood culture, to a reference laboratory.
Molecular detection.
As discussed by Song (360) the difficulties of obtaining a positive culture of non-spore-forming anaerobes and then accurately identifying them to species level make molecular methods potentially attractive. As described above, Aliyu et al. (10), using a real-time PCR targeting the rpoB gene sequence, were able to detect F. necrophorum in throat swabs of patients with sore throat. The same group (9) described a typical case of Lemierre's syndrome in a 27-year-old male with lung, kidney, spleen, and cerebral lesions. Cultures were negative, but using the same PCR they were able to detect F. necrophorum in aspirates from the renal and brain lesions. They then confirmed the specificity of their results by sequencing the amplicons. There are clearly practical issues with availability of a monoplex F. necrophorum-specific PCR, given the rarity of the condition. Jousimies-Somer et al. (213) commented that currently there are no commercially available methods but that in due time this would facilitate rapid and accurate diagnosis and identification of anaerobes such as F. necrophorum.
MANAGEMENT
Antibiotic Therapy
Effect of antibiotics.
Gunn (171) reported that with the availability of antibiotics, mortality from “Bacteroides septicemia” fell from 81% in the prechemotherapy era to 33%. Brocard and Guibé (54), describing their own experiences which encompassed both pre- and postantibiotic eras, were more sanguine and stated that penicillin had entirely transformed the prognosis of septicemia due to B. funduliformis and that patients who would formerly have inevitably succumbed were spectacularly healed.
Despite these endorsements, on first encountering the condition one is bound to be struck by the slow response to antibiotics even when they are shown to be active in vitro. In three series the median time from initiation of appropriate antibiotic therapy to resolution of fever ranged from 8 to 12 days (278, 245, 178).
Clinical resistance to antibiotic therapy.
A number of authors have described clinical resistance to penicillin despite activity in vitro. Moreno et al. (278) reported clinical resistance to penicillin, and Ahkee et al. (6) reported a typical Lemierre's syndrome case that did not respond to penicillin but subsequently responded to ticarcillin-clavulanic acid. Beldman et al. (32) described therapeutic failure with clindamycin and a subsequent response to penicillin. Ma et al. (254) described a patient who did not respond to clindamycin or 7 days of intravenous penicillin who settled rapidly after starting metronidazole.
Relapses.
Sanders et al. (327) described a classic case in whom intravenous penicillin led to resolution of fever after 10 days but relapse then occurred, which settled with metronidazole. Figueras et al. (142) reported relapse of bacteremia 5 days after stopping a 15-day course of intravenous penicillin and cefotaxime for ear-associated meningitis. An MR scan demonstrated a retropharyngeal abscess, but the infection settled on penicillin, cefotaxime, and metronidazole without drainage of the abscess. Hope et al. (197) described a relapse after therapy with ceftazidime. Trapp et al. (380) described a case of F. necrophorum septic arthritis in a child who relapsed clinically while receiving intravenous ampicillin-sulbactam, and repeat arthrotomy yielded F. necrophorum again. The infection settled with meropenem and clindamycin.
Factors accounting for poor response. (i) Antibiotic resistance.
F. necrophorum is intrinsically resistant to gentamicin and quinolones, and tetracyclines have relatively poor activity. As described in Epidemiology above, apart from erythromycin, in vitro resistance does not appear to be a major issue with F. necrophorum.
(ii) Antibiotic activity.
Kowalsky et al. (229), describing in vitro serum bactericidal studies, showed that clindamycin had weaker bactericidal activity as shown by a significantly smaller area under the bactericidal curve than metronidazole or imipenem. That study also provided some evidence in favor of combination therapy. Lechner et al. (235) quoted Stratton (371) that clindamycin exhibits only bacteriostatic activity in vivo.
(iii) Coinfecting β-lactamase-positive flora.
Numerous studies have show that the tonsillar flora may contain β-lactamase-producing organisms (66, 315, 382). Given that the initial tonsillar phase of infection is often mixed, the action of penicillin against F. necrophorum could be impaired by these organisms. However, this seems unlikely to be significant once the infection reaches the internal jugular vein and the bloodstream, where infection is most commonly purely F. necrophorum.
(iv) Penetration of antibiotic into clot and activity there.
Since septic thrombophlebitis is such a key part of the syndrome and since repeated episodes of bacteremia occur secondary to release of septic emboli from the internal jugular vein, the ability of antibiotics to penetrate and be active in fibrin clot seems likely to be highly relevant. Bergeron et al. (35) studied this, although not using F. necrophorum, and showed that clots were hard to penetrate by antibiotics, with variations between agents. For drugs which reached high fibrin clot levels rapidly, the killing was more impressive. They also showed that resistance could develop and that morphological and structural changes in bacteria were found within the different regions of the clot. Finally, in their model peak antibiotic levels in tissues or serum did not correlate with efficacy.
(v) Abscesses and necrotic lesions.
Septic pulmonary emboli, empyema, and abscesses with necrosis in sites such as lungs, liver, and muscle are such a feature of Lemierre's syndrome that it is hardly surprising that the response to antibiotics is poor. Clearly, wherever possible a key part of management is to drain abscesses. It is notable that in a fatal case, previously undiagnosed mediastinitis was found at postmortem (280).
(vi) L forms.
Langworth (232) quoted other authors suggesting that the presence of L forms of F. necrophorum may be responsible for persistent infection after penicillin therapy but also referred to studies suggesting that L-form isolates are not pathogenic for laboratory animals. It seems unlikely that this is a major factor.
Death versus delay in instigating antibiotic therapy.
There is some evidence that delay in initiation of antibiotics affects outcome. Thus, Leugers and Clover (245) found that two deaths occurred in 8 patients in whom antibiotic therapy was delayed for 4 days or more, contrasting with only one death in 29 patients who were promptly treated.
Antibiotic of choice.
It will never be possible to conduct controlled trials to identify the optimum agent(s). Decisions must be based on in vitro data together with anecdotal clinical evidence. On this basis, most authors would not advocate clindamycin or penicillin alone. The choice would seem to lie between a carbapenem, a penicillin/β-lactamase inhibitor combination, or metronidazole. A number of authors have expressed a preference for metronidazole (123, 198, 272, 277). Armstrong et al. (17) reported that in the series of cases that they identified in the literature, metronidazole was the most commonly used agent.
Perceived advantages of metronidazole (150) include excellent activity against all strains of Fusobacterium spp. and good penetration into tissue. Penetration of metronidazole into polymorphonuclear leukocytes has been described as equivalent to that in extracellular fluid. The drug also has excellent oral bioavailability, which means that a switch to oral therapy can be made when appropriate. Concern about the possible relevance in humans of mouse carcinogenicity studies (324) seem to have limited the use of metronidazole for anaerobic infection in the United States, in contrast to Europe, where usage has been widespread for many years (135).
Several anecdotes describe failure with conventional therapy and then dramatic improvement on adding or switching to metronidazole. Mitre and Rotheram (272) described a case with persistent fever and repeat positive blood cultures despite therapy with clindamycin then chloramphenicol. Introduction of metronidazole led to a rapid response. Spencer et al. (362), in describing a case of F. necrophorum infection with cerebral abscess, described failure to respond to ampicillin and chloramphenicol and then rapid improvement after adding metronidazole. Moore-Gillon et al. (277) commented, “…despite a lack of conclusive evidence our clinical impression is that definitive improvement coincided with introduction of metronidazole in at least 3 of our patients.” Most authors recommend use of metronidazole in combination with a penicillin to cover for mixed infection involving organisms such as oral streptococci.
Duration of therapy.
Armstrong et al. (17) noted that in their analysis of published cases, duration of treatment ranged from 9 to 84 days, with a median of 42 days. Given the wide variation in severity, the different sites of metastatic infection, and the rate of response to therapy, it is not surprising that there is no consistency concerning duration of therapy. Even with a good response, less than 2 weeks of therapy would seem to be unwise given the fact that relapses have been documented. However, many reports describe prolonged intravenous therapy, i.e., 6 weeks or more (314). Assuming that the infection settles clinically, this seems unjustified, and most cases could probably be switched to an oral regimen including metronidazole after a maximum of 2 to 3 weeks of intravenous therapy to complete a total of up to 6 weeks of therapy.
Anticoagulants
The use of anticoagulants has been controversial, and no controlled studies exist. Bach et al. (19) found that defervescence and clinical improvement were temporally associated with initiation of therapy with heparin. Similarly, Goldhagen et al. (161) reported that in one of their patients there was radiographic evidence of ongoing pulmonary emboli until full anticoagulation was achieved. Hong et al. instigated anticoagulation when serial sonograms showed a loose clot in the internal jugular vein (196). Nakamura et al. (281) commented on the considerable experience of use of heparin for treatment of septic pelvic thrombophlebitis with evidence of success and therefore, by implication, that the benefit might be transferable to the internal jugular vein. They also stated that “administration of heparin has shortened the course of Lemierre's syndrome and helped avoid surgical drainage,” although the sources they quoted remain anecdotal. In the current series of cases fulfilling the definition of Lemierre's syndrome, 23% received anticoagulation. Similarly, Armstrong et al. (17) reported that in 53 cases published in the 1990s, only 21% received anticoagulation and yet most had responded well. There are certainly neither objective data from studies nor a general consensus that anticoagulation is required for all cases with proven internal jugular vein thrombosis.
In cases where thrombosis involves the sigmoid or cavernous sinus, the situation is graver and anticoagulation has been more frequently used. Jones et al. (211) discussed the risks and benefits of anticoagulation in cavernous sinus thrombosis and the lack of data available. Doyle and Jackler (119) showed that there was clinical benefit when anticoagulation was used to treat suppurative thrombophlebitis of the sigmoid sinus propagating retrograde to the cavernous sinus.
de Veber et al. (111) studied anticoagulation therapy in pediatric patients with sinovenous thrombosis due to all causes. They suggested that anticoagulant therapy with low-molecular-weight heparin was safe and that it could have a role in therapy of children with sinovenous thrombosis. Jaremko et al. (206) concluded that anticoagulation probably decreases the risk of clot extension and possibly shortens recovery times. Hoehn (192) commented that one potential indication was a cerebral infarct or sinus venous thrombosis. She cautioned that there are hazards to use of anticoagulation in acutely septic patients who may have organ failure and concluded as follows: “…because there is controversy, perhaps the existence of underlying thrombophilia should guide our management.” Repanos et al. (316) quoted a review of the Cochrane database (366) that found only two small trials which suggested that anticoagulants are probably safe and may be beneficial for patients with sinus thrombosis, but the results were not conclusive.
Repanos et al. (316) also reviewed the options for patients with sinus thrombosis not responding to standard treatment. They quoted a study of regional infusion of thrombolytic therapy (173). Treatment apparently resulted in few complications, but a Cochrane review of this subject could neither identify the indications for this treatment nor establish that it was safe (89).
Surgery
Many reports describe how infection settled only after drainage of pus, whether it was a collection in the neck such as a peritonsillar or lateral pharyngeal abscess (97), an empyema, (169), a liver abscess (176, 227), septic arthritis (32, 156), or an abscess in a muscle (398). It is therefore a key part of management to assess the patient for evidence of a collection that needs to be drained.
In the preantibiotic era, in the face of repeated septic emboli from the internal jugular vein and mortality in excess of 90%, drastic measures were attempted, including ligation or excision of the internal jugular vein. Lemierre et al. (243) commented that Kissling (223) had claimed success in reducing mortality but that in his own experience it had not been of benefit. In the last 20 years there have been a significant number of reports of internal jugular vein ligation or excision (12, 19, 39, 78, 83, 86, 140, 164, 253, 288, 336, 339, 349, 388, 389, 399).
In some cases (253) internal jugular vein ligation was undertaken because of clinical and radiological evidence of ongoing septic emboli despite optimal medical therapy. Dramatic clinical improvement was noted in the postoperative period. Schwartz and Nguyen (336) reported a patient who on the 11th day of therapy, including anticoagulation, still had a swollen, tender neck. The affected portions of the external jugular vein were excised and purulent material in the supraclavicular fossa drained. This led to rapid clinical improvement. Nguyen-Dinh et al. (288) described continuing septicemia and extension of internal jugular vein thrombosis on repeat CT scan which led to surgical intervention. At operation a large cervical phlegmonous collection was found. The internal and anterior jugular veins were ablated. Cho et al. (86) described a patient in whom resolution of symptoms was not achieved after 2 weeks of therapy. Surgical intervention with excision of the involved portion of vein and drainage of surrounding abscesses led to rapid resolution.
In other reports, however, it would seem that ligation has been undertaken on the grounds of proven thrombophlebitis but apparently without giving medical therapy adequate time to be effective. Since large numbers of cases with proven thrombosis resolve without surgery, this seems unjustified. It is probably indicated only in patients with persistent septic embolization or evidence of extending thrombus despite aggressive medical therapy. This is likely to be relatively rare. In terms of the operative intervention of choice, Lustig et al. (253) commented that there are no studies comparing surgical ligation and excision and that therapeutic success had been achieved by both methods.
Adjunctive Therapy
Velez et al. (388) described a patient with severe and extensive tissue necrosis in the neck requiring three surgical procedures. After the third operation, a 2-week course of hyperbaric oxygen was instigated. This coincided with clinical improvement, but it is impossible to determine if the hyperbaric oxygen contributed or not. Similarly, Hodgson et al. (191) described an 18-year-old male who after 6 days of intensive medical therapy remained critically unwell with severe lung and neck lesions. Hyperbaric oxygen therapy was instigated, but as with the previous case it is not possible to determine whether it contributed to recovery.
The use of activated protein C for treatment of severe sepsis is controversial (397). Bhattacharya et al. (36) used activated protein C in a patient with typical Lemierre's syndrome associated with F. necrophorum who presented with hypotension, respiratory failure, and renal impairment. However, there are no data available on its efficacy specifically for F. necrophorum.
CONCLUSIONS
Human infection with F. necrophorum usually involves F. necrophorum subsp. funduliforme rather than F. necrophorum subsp. necrophorum, which causes infection in many animals. The two show significant differences, particularly in relation to virulence factors and behavior in experimental animal models.
Severe infection with F. necrophorum (necrobacillosis) is most often seen as a septicemic illness which follows tonsillitis in previously fit young adults and produces a characteristic clinical picture known variously as postanginal sepsis or Lemierre's syndrome. The key elements of this are a septic thrombophlebitis which propagates from the tonsillar veins to the internal jugular vein and then leads to septicemia and septic emboli which cause necrotic abscesses in lungs and other sites, such as bones, joints, and liver.
There has been no formal case definition for Lemierre's syndrome but I propose a definition based on an analysis of cases from the last 4 decades: (i) history of anginal illness or compatible clinical findings, (ii) evidence of either metastatic lung lesions or metastatic lesions in other sites, and (iii) either evidence of internal jugular vein thrombophlebitis or isolation of F. necrophorum or Fusobacterium sp. from blood culture or a normally sterile site.
F. necrophorum is not isolated in all clinically typical cases of Lemierre's syndrome, and other organisms such as peptostreptococci have been associated, although these may be secondary passengers. It is still unclear whether all cases involve F. necrophorum and negative cultures simply reflect factors such as timing of sampling, prior antibiotic therapy, etc.
F. necrophorum is unique among non-spore-forming anaerobes, first for its virulence and association with Lemierre's syndrome as a monomicrobial infection. In addition, there is no convincing evidence that F. necrophorum is part of the normal flora, and it seems probable that unlike other non-spore-forming anaerobes, this is an exogenously acquired infection. A recent PCR study detected the organism in throat swabs from 21% of healthy young adults. Further study of this is indicated. The source of infection is unclear; suggestions include acquisition from animals such as dogs or human-to-human transmission. The latter could account for the striking age incidence, which parallels that of infectious mononucleosis.
F. necrophorum bacteremia can be associated with a range of presentations from classical fulminating Lemierre's syndrome, through a transient bacteremia which leads weeks later to a focal abscess, to a relatively benign bacteremia referred to as “bacteremic tonsillitis” by one author.
In the upper respiratory tract, F. necrophorum is a common isolate from peritonsillar abscess. Recent evidence suggests that F. necrophorum can be limited to the throat and cause acute or particularly recurrent tonsillitis. Further study of this is merited.
Several factors predisposing to invasive infection have been identified. Approximately 10% of published cases are associated with infectious mononucleosis, which may facilitate invasion. Patients with infectious mononucleosis who present with the anginose form should be considered for metronidazole therapy. Erythromycin resistance is common in F. necrophorum, and this may be a predisposing factor, since among patients who received antibiotics prior to admission, there is an excess who have received macrolides to which many strains are resistant. Recent work suggests that underlying thrombophilia may be a factor in the development of the characteristic thrombophlebitis of the internal jugular vein.
Although sometimes included within Lemierre's syndrome, F. necrophorum infections arising from the ear affect a younger age group and behave differently, with fewer systemic complications but a much higher rate of intracranial complications. Similarly, cases of F. necrophorum/necrobacillosis arising from other sites such as the gut or genital tract tend to occur in older patients with underlying disease.
F. necrophorum and Lemierre's syndrome were relatively common in the preantibiotic era, and the condition seems to have virtually disappeared in the 1950s and 1960s following the widespread use of antibiotics for upper respiratory tract infection. In the last 15 years there has been a rise in incidence. This may be related to restriction in antibiotic use for sore throat. Clinicians should be aware that exclusion of streptococcal infection in a patient with severe tonsillar infection does not exclude a bacterial cause.
The key to diagnosis of Lemierre's syndrome is awareness of this rare but life-threatening condition. Imaging modalities such as ultrasound scanning for internal jugular venous thrombosis and CT scanning of the thorax to detect septic pulmonary emboli are vital adjuncts to diagnosis. Laboratory staff should be familiar with the very striking Gram stain morphology of F. necrophorum and its basic cultural characteristics, since an early alert from the laboratory to clinicians could avert delays of several days in reaching a diagnosis. In addition, commercial anaerobic identification kits may fail to achieve identification to species.
Despite some reports, in vitro resistance to penicillin is uncommon. However, the response to antibiotic therapy of established Lemierre's syndrome is slow. Anecdotal data suggest that metronidazole may be superior to other agents. The role of anticoagulants is unclear, but they probably should be reserved for cases not responding to antibiotics or with thrombosis which is extending to involve the cerebral sinuses. Drainage of collections of pus such as empyema, septic arthritis, and deep neck collections is a crucial part of management. The mortality from established infection remains at around 5%.
Supplementary Material
Acknowledgments
I thank Michele Foster for help with preparing the manuscript, Richard Ebrey for finding the photograph of André Lemierre, and the staff of Exeter Health Library for their considerable support in obtaining archive and other material.
Footnotes
Supplemental material for this article may be found at http://cmr.asm.org/.
REFERENCES
- 1.Aas, J. A., B. J. Paster, L. N. Stokes, I. Olsen, and F. E. Dewhirst. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721-5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abele-Horn, M., P. Emmerling, and J. F. E. Mann. 2001. Lemierre's syndrome with spondylitis and pulmonary and gluteal abscesses associated with Mycoplasma pneumoniae pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 20:263-266. [DOI] [PubMed] [Google Scholar]
- 3.Abt, I. A. 1932. Postanginal sepsis. J. Pediatr. 1:8-15. [Google Scholar]
- 4.Adams, J., T. Capistrant, K. Crossley, R. Johanssen, and S. Liston. 1983. Fusobacterium necrophorum septicaemia. JAMA 250:35. (Letter.) [PubMed] [Google Scholar]
- 5.Agarwal, R., P. S. Arunachalam, and D. A. Bosman. 2000. Lemierre's syndrome: a complication of acute oropharyngitis. J. Laryngol. Otol. 114:545-547. [DOI] [PubMed] [Google Scholar]
- 6.Ahkee, S., L. Srinatha, A. Huang, M. J. Raff, and J. A. Ramirez. 1994. Lemierre's syndrome: postanginal sepsis due to anaerobic oropharyngeal infection. Ann. Otol. Rhinol. Laryngol. 103:208-210. [DOI] [PubMed] [Google Scholar]
- 7.Albertyn, L. E., and M. K. Alcock. 1987. Diagnosis of internal jugular vein thrombosis. Radiology 162:505-508. [DOI] [PubMed] [Google Scholar]
- 8.Ali, M. T., P. Jain, and G. Narayanswami. 2003. Lemierre's syndrome: sepsis complicating a dental procedure. Chest 124:300. [Google Scholar]
- 9.Aliyu, S. H., P. F. K. Yong, M. J. Newport, H. Zhang, R. K. Marriott, M. D. Curran, and H. Ludlam. 2005. Molecular diagnosis of Fusobacterium necrophorum infection (Lemierre's syndrome). Eur. J. Clin. Microbiol. Infect. Dis. 24:226-229. [DOI] [PubMed] [Google Scholar]
- 10.Aliyu, S. H., R. K. Marriott, M. D. Curran, S. Parmar, N. Bentley, N. M. Brown, J. S. Brazier, and H. Ludlam. 2004. Real-time PCR investigation into the importance of Fusobacterium necrophorum as a cause of acute pharyngitis in general practice. J. Med. Microbiol. 53:1029-1035. [DOI] [PubMed] [Google Scholar]
- 11.Alston, J. M. 1955. Necrobacillosis in Great Britain. Br. Med. J. 1955:1524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez, A., and J. R. Schreiber. 1995. Lemierre's syndrome in adolescent children—anaerobic sepsis with internal jugular vein thrombophlebitis following pharyngitis. Pediatrics 96:354-359. [PubMed] [Google Scholar]
- 13.Amoako, K. K., Y. Goto, and T. Shinjo. 1993. Comparison of extracellular enzymes of Fusobacterium necrophorum subsp. necrophorum and Fusobacterium necrophorum subsp. funduliforme. J. Clin. Microbiol. 31:2244-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrianakis, I. A., A. N. Kotanidou, M. T. Pitaridis, G. J. Saroglou. D. N. Exarhos, C. S. Roussos, and I. P. Bellenis. 2002. Life-threatening bilateral empyema and mediastinitis complicating infectious mononucleosis. Intens. Care Med. 28:663-664. [DOI] [PubMed] [Google Scholar]
- 15.Aoki, M., R. C. Noble, E. J. Scott, and G. V. Osetinsky. 1993. Lemierre's syndrome caused by Fusobacterium necrophorum: a case report. J. Ky. State Med. Assoc. 91:141-142. [PubMed] [Google Scholar]
- 16.Applebaum, P. C., S. K. Spangler, and M. R. Jacobs. 1990. β-Lactamase production and susceptibilities to amoxicillin, amoxicillin-clavulanate, ticarcillin, ticarcillin-clavulanate, cefoxitin, imipenem, and metronidazole of 320 non-Bacteroides fragilis Bacteroides isolates and 129 fusobacteria from 28 U.S. centers. Antimicrob. Agents Chemother. 34:1546-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong, A. W., K. Spooner, and J. W. Sanders. 2000. Lemierre's syndrome. Curr. Infect. Dis. Report. 2:168-173. [DOI] [PubMed] [Google Scholar]
- 18.Auber, A. E., and P. A. Mancuso. 2000. Lemierre syndrome: magnetic resonance imaging and computed tomographic appearance. Mil. Med. 165:638-640. [PubMed] [Google Scholar]
- 19.Bach, M. C., J. H. Roediger, and H. M. Rinder. 1988. Septic anaerobic jugular phlebitis with pulmonary embolism: problems in management. Rev. Infect. Dis. 10:424-427. [DOI] [PubMed] [Google Scholar]
- 20.Bader-Meunier, B., G. Pinto, M. Tardieu, D. Pariente, S. Bobin, and J.-P. Dommergues. 1994. Mastoiditis, meningitis and venous sinus thrombosis caused by Fusobacterium necrophorum. Eur. J. Pediatr. 153:339-341. [DOI] [PubMed] [Google Scholar]
- 21.Baker, A. S., and W. W. Montgomery. 1987. Oropharyngeal space infections, p. 227-265. In J. S. Remington and M. N. Sevartz (ed.), Current clinical topics in infectious diseases, vol. 8. McGraw-Hill, New York, NY. [PubMed] [Google Scholar]
- 22.Bang, B. 1890. Om Aarsagen til local Nekrose. Maanedsskrift Dyrlæger 2:235-259. [Google Scholar]
- 23.Bang, B. L. F. 1897. Die aetiologie des seuchenhaften (infectiosen) verwerfens. Z. Thiermedizin 1:241-278. [Google Scholar]
- 24.Baquero, F., and M. Reig. 1992. Resistance of anaerobic bacteria to antimicrobial agents in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 11:1016-1020. [DOI] [PubMed] [Google Scholar]
- 25.Bartell, J. M. 2001. Lemierre's syndrome. Wisc. Med. J. Proceedings of the 2001 Annual Meeting of the American College of Physicians. http://www.acponline.org/chapters/wi/associates/2001/vignette18.htm.
- 26.Bartlett, J. G., A. B. Onderdonk, E. Drude, C. Goldstein, M. Anderka, S. Alpert, and W. M. McCormack. 1977. Quantitative bacteriology of the vaginal flora. J. Infect. Dis. 136:271-277. [DOI] [PubMed] [Google Scholar]
- 27.Bartlett, J. G., and S. L. Gorbach. 1976. Anaerobic infections of the head and neck. Otolaryng. Clin. N. Am. 9:655-678. [PubMed] [Google Scholar]
- 28.Bartlett, J. G., and S. M. Finegold. 1974. Anaerobic infections of the lung and pleural space. Am. Rev. Respir. Dis. 110:56-77. [DOI] [PubMed] [Google Scholar]
- 29.Batty, A., and M. W. D. Wren. 2005. Prevalence of Fusobacterium necrophorum and other upper respiratory tract pathogens isolated from throat swabs. Br. J. Biomed. Sci. 62:66-70. [DOI] [PubMed] [Google Scholar]
- 30.Batty, A., M. W. D. Wren, and M. Gal. 2005. Fusobacterium necrophorum as the cause of recurrent sore throat: comparison of isolates from persistent sore throat syndrome and Lemierre's disease. J. Infect. 51:299-306. [DOI] [PubMed] [Google Scholar]
- 31.Beerens, H. 1954. Procédé de différenciation entre Spherophorus necrophorus (Schmorl 1891) et Spherophorus funduliformis (Hallé 1898). Ann. Inst. Pasteur Paris 86:384-386. [PubMed] [Google Scholar]
- 32.Beldman, T. F. J., H. A. Teunisse, and T. J. Schouten. 1997. Septic arthritis of the hip by Fusobacterium necrophorum after tonsillectomy: a form of Lemierre syndrome? Eur. J. Pediatr. 156:856-857. [DOI] [PubMed] [Google Scholar]
- 33.Bennett, K. W., and A. Eley. 1993. Fusobacteria: new taxonomy and related diseases. J. Med. Microbiol. 39:246-254. [DOI] [PubMed] [Google Scholar]
- 34.Bentham, J. R., A. J. Pollard, C. A. Milford, P. Anslow, and M. G. Pike. 2004. Cerebral infarct and meningitis secondary to Lemierre's syndrome. Pediatr. Neurol. 30:281-283. [DOI] [PubMed] [Google Scholar]
- 35.Bergeron, M. G., J. Robert, and D. Beauchamp. 1993. Pharmacodynamics of antibiotics in fibrin clots. J. Antimicrob. Chemother. 31(Suppl. D):113-136. [DOI] [PubMed] [Google Scholar]
- 36.Bhattacharya, S., S. A. Livsey, M. Wiselka, and S. S. Bukhari. 2005. Fusobacteriosis presenting as community acquired pneumonia. J. Infect. 50:236-239. [DOI] [PubMed] [Google Scholar]
- 37.Bilfinger, T. V., C. K. Hayden, K. T. Oldham, and T. E. Lobe. 1986. Pyogenic liver abscesses in nonimmunocompromised children. South Med. J. 79:37-40. [DOI] [PubMed] [Google Scholar]
- 38.Bisno, A. L., M. A. Gerber, J. M. Gwaltney, Jr., E. L. Kaplan, and R. H. Schwartz. 2002. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin. Infect. Dis. 35:113-125. [DOI] [PubMed] [Google Scholar]
- 39.Bliss, S. J., S. A. Flanders, and S. Saint. 2004. A pain in the neck. N. Engl. J. Med. 350:1037-1042. [DOI] [PubMed] [Google Scholar]
- 40.Blomquist, I. K., and A. S. Bayer. 1988. Life-threatening deep fascial space infections of the head and neck. Infect. Dis. Clin. N. Am. 2:237-264. [PubMed] [Google Scholar]
- 41.Boharas, S. 1943. Postanginal sepsis. Arch. Intern. Med. 71:844-853. [Google Scholar]
- 42.Bourbeau, P. P. 2003. Role of the microbiology laboratory in diagnosis and management of pharyngitis. J. Clin. Microbiol. 41:3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourgault, A.-M., F. Lamothe, P. Dolcé, L. Saint-Jean, and P. Saint-Antoine. 1997. Fusobacterium bacteremia: clinical experience with 40 cases. Clin. Infect. Dis. 25(Suppl. 2):S181-S183. [DOI] [PubMed] [Google Scholar]
- 44.Bouvier, D. P., R. D. Armstrong, D. M. Bradshaw, and R. E. Hoenzsch. 1995. Fusobacterium necrophorum septicaemia without a primary focus. South. Med. J. 78:1521-1522. [DOI] [PubMed] [Google Scholar]
- 45.Boyanova, L., V. Djambazov, G. Gergova, D. Iotov, D. Petrov, D. Osmanliev, Z. Minchev, and I. Mitov. 2004. Anaerobic microbiology in 198 cases of pleural empyema: a Bulgarian study. Anaerobe 10:261-267. [DOI] [PubMed] [Google Scholar]
- 46.Boz, G. A., S. Iskender, R. Caylan, K. Aydin, and I. Koksal. 2005. A case of Lemierre's syndrome following Epstein-Barr virus infection. Anaerobe 11:185-187. [DOI] [PubMed] [Google Scholar]
- 47.Braun, I. F., J. C. Hoffman, Jr., J. A. Malko, R. I. Pettigrew, W. Daniels, and P. C. Davis. 1985. Jugular venous thrombosis: MR imaging. Radiology 157:357-360. [DOI] [PubMed] [Google Scholar]
- 48.Brazier, J. S. 1986. Yellow fluorescence of fusobacteria. Lett. Appl. Microbiol. 2:125-126. [Google Scholar]
- 49.Brazier, J. S. 2002. Fusobacterium necrophorum infections in man. Rev. Med. Microbiol. 13:141-149. [DOI] [PubMed] [Google Scholar]
- 50.Brazier, J. S. 2006. Human infections with Fusobacterium necrophorum. Anaerobe. 12:165-172. [DOI] [PubMed] [Google Scholar]
- 51.Brazier, J. S., D. M. Citron, and E. J. C. Goldstein. 1991. A selective medium for Fusobacterium spp. J. Appl. Bacteriol. 71:343-346. [DOI] [PubMed] [Google Scholar]
- 52.Brazier, J. S., V. Hall, E. Yusuf, and B. I. Duerden. 2002. Fusobacterium necrophorum infections in England and Wales 1990-2000. J. Med. Microbiol. 51:269-272. [DOI] [PubMed] [Google Scholar]
- 53.Broberg, T., A. Murr, and N. Fischbein. 1999. Devastating complications of acute pediatric bacterial sinusitis. Otolaryng. Head Neck Surg. 120:575-579. [DOI] [PubMed] [Google Scholar]
- 54.Brocard, H., and C. Guibé. 1957. Les septicémies a Bacillus funduliformis. Rev. Praticien. 7:1285-1300. [PubMed] [Google Scholar]
- 55.Brook, I. 1981. Aerobic and anaerobic bacteriology of chronic mastoiditis in children. Am. J. Dis. Child. 135:478-479. [DOI] [PubMed] [Google Scholar]
- 56.Brook, I. 1992. Aerobic and anaerobic bacteriology of intracranial abscesses. Pediatr. Neurol. 8:210-214. [DOI] [PubMed] [Google Scholar]
- 57.Brook, I. 1993. Infections caused by beta-lactamase-producing Fusobacterium spp. in children. Pediatr. Infect. Dis. J. 12:532-533. [DOI] [PubMed] [Google Scholar]
- 58.Brook, I. 2003. Microbiology and management of deep facial infections and Lemierre syndrome. J. Otorhinolaryngol. Relat. Spec. 65:117-120. [DOI] [PubMed] [Google Scholar]
- 59.Brook, I. 2003. Microbiology and management of periodontal infections. Gen. Dent. 51:424-428. [PubMed] [Google Scholar]
- 60.Brook, I. 2005. The association of anaerobic bacteria with infectious mononucleosis. Anaerobe 11:308-311. [DOI] [PubMed] [Google Scholar]
- 61.Brook, I. 2005. The role of anaerobic bacteria in acute and chronic mastoiditis. Anaerobe 11:252-257. [DOI] [PubMed] [Google Scholar]
- 62.Brook, I., and E. H. Frazier. 1996. Microbiology of mediastinitis. Arch. Intern. Med. 156:333-336. [PubMed] [Google Scholar]
- 63.Brook, I., and P. A. Foote, Jr. 1990. Microbiology of “normal tonsils.” Ann. Oto. Rhinol. Laryngol. 99:980-983. [DOI] [PubMed] [Google Scholar]
- 64.Brook, I., and P. Burke. 1992. The management of acute, serous and chronic otitis media: the role of anaerobic bacteria. J. Hosp. Infect. 22(Suppl. A):75-87. [DOI] [PubMed] [Google Scholar]
- 65.Brook, I., and S. M. Finegold. 1979. Bacteriology of chronic otitis media. JAMA 241:487-488. [PubMed] [Google Scholar]
- 66.Brook, I., P. Yocum, and E. M. Friedman. 1981. Aerobic and anaerobic bacteria in tonsils of children with recurrent tonsillitis. Ann. Otol. Rhinol. Laryngol. 90:261-263. [DOI] [PubMed] [Google Scholar]
- 67.Brook, I., and K. Shah. 2001. Bacteriology of adenoids and tonsils in children with recurrent adenotonsillitis. Ann. Otol. Rhinol. Laryngol. 110:844-848. [DOI] [PubMed] [Google Scholar]
- 68.Brook, I., P. Yocum, and K. Shah. 1980. Surface vs core-tonsillar aerobic and anaerobic flora in recurrent tonsillitis. JAMA 244:1696-1698. [PubMed] [Google Scholar]
- 69.Brook, I., S. F. Anthony, and S. M. Finegold. 1978. Aerobic and anaerobic bacteriology of acute otitis media in children. J. Pediatr. 92:13-15. [DOI] [PubMed] [Google Scholar]
- 70.Brown, R., H. G. Lough, and I. R. Poxton. 1997. Phenotypic characteristics and lipopolysaccharides of human and animal isolates of Fusobacterium necrophorum. J. Med. Microbiol. 46:873-878. [DOI] [PubMed] [Google Scholar]
- 71.Bullen, J. J., H. J. Rogers, P. B. Spalding, and C. G. Ward. 2005. Iron and infection: the heart of the matter. FEMS Immunol. Med. Microbiol. 43:325-330. [DOI] [PubMed] [Google Scholar]
- 72.Busch, D. F., L. A. Kureshi, V. L. Sutter, and S. M. Finegold. 1976. Susceptibility of respiratory tract anaerobes to orally administered penicillins and cephalosporins. Antimicrob. Agents Chemother. 10:713-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carpenter, J. P., G. A. Holland, R. A. Baum, R. S. Owen, J. T. Carpenter, and C. Cope. 1993. Magnetic resonance venography for the detection of deep venous thrombosis: comparison with contrast venography and duplex Doppler ultrasonography. J. Vasc. Surg. 18:734-741. [DOI] [PubMed] [Google Scholar]
- 74.Carrie, S., and P. A. Fenton. 1994. Necrobacillosis—an unusual case of pharyngotonsillitis. J. Laryngol. Otol. 108:1097-1098. [DOI] [PubMed] [Google Scholar]
- 75.Cartwright, K. A., D. M. Jones, A. J. Smith, J. M. Stuart, E. B. Kaczmarski, and S. R. Palmer. 1991. Influenza A and meningococcal disease. Lancet 338:554-557. [DOI] [PubMed] [Google Scholar]
- 76.Cartwright, K. A. V. 1995. Meningococcal carriage and disease, p. 115-146. In K. A. V. Cartwright (ed.), Meningococcal disease, vol. 5. John Wiley & Sons, New York, NY. [Google Scholar]
- 77.Celig, D. M., and P. C. Schreckenberger. 1991. Clinical evaluation of the RapID-ANA II panel for identification of anaerobic bacteria. J. Clin. Microbiol. 29:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Celikel, T. H., and P. P. Muthuswamy. 1984. Septic pulmonary emboli secondary to internal jugular vein phlebitis (postanginal sepsis) caused by Eikenella corrodens. Am. Rev. Respir. Dis. 130:510-513. [DOI] [PubMed] [Google Scholar]
- 79.Chalstrey, S. E., H. O. L. Williams, and G. Reilly. 1992. Necrobacillosis—an unusual cause of cervical abscess. J. Laryngol. Otol. 106:374-375. [DOI] [PubMed] [Google Scholar]
- 80.Chan, E. D., M. H. Hermanoff, and E. Connick. 1999. Lemierre's syndrome: review of the English literature over the past 25 years. Clin. Pulm. Med. 6:333-338. [Google Scholar]
- 81.Chand, D. H., R. C. Brady, and J. J. Bissler. 2001. Hemolytic uremic syndrome in an adolescent with Fusobacterium necrophorum bacteremia. Am. J. Kidney Dis. 37:1-4. [DOI] [PubMed] [Google Scholar]
- 82.Chang, P. S., J. P. Harris, N. Bhumbra, M. Puczynski, N. Kherrallah, T. J. Lewis, N. Shilen, and S. Rana. 2006. Index of suspicion. Pediatr. Rev. 27:73-78. [DOI] [PubMed] [Google Scholar]
- 83.Charles, K., W. R. Flinn, and D. G. Neschis. 2005. Lemierre's syndrome: a potentially fatal complication that may require vascular surgical intervention. J. Vasc. Surg. 42:1023-1025. [DOI] [PubMed] [Google Scholar]
- 84.Chemlal, K., I. Caby, N. Memain, D. Aubert, and F. Bruneel. 2000. Syndrome de Lemierre dû à Streptococcus intermedius: une association inhabituelle. Presse Méd. 29:1601-1602. [PubMed] [Google Scholar]
- 85.Chirinos, J. A., D. M. Lichtstein, J. Garcia, and L. J. Tamariz. 2002. The evolution of Lemierre syndrome. Report of 2 cases and review of the literature. Medicine 81:458-465. [DOI] [PubMed] [Google Scholar]
- 86.Cho, Y. P., S.-J. Choi, B. H. Jung, J.-W. Hwang, M. S. Han, Y. H. Kim, T.-W. Kwon, and S. G. Lee. 2006. Lemierre's syndrome in a patient with antiphospholipid syndrome. Ann. Vasc. Surg. 20:274-277. [DOI] [PubMed] [Google Scholar]
- 87.Chow, A. W. 1992. Life-threatening infections of the head and neck. Clin. Infect. Dis. 14:991-1004. [DOI] [PubMed] [Google Scholar]
- 88.Chow, A. W., S. M. Roser, and F. A. Brady. 1978. Orofacial odontogenic infections. Ann. Intern. Med. 88:392-402. [DOI] [PubMed] [Google Scholar]
- 89.Ciccone, A., P. Canhao, F. Falcao, J. M. Ferro, and R. Sterzi. 2004. Thrombolysis for cerebral vein and dural sinus thrombosis. Cochrane Database Syst. Rev. 1:CD003693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Citron, D. M. 2002. Update on the taxonomy and clinical aspects of the genus Fusobacterium. Clin. Infect. Dis. 35(Suppl. 1):S22-S27. [DOI] [PubMed] [Google Scholar]
- 91.Civen, R., H. Jousimies-Somer, M. Marina, L. Borenstein, H. Shah, and S. M. Finegold. 1995. A retrospective review of cases of anaerobic empyema and update of bacteriology. Clin. Infect. Dis. 20(Suppl. 2):S224-S229. [DOI] [PubMed] [Google Scholar]
- 92.Clarke, M. G., N. J. Kennedy, and K. Kennedy. 2003. Serious consequences of a sore throat. Ann. R. Coll. Surg. Engl. 85:242-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Claus, H. C. 1931. Über 100 Fälle von Septico-Pyämie nach Angina. Med. Klin. Wochenschr. Praktische Árzte 35:1269-1271. [Google Scholar]
- 94.Conrads, G., M. C. Claros, D. M. Citron, C. V. Merriam, Y. Warren, and E. J. Goldstein. 2002. 16S-23S rDNA internal transcribed spacer sequences for analysis of the phylogenetic relationships among species of the genus Fusobacterium. Int. J. Syst. Evol. Microbiol. 52:493-499. [DOI] [PubMed] [Google Scholar]
- 95.Constantin, J.-M., J.-P. Mira, R. Guerin, S. Cayot-Constantin, O. Lesens, F. Gourdon, J.-P. Romaszko, P. Linval, H. Laurichesse, and J.-E. Bazin. 2006. Lemierre's syndrome and genetic polymorphisms: a case report. BMC Infect. Dis. 17:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cook, R. J., R. W. Ashton, G. L. Aughenbaugh, and J. H. Ryu. 2005. Septic pulmonary embolism. Presenting features and clinical course of 14 patients. Chest 128:162-166. [DOI] [PubMed] [Google Scholar]
- 97.Cosgrove, E. F., S. M. Colodny, and R. R. Pesce. 1993. Adult respiratory distress syndrome as a complication of postanginal sepsis. Chest 103:1628-1629. [DOI] [PubMed] [Google Scholar]
- 98.Courmont, P., and A. Cade. 1900. Sur une septico-pyohémie de l'homme simulant la peste et causée par un strepto-bacille anaérobie. Arch. de Méd. Exp. 12:393-418. [Google Scholar]
- 99.Coyle-Dennis, J. E., and L. H. Lauerman. 1979. Correlations between leukocidin production and virulence of two isolates of Fusobacterium necrophorum. Am. J. Vet. Res. 40:274-276. [PubMed] [Google Scholar]
- 100.Cron, R. Q., and K. H. Webb. 1995. Necrobacillosis: an unusual cause of purulent otitis media and sepsis. Pediatr. Emerg. Care 11:379-380. [DOI] [PubMed] [Google Scholar]
- 101.Croucher, S. C., A. P. Houston, C. E. Bayliss, and R. J. Turner. 1983. Bacterial populations associated with different regions of the human colon wall. Appl. Environ. Microbiol. 45:1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cunningham, J. S. 1930. Human infection with Actinomyces necrophorus. Arch. Pathol. 9:843-868. [Google Scholar]
- 103.Dack, G. M. 1940. Non-sporeforming anaerobic bacteria of medical importance. Bacteriol. Rev. 4:227-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dagan, R., and K. R. Powell. 1987. Postanginal sepsis following infectious mononucleosis. Arch. Intern. Med. 147:1581-1583. [PubMed] [Google Scholar]
- 105.Dalamaga, M., K. Karmaniolas, C. Chavelas, S. Liatis, H. Matekovits, and I. Migdalis. 2003. Fusobacterium necrophorum septicaemia following Epstein Barr Virus infectious mononucleosis. Anaerobe 9:285-287. [DOI] [PubMed] [Google Scholar]
- 106.de Lima, J. E., Jr., and M. Levin. 2003. Lemierre's syndrome: post-anginal septicaemia. Pediatr. Radiol. 33:281-283. [DOI] [PubMed] [Google Scholar]
- 107.Dellamonica, P., and E. Bernard. 1999. ‘Deep throat’ cellulitis. J. Infect. 38:134. (Letter.) [DOI] [PubMed] [Google Scholar]
- 108.Del Mar, C. B., P. P. Glasziou, and A. B. Spinks. 2004. Antibiotics for sore throat. Cochrane Database Syst. Rev. 2:CD000023. [DOI] [PubMed] [Google Scholar]
- 109.de Rautlin de la Roy, Y., V. Toma, B. Grignon, G. Grollier, and M-C. Paute. 1978. Abcès cérébral à Fusobacterium necrophorum. Importance des conditions de prélèvement et d'analyse bactériologique. Nouv. Presse Med. 7:3454-3455. [PubMed] [Google Scholar]
- 110.De Sena, S., D. L. Rosenfield, S. Santos, and I. Keller. 1996. Jugular thrombophlebitis complicating bacterial pharyngitis (Lemierre's syndrome). Pediatr. Radiol. 26:141-144. [DOI] [PubMed] [Google Scholar]
- 111.de Veber, G., A. Chan, P. Monagle, V. Marzinotto, D. Armstrong, P. Massicotte, M. Leaker, and M. Andrew. 1998. Anticoagulation therapy in pediatric patients with sinovenous thrombosis. Arch. Neurol. 55:1533-1537. [DOI] [PubMed] [Google Scholar]
- 112.de Veber, G., M. Andrew, C. Adams, B. Bjornson, F. Booth, D. J. Buckley, C. S. Camfield, M. David, P. Humphreys, P. Langevin, E. A. MacDonald, and J. Gillett. 2001. Cerebral sinovenous thrombosis in children. N. Engl. J. Med. 345:417-423. [DOI] [PubMed] [Google Scholar]
- 113.de Veber, G., P. Monagle, A. Chan, D. MacGregor, R. Curtis, S. Lee, P. Vegh, M. Adams, V. Marzinotto, M. Leaker, P. Massicotte, D. Lillicrap, and M. Andrew. 1998. Prothrombotic disorders in infants and children with cerebral thromboembolism. Arch. Neurol. 55:1539-1543. [DOI] [PubMed] [Google Scholar]
- 114.De Vos, A. I., R. N. van Rossem, E. P. M. van Elzakker, J. M. A. Nijhuis-Heddes, and E. Maartense. 2000. Lemierre's syndrome sepsis complicating an anaerobic oropharyngeal infection. Neth. J. Med. 59:181-183. [DOI] [PubMed] [Google Scholar]
- 115.Dhawan, B., R. Chaudhry, A. Pandey, N. Nisar, and M. Singh. 1998. Anaerobic septicaemia by Fusobacterium necrophorum: Lemierre's syndrome. Indian J. Pediatr. 65:469-472. [DOI] [PubMed] [Google Scholar]
- 116.Dixon, O. J., and F. C. Helwig. 1930. Thrombophlebitis of the internal jugular vein as a complication of tonsillitis. Ann. Otol. Rhinol. Laryn. 39:1137-1149. [Google Scholar]
- 117.Dodd, J. D., C. A. Souza, and N. L. Műller. 2006. High-resolution MDCT of pulmonary septic embolism: evaluation of the feeding vessel sign. Am. J. Roentgenol. 187:623-629. [DOI] [PubMed] [Google Scholar]
- 118.Dorsey, S. T. 2002. A critically ill young man with septic emboli to the lungs. J. Emerg. Med. 22:415-417. [DOI] [PubMed] [Google Scholar]
- 119.Doyle, K. J., and R. K. Jackler. 1991. Otogenic cavernous sinus thrombosis. Otolaryng. Head Neck. 104:873-877. [DOI] [PubMed] [Google Scholar]
- 120.Drasar, B. S., and B. I. Duerden. 1991. Anaerobes in the normal flora of man, p. 162-179. In B. I. Duerden and B. S. Drasar (ed.), Anaerobes in human disease, Edward Arnold, London, United Kingdom.
- 121.Duboc, H. 1994. Lemierre and Sohier's mission (3-13 January 1942). Hist. Sci. Med. 28:33-39. [PubMed] [Google Scholar]
- 122.Ducroz, V., C. le Pajolec, E. Harbourn, and S. Bobin. 1993. Mastoïdites aiguës a germes anaérobies revue de la literature à propos d'un cas. Ann. Otolaryng. 110:55-59. [PubMed] [Google Scholar]
- 123.Dykhuizen, R. S., E. S. Olson, S. Clive, and J. G. Douglas. 1994. Necrobacillosis (Lemierre's syndrome): a rare cause of necrotizing pneumonia. Eur. Respir. J. 7:2246-2248. [DOI] [PubMed] [Google Scholar]
- 124.Ellis, G. R., D. I. Gozzard, D. N. Looker, and G. J. Green. 1998. Postanginal septicaemia (Lemierre's disease) complicated by haemophagocytosis. J. Infect. 36:340-341. [DOI] [PubMed] [Google Scholar]
- 125.Embree, J. E., T. Williams, and B. J. Law. 1988. Hepatic abscesses in a child caused by Fusobacterium necrophorum. Pediatr. Infect. Dis. J. 7:359-360. [DOI] [PubMed] [Google Scholar]
- 126.Emery, D. L., J. A. Vaughan, B. L. Clark, J. H. Dufty, and D. J. Stewart. 1985. Cultural characteristics and virulence of strains of Fusobacterium necrophorum isolated from the feet of cattle and sheep. Aust. Vet. J. 62:43-46. [DOI] [PubMed] [Google Scholar]
- 127.Engelke, C., E. J. Rummeny, and K. Marten. 2004. A patient with prolonged fever after pharyngotonsillitis. Br. J. Radiol. 77:627-628. [DOI] [PubMed] [Google Scholar]
- 128.Enwonwu, C. O. 1995. Noma: a neglected scourge of children in sub-Saharan Africa. Bull. W. H. O. 73:541-545. [PMC free article] [PubMed] [Google Scholar]
- 129.Enwonwu, C. O., W. A. Falker, Jr., and E. O. Idigbe. 2000. Oro-facial gangrene (noma/cancrum oris): pathogenetic mechanisms. Crit. Rev. Oral Biol. Med. 11:159-171. [DOI] [PubMed] [Google Scholar]
- 130.Epaulard, O., J.-P. Brion, J.-P. Stahl, B. Colombe, and M. Maurin. 2006. The changing pattern of fusobacterium infections in humans: recent experience with fusobacterium bacteraemia. Clin. Microbiol. Infect. 12:178-181. [DOI] [PubMed] [Google Scholar]
- 131.Epstein, M., A. D. J. Pearson, S. J. Hudson, R. Bray, M. Taylor, and J. Beesley. 1992. Necrobacillosis with pancytopenia. Arch. Dis. Child. 67:958-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Escardó, J. A., A. Feyi-Waboso, C. M. Lane, and J. E. Morgan. 2000. Orbital cellulitis caused by Fusobacterium necrophorum. Am. J. Ophthal. 131:280-281. [DOI] [PubMed] [Google Scholar]
- 133.Esposito, N. 2004. Final diagnosis—Fusobacterium necrophorum bacteraemia. http://path.upmc.edu/cases/case385/dx.html.
- 134.Estrera, A. S., M. J. Landay, J. M. Grisham, D. P. Sinn, and M. R. Platt. 1983. Descending necrotizing mediastinitis. Surg. Gynecol. Obstet. 157:545-552. [PubMed] [Google Scholar]
- 135.Eykyn, S. J. 1983. The therapeutic use of metronidazole in anaerobic infection: six years’ experience in a London hospital. Surgery 93:209-214. [PubMed] [Google Scholar]
- 136.Eykyn, S. J. 1989. Necrobacillosis. Scand. J. Infect. Dis. 62:41-46. [PubMed] [Google Scholar]
- 137.Falkler, W. A., Jr., C. O. Enwonwu, and E. O. Idigbe. 1999. Isolation of Fusobacterium necrophorum from cancrum oris (noma). Am. J. Trop. Med. Hyg. 60:150-156. [DOI] [PubMed] [Google Scholar]
- 138.Falkler, W. A., C. O. Enwonwu, A. J. Ewell, and E. O. Idigbe. 2000. Isolation of fusobacteria from the oral cavities of malnourished Nigerian children living in agricultural and herding villages. Oral Dis. 6:103-105. [DOI] [PubMed] [Google Scholar]
- 139.Faussat, J. M., A. Coste, G. Roger, B. Page, H. Marrek, and P. Roulleau. 1993. Les thrombophlébites septiques de la veine jugulaire interne à porte d'entrée oro-pharyngée. Ann. Otolaryng. 110:445-449. [PubMed] [Google Scholar]
- 140.Fiesseler, F. W., P. B. Richman, and R. L. Riggs. 2001. Pharyngitis followed by hypoxia and sepsis: Lemierre syndrome. Am. J. Emerg. Med. 19:320-322. (Letter.) [DOI] [PubMed] [Google Scholar]
- 141.Fiévez, L. 1963. Etude comparée des souches de Sphaerophorus nécrophorus isolées chez l'homme et l'animal. Presses Académiques Européennes, Brussels, Belgium.
- 142.Figueras, G., O. Garcia, O. Vall, X. Massaguer, and M. Salvadó. 1995. Otogenic Fusobacterium necrophorum meningitis in children. Pediatr. Infect. Dis. J. 14:627-628. [DOI] [PubMed] [Google Scholar]
- 143.Finegold, S. M. 1977. Anaerobic bacteria in human disease, p. 13-14. Academic Press, New York, NY.
- 144.Finegold, S. M. 1991. Role of anaerobic bacteria in infections of the tonsils and adenoids. Ann. Otol. Rhinol. Laryng. 154(Suppl.):30-33. [DOI] [PubMed] [Google Scholar]
- 145.Finegold, S. M. 1992. Anaerobic gram negative rods: Bacteroides and Fusobacterium, p. 1571-1580. In S. L. Gorbach, J. G. Bartlett, and N. R. Blacklow (ed.), Infectious diseases. W. B. Saunders, Philadelphia, PA.
- 146.Flügge, C. 1886. Zweiter Abschnitt. II. Bacillen. B. Für Thiere pathogene Bacillen Bacillus necrophorus (Löffler), p. 273. In Die mikroorganismen. Mit besonder berücksichtigung der aetiologie der infectionskrankheiten. FCW Vogel, Leipzig, Germany.
- 147.Forrester, L. J., B. J. Campbell, J. N. Berg, and J. T. Barrett. 1985. Aggregation of platelets by Fusobacterium necrophorum. J. Clin. Microbiol. 22:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Foulkes, G. D., C. E. Johnson, and H. P. Katner. 1990. Fusobacterium osteomyelitis associated with intraosseous gas. Clin. Orthop. Relat. Res. 251:246-248. [PubMed] [Google Scholar]
- 149.Fränkel, E. 1925. [Title unavailable.] Virch. Arch. 254:639. [Google Scholar]
- 150.Freeman, C. D., N. E. Klutman, and K. C. Lamp. 1997. Metronidazole a therapeutic review and update. Drugs 54:679-708. [DOI] [PubMed] [Google Scholar]
- 151.Garcia, G. G., K. K. Amoako, D. L. Xu, T. Inoue, Y. Goto, and T. Shinjo. 1999. Chemical composition of endotoxins produced by Fusobacterium necrophorum subsp. necrophorum and F. necrophorum subsp. funduliforme. Microbios 100:175-179. [PubMed] [Google Scholar]
- 152.Garcia, M. M., K. M. Charlton, and K. A. McKay. 1975. Characterization of endotoxin from Fusobacterium necrophorum. Infect. Immun. 11:371-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garcia Del Castillo, E., J. M. Morales, J. Crespo, M. Ruiz, J. Carballo, and C. Gomez. 1995. Thrombophlebitis of the cavernous sinus secondary to polysinusitis. Report of a new case. Acta Otorrhinolaringol. Esp. 46:60-62. [PubMed] [Google Scholar]
- 154.Garimella, S., A. Inaparthy, and T. Herchline. 2004. Meningitis due to Fusobacterium necrophorum in an adult. BMC Infect. Dis. http://www.biomedcentral.com/1471-2334/4/24. [DOI] [PMC free article] [PubMed]
- 155.Garnham, F., and P. Longstaff. 2005. “My back is killing me.” Emerg. Med. J. 22:824-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gerst, C., and J. M. Primrose. 1997. Septic arthritis of the ankle from Fusobacterium necrophorum. Acad. Emerg. Med. 4:1172-1173. (Letter.) [DOI] [PubMed] [Google Scholar]
- 157.Ghaffar, F. A., M. Wördemann, and G. H. McCracken, Jr. 2001. Acute mastoiditis in children: a seventeen-year experience in Dallas, Texas. Pediatr. Infect. Dis. J. 20:376-380. [DOI] [PubMed] [Google Scholar]
- 158.Go, C., J. M. Bernstein, A. L. de Jong, M. Sulek, and E. M. Friedman. 2000. Intracranial complications of acute mastoiditis. Int. J. Pediatr. Otorhinolaryngol. 52:143-148. [DOI] [PubMed] [Google Scholar]
- 159.Gold, W. L., M. K. Kapral, M. R. Witme, W. A. Mahon, M. Ostrowski, and H. Villend. 1995. Postanginal septicaemia as a life-threatening complication of infectious mononucleosis. Clin. Infect. Dis. 20:1439-1440. (Letter.) [DOI] [PubMed] [Google Scholar]
- 160.Goldenberg, N. A., R. Knapp-Clevenger, T. Hays, and M. J. Manco-Johnson. 2005. Lemierre's and Lemierre's-like syndromes in children: survival and thromboembolic outcomes. Pediatrics 116:543-548. [DOI] [PubMed] [Google Scholar]
- 161.Goldhagen, J., B. A. Alford, L. H. Prewitt, L. Thompson, and M. K. Hostetter. 1988. Suppurative thrombophlebitis of the internal jugular vein: report of three cases and review of the pediatric literature. Pediatr. Infect. Dis. J. 7:410-414. [DOI] [PubMed] [Google Scholar]
- 162.Golledge, C. L., M. H. Beaman, T. Weeramanthri, and T. V. Riley. 1990. Necrobacillosis-primary anaerobic septicaemia due to Fusobacterium necrophorum. Aust. N.Z. J. Med. 20:702-705. [DOI] [PubMed] [Google Scholar]
- 163.Golpe, R., B. Marin, and M. Alonso. 1999. Lemierre's syndrome (necrobacillosis). Postgrad. Med. J. 75:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gong, J., and J. Garcia. 1999. Lemierre's syndrome. Eur. Radiol. 9:672-674. [DOI] [PubMed] [Google Scholar]
- 165.Gorbach, S. L., and J. G. Bartlett. 1974. Anaerobic infections (second of three parts). N. Engl. J. Med. 290:1237-1245. [DOI] [PubMed] [Google Scholar]
- 166.Gowan, R. T., R. J. Mehran, P. Cardinal, and G. Jones. 2000. Thoracic complications of Lemierre syndrome. Can. Respir. J. 7:481-485. [DOI] [PubMed] [Google Scholar]
- 167.Goyal, M., R. Sharma, Y. Jain, and M. Berry. 1995. Unusual radiological manifestations of Lemierre's syndrome: a case report. Ped. Radiol. 25:S105-S106. [PubMed] [Google Scholar]
- 168.Gudinchet, F., P. Maeder, P. Neveceral, and P. Schnyder. 1997. Lemierre's syndrome in children. Chest 112:271-273. [DOI] [PubMed] [Google Scholar]
- 169.Guidet, B., G. Offenstadt, P. Pinta, D. Lesage, and A. Tembely. 1981. Une infection oubliée: le syndrome angine-infarctus pulmonaire à Fusobacterium necrophorum. Un cas avec insuffisance respiratoire aiguë. Méd. Maladies Infect. 11:462-464. [Google Scholar]
- 170.Guillemot, L., J. Hallé, and E. Rist. 1899. Recherches bactériologiques et expérimentales sur les pleurisies putrides. Arch. Méd. Exp. Anat. Pathol. 5:1904. [Google Scholar]
- 171.Gunn, A. A. 1956. Bacteroides septicaemia. J. R. Coll. Surg. 2:41. [PubMed] [Google Scholar]
- 172.Gupta, M., F. V. Castello, and H. H. Kesarwala. 1995. Respiratory failure caused by Lemierre's syndrome. Clin. Pediatr. 34:275-277. [DOI] [PubMed] [Google Scholar]
- 173.Gurley, M. B., T. S. King, and F. Y. Tsai. 1996. Sigmoid sinus thrombosis associated with internal jugular venous occlusion: direct thrombolytic treatment. J. Endovasc. Surg. 3:306-314. [DOI] [PubMed] [Google Scholar]
- 174.Haasper, C., F. W. Tecklenburg, J. B. Cochran, D. M. Habib, and C. D. Smith. 2004. A pain in the neck can lead to pain in the belly: Lemierre's syndrome. Int. Pediatr. 19:185-187. [Google Scholar]
- 175.Hadlock, F. P., R. J. Wallace, Jr., and M. Rivera. 1979. Pulmonary septic emboli secondary to parapharyngeal abscess: postanginal sepsis. Radiology 130:29-33. [DOI] [PubMed] [Google Scholar]
- 176.Hagelskjaer, L., and G. Pedersen. 1993. Fusobacterium necrophorum septicemia complicated by liver abscess. APMIS 101:904-906. [DOI] [PubMed] [Google Scholar]
- 177.Hagelskjaer, L. H., J. Prag, J. Malczynski, and J. H. Kristensen. 1998. Incidence and clinical epidemiology of necrobacillosis, including Lemierre's syndrome, in Denmark 1990-1995. Eur. J. Clin. Microbiol. Infect. Dis. 17:561-565. [DOI] [PubMed] [Google Scholar]
- 178.Hagelskjaer Kristensen, L., and J. Prag. 2000. Human necrobacillosis, with emphasis on Lemierre's syndrome. Clin. Infect. Dis. 31:524-532. [DOI] [PubMed] [Google Scholar]
- 179.Halasa, N. B., M. R. Griffin, Y. Zhu, and K. M. Edwards. 2002. Decreased number of antibiotic prescriptions in office based settings from 1993 to 1999 in children less than five years of age. Pediatr. Infect. Dis. J. 21:1023-1028. [DOI] [PubMed] [Google Scholar]
- 180.Hall, C. 1939. Sepsis following pharyngeal infections. Ann. Otol. Rhinol. Laryngol. 48:905-925. [Google Scholar]
- 181.Hall, V., B. I. Duerden, J. T. Magee, H. C. Ryley, and J. S. Brazier. 1997. A comparative study of Fusobacterium necrophorum strains from human and animal sources by phenotypic reactions, pyrolysis mass spectrometry and SDS-PAGE. J. Med. Microbiol. 46:865-871. [DOI] [PubMed] [Google Scholar]
- 182.Hallé, J. 1898. Recherches sur la bactériologie du canal génital de la femme. Faculté de Médicine de Paris, Paris, France.
- 183.Han, X. Y., J. S. Weinberg, S. S. Prabhu, S. J. Hassenbusch, G. N. Fuller, J. J. Tarrand, and D. P. Kontoyiannis. 2003. Fusobacterial brain abscess: a review of five cases and an analysis of possible pathogenesis. J. Neurosurg. 99:693-700. [DOI] [PubMed] [Google Scholar]
- 184.Handler, S. D., and W. S. Warren. 1979. Peritonsillar abscess: A complication of corticosteroid treatment in infectious mononucleosis. Int. J. Pediatr. Otorhi. 1:265-268. [DOI] [PubMed] [Google Scholar]
- 185.Harar, R. P. S., A. MacDonald, D. Pullen, S. Ganesan, and A. J. Prior. 1996. Lemierre's syndrome: are we underdiagnosing this life-threatening infection? J. Otorhinolaryngol. Relat. Spec. 58:178-181. [DOI] [PubMed] [Google Scholar]
- 186.Hardie, J. 1983. Microbial flora of the oral cavity, p. 162-196. In G. S. Shuster (ed.), Oral microbiology and infectious disease. Williams and Wilkins, Baltimore, MD.
- 187.Hardingham, M. 1987. Peritonsillar infections. Otolaryng. Clin. N. Am. 20:273-278. [PubMed] [Google Scholar]
- 188.Harrington, E., and M. A. Bleicher. 1980. Cryptogenic hepatic abscess in two uncompromised children. J. Pediatr. Surg. 15:660-662. [DOI] [PubMed] [Google Scholar]
- 189.Hentges, D. J. 1993. The anaerobic microflora of the human body. Clin. Infect. Dis. 16(Suppl. 4):S175-S180. [DOI] [PubMed] [Google Scholar]
- 190.Hirschel, B., A.-F. Allaz, and C.-A. Siegrist. 1983. Septicémie à anaérobes après infections oropharyngées (septicémie post-angine de Lemierre): un syndrome oublié. Schweiz Med. Wschr. 113:2008-2011. [PubMed] [Google Scholar]
- 191.Hodgson, R., M. Emig, and J. Pisarello. 2003. Hyperbaric oxygen (HBO2) in the treatment of Lemierre syndrome. Undersea Hyperb. Med. 30:87-91. [PubMed] [Google Scholar]
- 192.Hoehn, K. S. 2005. Lemierre's syndrome: the controversy of anticoagulation. Pediatrics. 115:1415-1416. [DOI] [PubMed] [Google Scholar]
- 193.Hoehn, S., and T. E. Dominguez. 2002. Lemierre's syndrome: an unusual cause of sepsis and abdominal pain. Crit. Care. Med. 30:1644-1647. [DOI] [PubMed] [Google Scholar]
- 194.Hofstad, T., and I. Olsen. 2006. Fusobacterium and Leptotrichia, p. 1945-1956. In S. P. Boriello, P. R. Murray, G. Funke, (ed.), Topley & Wilson bacteriology, 10th ed., vol. 2. Oxford University Press, New York, NY. [Google Scholar]
- 195.Holland, B. W., W. F. McGuirt, Jr., and N. C. Winston-Salem. 2000. Imaging quiz case 2. Arch. Otol. Head Neck Surg. 126:1500-1504. [PubMed] [Google Scholar]
- 196.Hong, P., J. MacCormick, A. Lamothe, and M. Corsten. 2005. Lemierre syndrome: presentation of three cases. J. Otolaryngol. 34:352-358. [DOI] [PubMed] [Google Scholar]
- 197.Hope, A., N. Bleach, and S. Ghiacy. 2002. Lemierre's syndrome as a consequence of acute supraglottitis. J. Laryngol. Otol. 116:216-218. [DOI] [PubMed] [Google Scholar]
- 198.Hughes, C. E., R. K. Spear, C. E. Shinabarger, and I. C. Tuna. 1994. Septic pulmonary emboli complicating mastoiditis: Lemierre's syndrome revisited. Clin. Infect. Dis. 18:633-635. [DOI] [PubMed] [Google Scholar]
- 199.Hwang, J.-J., Y.-J. Lau, B.-S. Hu, Z.-Y. Shi, and Y.-H. Lin. 2002. Haemophilus parainfluenzae and Fusobacterium necrophorum liver abscess: a case report. J. Microbiol. Immunol. Infect. 35:65-67. [PubMed] [Google Scholar]
- 200.Ieven, M., K. Vael, M. De Mayer, A. De Schepper, and S. Pattyn. 1993. Three cases of Fusobacterium necrophorum septicaemia. Eur. J. Clin. Microbiol. 12:705-706. [DOI] [PubMed] [Google Scholar]
- 201.Inoue, T., M. Kanoe, N. Goto, K. Matsumura, and K. Nakano. 1985. Chemical and biological properties of lipopolysaccharides from Fusobacterium necrophorum biovar A and biovar B strains. Nippon Juigaku Zasshi 47:639-645. [DOI] [PubMed] [Google Scholar]
- 202.Irigoyen, M. M., M. Katz, and J. G. Larsen. 1983. Postanginal sepsis in adolescence. Pediatr. Infect. Dis. 2:248-250. [PubMed] [Google Scholar]
- 203.Islam, A. K. M. S., and J. M. Shneerson. 1980. Primary meningitis caused by Bacteroides fragilis and Fusobacterium necrophorum. Postgrad. Med. J. 56:351-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jacobs, J. A., J. J. E. Hendriks, P. D. M. M. Verschure, A. M. van der Wurff, G. Freling, G. D. Vos, and E. E. Stobberingh. 1993. Meningitis due to Fusobacterium necrophorum subspecies necrophorum. Case report and review of the literature. Infection 21:57-60. [DOI] [PubMed] [Google Scholar]
- 205.Jaffe, R. B., and E. B. Koschmann. 1970. Septic pulmonary emboli. Radiology 96:527-532. [DOI] [PubMed] [Google Scholar]
- 206.Jaremko, J. L., A. Kirton, and J. L. Brenner. 2003. A 12-year-old girl with pharyngitis, meningitis and sinovenous thrombosis. Can. Med. Assoc. J. 169:811-812. [PMC free article] [PubMed] [Google Scholar]
- 207.Jensen, A., L. H. Kristensen, and J. Prag. 2007. Fusobacterium necrophorum in young patients with tonsillitis and healthy controls examined by real-time PCR. Clin. Microbiol. Infect. 13:695-701. [DOI] [PubMed] [Google Scholar]
- 208.Jensen, J. S., B Bruun, and B. Gahrn-Hansen. 1999. Unexpected cross-reaction with Fusobacterium necrophorum in a PCR for detection of mycoplasmas. J. Clin. Microbiol. 37:828-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jokipii, A. M., P. Karma, K. Ojala, and L. Jokipii. 1977. Anaerobic bacteria in chronic otitis media. Arch. Otolaryngol. 103:278-280. [DOI] [PubMed] [Google Scholar]
- 210.Jones, J. W., T. Riordan, and M. S. Morgan. 2001. Investigation of postanginal sepsis and Lemierre's syndrome in the South West Peninsula. Commun. Dis. Public Health 4:278-282. [PubMed] [Google Scholar]
- 211.Jones, T. H., V. Bergvall, and J. P. P. Bradshaw. 1990. Carotid artery stenosis and thrombosis secondary to cavernous sinus thromboses in Fusobacterium necrophorum meningitis. Postgrad. Med. J. 66:747-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Jousimies-Somer, H., S. Savolainen, A. Mäkitie, and J. Ylikoski. 1993. Bacteriologic findings in peritonsillar abscesses in young adults. Clin. Infect. Dis. 16(Suppl. 4):S292-S298. [DOI] [PubMed] [Google Scholar]
- 213.Jousimies-Somers, H. R., P. H. Summanen, H. Wexler, S. M. Finegold, S. E. Gharbia, and H. N. Shah. 2006. Bacteroides, Porphyromonas, Prevotella, Fusobacterium and other anaerobic gram negative bacilli, p. 880-901. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, DC.
- 214.Justin-Besançon, L. 1956. L'enseignement de la clinique au chevet du malade. L'exemple d'un grand clinician: Le Professeur André Lemierre. Sem. Hôp. Paris 74:387-392. [PubMed] [Google Scholar]
- 215.Kanoe, M. 1990. Fusobacterium necrophorum hemolysin in bovine hepatic abscess. Zentbl. Vet. B 37:770-773. [DOI] [PubMed] [Google Scholar]
- 216.Kanoe, M., M. Yamanaka, and M. Inoue. 1989. Effects of Fusobacterium necrophorum on the mesenteric microcirculation of guinea pigs. Med. Microbiol. Immunol. 178:99-104. [DOI] [PubMed] [Google Scholar]
- 217.Kanoe, M., Y. Koyanagi, C. Kondo, K. Mamba, T. Makita, and K. Kai. 1998. Location of haemagglutinin in bacterial cells of Fusobacterium necrophorum subsp. necrophorum. Microbios 96:33-38. [PubMed] [Google Scholar]
- 218.Kara, E., A. Sakarya, C. Keleçs, H. Borand, G. Pekindil, and C. Gőktan. 2004. Case of Lemierre's syndrome presenting with thyroid abscess. Eur. J. Clin. Microbiol. Infect. Dis. 23:570-572. [DOI] [PubMed] [Google Scholar]
- 219.Karanas, Y. L., K. K. Yim, B. A. Shuster, and W. C. Lineaweaver. 1995. Lemierre's syndrome: a case of postanginal septicaemia and bilateral flank abscesses. Ann. Plast. Surg. 35:525-528. [DOI] [PubMed] [Google Scholar]
- 220.Karkos, P. D., A. Karkanevatos, S. Panagea, A. Dingle, and J. E. Davies. 2004. Lemierre's syndrome: how a sore throat can end in disaster. Eur. J. Emerg. Med. 11:228-230. [DOI] [PubMed] [Google Scholar]
- 221.Katz, A., E. Leibovitz, D. Greenberg, S. Raiz, M. Greenwald-Maimon, A. Leiberman, and R. Dagan. 2003. Acute mastoiditis in southern Israel: a twelve year retrospective study (1990 through 2001). Pediatr. Infect. Dis. J. 22:878-882. [DOI] [PubMed] [Google Scholar]
- 222.Kern, W., M. Dolderer, D. Krieger, M. Buchler, and P. Kern. 1992. Lemierre's syndrome with splenic abscesses. Dtsch. Med. Wochenschr. 117:1513-1517. [DOI] [PubMed] [Google Scholar]
- 223.Kissling, K. K. 1929. Ueber postanginöse Sepsis. Műnchener Med. Wochenschr. 28:1163-1168. [Google Scholar]
- 224.Kitch, T. T., and P. C. Appelbaum. 1989. Accuracy and reproducibility of the 4-hour ATB 32A method for anaerobe identification. J. Clin. Microbiol. 27:2509-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Klinge, L., U. Vester, J. Schaper, and P. F. Hoyer. 2002. Severe Fusobacteria infections (Lemierre syndrome) in two boys. Eur. J. Pediatr. 161:616-618. [DOI] [PubMed] [Google Scholar]
- 226.Knorr, M. 1923. Ueber die fusospirilläre symbiose, die Gattung Fusobacterium (K. B. Lehmann) und Spirillum sputigenum. II Mitteilung. Die Gattung Fusobacterium. Zentbl. Bakteriol. Parasitenkd. Infekt. Hyg. Abt. 1 Orig. 89:4-22. [Google Scholar]
- 227.Koay, C. B., T. Heyworth, and P. Burden. 1995. Lemierre syndrome—a forgotten complication of acute tonsillitis. J. Laryngol. Otol. 109:657-661. [DOI] [PubMed] [Google Scholar]
- 228.Könönen, E., A. Kanervo, A. Takala, S. Asikainen, and H. Jousimies-Somer. 1999. Establishment of oral anaerobes during the first year of life. J. Dent. Res. 78:1634-1639. [DOI] [PubMed] [Google Scholar]
- 229.Kowalsky, S. F., R. M. Echols, and E. M. McCormick. 1990. Comparative serum bactericidal activity of ceftizoxime/metronidazole, ceftizoxime, clindamycin, and imipenem against obligate anaerobic bacteria. J. Antimicrob. Chemo. 25:767-775. [DOI] [PubMed] [Google Scholar]
- 230.Kuduvalli, P. M., C. M. Jukka, M. Stallwood, C. Battersby, T. Neal, G. Masterson, and G. Marx. 2005. Fusobacterium necrophorum-induced sepsis: an unusual case of Lemierre's syndrome. Acta Anaesth. Scand. 49:572-575. [DOI] [PubMed] [Google Scholar]
- 231.Kuhlman, J. E., E. K. Fishman, and C. Teigen. 1990. Pulmonary septic emboli: diagnosis with CT. Radiology 174:211-213. [DOI] [PubMed] [Google Scholar]
- 232.Langworth, B. F. 1977. Fusobacterium necrophorum: its characteristics and role as an animal pathogen. Bacteriol. Rev. 41:373-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Larsen, P. D., S. A. Chartrand, and E. D. Adickes. 1997. Fusobacterium necrophorum meningitis associated with cerebral vessel thrombosis. Pediatr. Infect. Dis. J. 16:330-331. [DOI] [PubMed] [Google Scholar]
- 234.Lau, E. S., and R. Shuckett. 1993. Fusobacterium septic arthritis of the sternoclavicular joint. J. Rheumatol. 20:1979-1981. [PubMed] [Google Scholar]
- 235.Lechner, A., M. Bender, and H. Mittermayer. 2000. Lemierre's syndrome: two cases of septicemia due to Fusobacterium necrophorum. Anaerobe 6:139-141. [Google Scholar]
- 236.Lee, B. K., F. Lopez, M. Genovese, and J. S. Loutit. 1997. Lemierre's syndrome. South. Med. J. 90:640-643. [DOI] [PubMed] [Google Scholar]
- 237.Lee, S. J., S. I. Cha, C. H. Kim, J. Y. Park, T. H. Jung, K. N. Jeon, and G. W. Kim. 2006. Septic pulmonary embolism in Korea: microbiology clinicoradiologic features, and treatment outcome. J. Infect. 54:230-234. [DOI] [PubMed] [Google Scholar]
- 238.Lee, Y., L. H. Straffon, K. B. Welch, and W. J. Loesche. 2005. The transmission of anaerobic periodontopathic organisms. J. Dent. Res. 85:182-186. [DOI] [PubMed] [Google Scholar]
- 239.Lemierre, A. 1936. On certain septicaemias due to anaerobic organisms. Lancet ii:701-703. [Google Scholar]
- 240.Lemierre, A. 1936. Sur un cas de septico-pyohémie à Bacillus funduliformis. Bull. Mém. Soc. Méd. Hôp. 19:366-385. [Google Scholar]
- 241.Lemierre, A., A. Guy, and M. Rudolf. 1929. Septicémies à Bacillus fragilis. Presse Méd. 37:1669. [Google Scholar]
- 242.Lemierre, A., A. Laporte, et Bloch-Michel. 1936. Phlegmon gazeux cervical á ≪Bacillus funduliformis≫. Bull. Mém. Soc. Méd. Hôp. 19:1558-1562. [Google Scholar]
- 243.Lemierre, A., R. Grégoire, A. Laporte, et R. Couvelaire. 1938. Les aspects chirurgicaux des infections à Bacilllus funduliformis. Acad. Méd. 119:352-359. [Google Scholar]
- 244.Le Moal, G., C. Landron, G. Grollier, B. Bataille, F. Roblot, P. Nassans, and B. Becq-Giraudon. 2003. Characteristics of brain abscess with isolation of anaerobic bacteria. Scand. J. Infect. Dis. 35:318-321. [DOI] [PubMed] [Google Scholar]
- 245.Leugers, C. M., and R. Clover. 1995. Lemierre syndrome: postanginal sepsis. J. Am. Board Fam. Pract. 8:384-391. [PubMed] [Google Scholar]
- 246.Reference deleted.
- 247.Liu, A. C. Y., and J. D. Argent. 2002. Necrobacillosis—a resurgence? Clin. Radiol. 57:332-338. [DOI] [PubMed] [Google Scholar]
- 248.Loeffler, F. 1884. Die Diphtherie beim Kalbe. Mitteilungen Kaiserlichen Gesundheitsamte 2:489-499. [Google Scholar]
- 249.Loesche, W. J. 1972. Dental infections. Proceedings of the international conference on anaerobic bacteria. Atlanta, GA.
- 250.Long, J. W. 1912. Excision of internal jugular vein for streptococcic thrombi vein and cavernous sinus causing paralysis of orbital muscles. Surg. Gynaecol. Obst. 14:86. [Google Scholar]
- 251.Lorber, B. 2006. Bacteroides, Prevotella, Porphyromonas and Fusobacterium species (and other medically important anaerobic gram-negative bacilli), p. 2838-2846. In G. L. Mandell, J. E. Bennett, and R Dolin (ed.), Principles and practice of infectious diseases, 6th ed. Elsevier Churchill Livingstone, Philadelphia, PA.
- 252.Loupa, C., D. Voyatzoglou, H. Papadaki, G. Kouppari, and G. Saroglou. 2000. A 32-year-old woman with fever, sore throat, jaundice and pulmonary infiltrates. Clin. Microbiol. Infect. 6:325-326. [DOI] [PubMed] [Google Scholar]
- 253.Lustig, L. R., B. C. Cusick, S. W. Cheung, and K. C. Lee. 1995. Lemierre's syndrome: two cases of postanginal sepsis. Otolaryngol. Head Neck Surg. 112:767-772. [DOI] [PubMed] [Google Scholar]
- 254.Ma, M., E. C. Jauch, and M. Cousins Johnson. 2003. A case of Lemierre's syndrome. Eur. J. Emerg. Med. 10:139-142. [DOI] [PubMed] [Google Scholar]
- 255.MacDonald, A. A., R. P. S. Harar, and A. J. Prior. 1995. Necrobacillosis: are we missing the early stages of this life-threatening infection? Lancet 346:1705. (Letter.) [DOI] [PubMed] [Google Scholar]
- 256.MacDonald, K. L., M. T. Osterholm, C. W. Hedberg, C. G. Schrock, G. F. Peterson, J. M. Jentzen, S. A. Leonard, and P. M. Schlievert. 1987. Toxic shock syndrome. A newly recognized complication of influenza and influenzalike illness. JAMA 257:1053-1058. [DOI] [PubMed] [Google Scholar]
- 257.Mancao, M., E. Manci, M. Figarola, and B. Estrada. 2005. Fusobacterium necrophorum mediastinal abscess presenting as an anterior chest wall mass in a child: a case report. Clin. Pediatr. 44:73-75. [DOI] [PubMed] [Google Scholar]
- 258.Mañé, S., M. Torres, J. Bugés, A. Rivas, C. Bruno, E. Rodriguez, J. A. Martinez, and A. Nubiola. 1992. Scintigraphic demonstration of jugular obstruction in a case of Lemierre syndrome. Clin. Nucl. Med. 17:233-235. [DOI] [PubMed] [Google Scholar]
- 259.Maniglia, R. J., T. Roth, and E. A. Blumberg. 1997. Polymicrobial brain abscess in a patient infected with human immunodeficiency virus. Clin. Infect. Dis. 24:449-451. [DOI] [PubMed] [Google Scholar]
- 260.Mann, K. A. 1994. Lemierre's syndrome following infectious mononucleosis. Clin. Microbiol. Newsl. 16:158-159. [Google Scholar]
- 261.Marangos, M. N., D. A. Fuchs-Ertman, H. P. Kourea, K. J. Meurer, P. A. Lerwick, S. H. Zimmerman, and A. J. Valenti. 2000. Cerebral infarct and adult respiratory distress syndrome in a patient with postanginal sepsis. Eur. Soc. Clin. Microbiol. Infect. Dis. 6:337-339. [DOI] [PubMed] [Google Scholar]
- 262.Marklund, G., B. Carlsson, and C. Lundberg. 1984. Low secretory IgA concentrations in oral secretions during the acute phase of infectious mononucleosis. Scand. J. Infect. Dis. 16:241-246. [DOI] [PubMed] [Google Scholar]
- 263.Marsh, P. D., and M. V. Martin. 1984. Oral microbiology, 2nd ed., p. 11-25. Van Norstrad Reinhold (UK) Co., Ltd., London, United Kingdom.
- 264.Martin, M. J., and E. D. Wright. 1995. A case of Fusobacterium necrophorum sepsis. J. Infect. 31:151-152. [DOI] [PubMed] [Google Scholar]
- 265.Matten, E. C., and L. Grecu. 2006. Unilateral empyema as a complication of infectious mononucleosis: a pathogenic variant of Lemierre's syndrome. J. Clin. Microbiol. 44:659-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.McLean, A. S., and K. Tyler. 1998. Cardiac tamponade in a postpartum woman with Lemierre's syndrome. Anaesth. Intens. Care 26:582-583. [DOI] [PubMed] [Google Scholar]
- 267.Medical Research Council. 1938. Epidemics in schools. X. Tonsillectomy. Spect. Rept. Ser. 1938:118-125. [Google Scholar]
- 268.Mehta, N., and G. Aisenberg. 2006. Necrobacillosis without Lemierre's syndrome. Lancet 367:1702. [DOI] [PubMed] [Google Scholar]
- 269.Meis, J. F. G. M., T. W. Polder, P. van de Kar, and J. A. A. Hoogkamp-Korstanje. 1993. Multiple brain abscesses and bacteremia in a child due to Fusobacterium necrophorum. Infection 21:174-176. [DOI] [PubMed] [Google Scholar]
- 270.Migirov, L., S. Duvdevani, and J. Kronenberg. 2005. Otogenic intracranial complications: a review of 28 cases. Acta Otolaryngol. 125:819-822. [DOI] [PubMed] [Google Scholar]
- 271.Mitchelmore, I. J., A. J. Prior, P. Q. Montgomery, and S. Tabaqchali. 1995. Microbiological features and pathogenesis of peritonsillar abscesses. Eur. J. Clin. Microbiol. Infect. Dis. 14:870-877. [DOI] [PubMed] [Google Scholar]
- 272.Mitre, R. J., and E. B. Rotheram, Jr. 1974. Anaerobic septicaemia from thrombophlebitis of the internal jugular vein. JAMA 230:1168-1169. [PubMed] [Google Scholar]
- 273.Miyazato, S., T. Shinjo, H. Yago, and N. Nakamura. 1978. Fimbriae (pili) detected in Fusobacterium necrophorum. Nippon Juigaku Zasshi 40:619-621. [DOI] [PubMed] [Google Scholar]
- 274.Møller, K., and B. Dreijer. 1997. Post-anginal sepsis (Lemierre's disease): a persistent challenge. Presentation of 4 cases. Scand. J. Infect. Dis. 29:191-194. [DOI] [PubMed] [Google Scholar]
- 275.Moore, B. A., C. Dekle, and J. Werkhaven. 2002. Bilateral Lemierre's syndrome: a case report and literature review. Ear Nose Throat J. 81:234-252. [PubMed] [Google Scholar]
- 276.Moore, W. E. C., L. V. Holdeman, R. M. Smibert, I. J. Good, J. A. Burmeister, K. G. Palcanis, and R. R. Ranney. 1982. Bacteriology of experimental gingivitis in young adult humans. Infect. Immun. 38:651-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Moore-Gillon, J., T. H. Lee, S. J. Eykyn, and I. Phillips. 1984. Necrobacillosis: a forgotten disease. Br. Med. J. 288:1526-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Moreno, S., J. G. Altozano, B. Pinilla, J. C. López. B. de Quirós, A. Ortega, and E. Bouza. 1989. Lemierre's disease: postanginal bacteremia and pulmonary involvement caused by Fusobacterium necrophorum. Rev. Infect. Dis. 11:319-324. [DOI] [PubMed] [Google Scholar]
- 279.Morizono, S., M. Enjoji, N. Sonoda, M. Fukushima, M. Kuniyoshi, K. Kotoh, M. Nakamuta, and H. Nawata. 2005. Lemierre's syndrome: Porphyromonas asaccharolytica as a putative pathogen. Intern. Med. 44:350-353. [DOI] [PubMed] [Google Scholar]
- 280.Mukau, L. 1985. Dissecting retropharyngeal abscess due to Fusobacterium necrophorum in an adult. South. Med. J. 78:476-478. [DOI] [PubMed] [Google Scholar]
- 281.Nakamura, S., S. Sadoshima, Y. Doi, M. Yoshioka, S. Yamashita, H. Gotoh, and K. Onoyama. 2000. Internal jugular vein thrombosis, Lemierre's syndrome; oropharyngeal infection with antibiotic and anticoagulation therapy. J. Vasc. Dis. 51:173-177. [DOI] [PubMed] [Google Scholar]
- 282.Narayanan, S., G. C. Stewart, M. M. Chengappa, L. Willard, W. Shuman, M. Wilkerson, and T. G. Nagaraja. 2002. Fusobacterium necrophorum leukotoxin induces activation and apoptosis of bovine leukocytes. Infect. Immun. 70:4609-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Narayanan, S. K., M. M. Chengappa, G. C. Stewart, and T. G. Nagaraja. 2003. Immunogenicity and protective effects of truncated recombinant leukotoxin proteins of Fusobacterium necrophorum in mice. Vet. Microbiol. 93:335-347. [DOI] [PubMed] [Google Scholar]
- 284.Narayanan, S. K., T. G. Nagaraja, M. M. Chengpappa, and G. C. Stewart. 2001. Cloning, sequencing, and expression of the leukotoxin gene from Fusobacterium necrophorum. Infect. Immun. 69:5447-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Narongwanichgarn, W., N. Misawa, J. H. Jin, K. K. Amoako, E. Kawaguchi, T. Shinjo, T. Haga, and Y. Goto. 2003. Specific detection and differentiation of two subspecies of Fusobacterium necrophorum by PCR. Vet. Microbiol. 93:183-195. [DOI] [PubMed] [Google Scholar]
- 286.Narsinghani, U., M. B. Schmidt, R. F. Jacobs, and K. S. Anand. 2001. Radiological case of the month. Arch. Pediatr. Adolesc. Med. 155:965-966. [DOI] [PubMed] [Google Scholar]
- 287.Nastro, L. J., and S. M. Finegold. 1973. Endocarditis due to anaerobic gram-negative bacilli. Am. J. Med. 54:482-496. [DOI] [PubMed] [Google Scholar]
- 288.Nguyen-Dinh, K. V., K. Marsot-Dupuch, F. Portier, and B. Lamblin. 2002. Lemierre syndrome: usefulness of CT in detection of extensive occult thrombophlebitis. J. Neuroradiol. 29:132-135. [PubMed] [Google Scholar]
- 289.Nicholson, L. A., C. J. Morrow, L. A. Corner, and A. L. Hodgson. 1994. Phylogenetic relationship of Fusobacterium necrophorum A, AB, and B biotypes based upon 16S rRNA gene sequence analysis. Int. J. Syst. Bacteriol. 44:315-319. [DOI] [PubMed] [Google Scholar]
- 290.Nord, C. E. 1995. The role of anaerobic bacteria in recurrent episodes of sinusitis and tonsillitis. Clin. Infect. Dis. 20:1512-1524. [DOI] [PubMed] [Google Scholar]
- 291.Nord, C. E., A. Heimdahl, and K. Tunér. 1988. Beta-lactamase producing anaerobic bacteria in the oropharynx and their clinical relevance. Scand. J. Infect. Dis. Suppl. 57:50-54. [PubMed] [Google Scholar]
- 292.Nuermberger, E. 4 August 2000. A previously healthy 23 year-old woman with sepsis. Case rounds case 17. http://hopkins-id.edu/education/id_caserounds/caserounds17.html.
- 293.Oestreicher-Kedem, Y., E. Raveh, L. Kornreich, I. Yaniv, and H. Tamary. 2004. Prothrombotic factors in children with otitis media and sinus thrombosis. Laryngoscope 114:90-95. [DOI] [PubMed] [Google Scholar]
- 294.Oh, T.-J., R. Eber, and H.-L. Wang. 2002. Periodontal diseases in the child and adolescent. J. Clin. Periodontol. 29:400-410. [DOI] [PubMed] [Google Scholar]
- 295.Okada, Y., M. Kanoe, Y. Yaguchi, T. Watanabe, H. Ohmi, and K. Okamoto. 1999. Adherence of Fusobacterium necrophorum subspecies necrophorum to different animal cells. Microbios 99:95-104. [PubMed] [Google Scholar]
- 296.Olson, J. L., and N. Mandava. 2006. Bilateral intraocular involvement in Lemierre's syndrome. Br. J. Ophthalmol. 90:249-250 (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Oteo, J., B. Aracil, C. Barros, J. I. Alós, and J. L. Gómez-Garcés. 1999. Bacteremic pharyngotonsillitis by Fusobacterium necrophorum: a prelude to Lemierre's syndrome. Clin. Microbiol. Newsl. 21:126-128. [Google Scholar]
- 298.Paaske, P. B., B. M. Rasmussen., and P. Illum. 1994. Fusobacterium pneumonia and death following uvulo-palato-pharyngoplasty. Head Neck 16:450-452. [DOI] [PubMed] [Google Scholar]
- 299.Page, Y., C. Comtet, B. Tardy, F. Zeni, D. Thevent, F. Lucht, and J.-C. Bertrand. 1990. Disseminated intravascular coagulation in Fusobacterium necrophorum septicaemia. Scand. J. Infect. Dis. 22:743-747. [DOI] [PubMed] [Google Scholar]
- 300.Palmer, A. L., J. D. Strain, D. B. Henry, F. M. Karrer, and E. A. F. Simoes. 1995. Postanginal sepsis after oropharyngeal trauma. Pediatr. Infect. Dis. J. 14:249-251. [DOI] [PubMed] [Google Scholar]
- 301.Park, D., K. Rezajooi, and I. Sabin. 2006. Lemierre's syndrome an unusual manifestation of spinal infection. J. Bone Joint Surg. 88B:261-262. [DOI] [PubMed] [Google Scholar]
- 302.Parulekar, S. S., D. F. Paonessa, A. Rapoport, and J. Kouyoumgian. 1978. Epstein-Barr virus, the ravager. Ann. Otol. 87:729-735. [DOI] [PubMed] [Google Scholar]
- 303.Paster, B. J., S. K. Boches, J. L. Galvin, R. E. Ericson, C. N. Lau, V. A. Levanos, A. Sahasrabudhe, and F. E. Dewhirst. 2001. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183:3770-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Pham, H. C. 1935. Les septicémies dues au Bacillus funduliformis. Thèse pour le Doctorat en Médicine. Faculté de Médicine, Paris, France.
- 305.Pickering, M. C., T. Barkham. J. C. Mason, S. Shaunak, and K. A. Davies. 2000. Bilateral gluteal abscesses as a unique manifestation of Fusobacterium septicaemia. Rheumatology 39:224-225. (Letter.) [DOI] [PubMed] [Google Scholar]
- 306.Pitkäranta, A., P. Lindahl, M. Raade, and R. Puohiniemi. 2004. Orbital abscess caused by Fusobacterium necrophorum. Int. J. Pediatr. Otorhi. 68:585-587. [DOI] [PubMed] [Google Scholar]
- 307.Portman, M., D. Ingall, G. Westenfelder, and R. Yogev. 1984. Peritonsillar abscess complicating infectious mononucleosis. J. Pediatr. 104:742-744. [DOI] [PubMed] [Google Scholar]
- 308.Potter, M. N., H. C. Drysdale, P. A. Price, and A. C. Buck. 1988. Disseminated intravascular coagulation with Fusobacterium necrophorum infection. Postgrad. Med. J. 64:155-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Prévot, A. R. 1938. Etudes de sytématique bactérienne III. Invalidité du genre Bacteroides Castellani et Chambers démembrement et reclassification. Ann. Inst. Pasteur Paris 60:285-307. [Google Scholar]
- 310.Pulcini, C., F. Vandenbos, S. Roth, V. Mondain-Miton, E. Bernard, P.-M. Roger, F. de Salvador-Guillouet, H. Hyvernat, F. Girard-Pipau, M. Mattéi, and P. Dellamonica. 2003. Syndrome de Lemierre: à propos de 6 cas. Rev. Méd. Intern. 24:17-23. [DOI] [PubMed] [Google Scholar]
- 311.Quentin, C., M.-A. Desailly-Chanson, and C. Bebear. 1991. Evaluation of AN-Ident. J. Clin. Microbiol. 29:231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Ramirez, S., T. G. Hild, C. N. Rudolph, J. R. Sty, S. C. Kehl, P. Havens, K. Henrickson, and M. J. Chusid. 2003. Increased diagnosis of Lemierre's syndrome and other Fusobacterium necrophorum infections at a children's hospital. Pediatrics 112:380-385. [DOI] [PubMed] [Google Scholar]
- 313.Rathore, M. H., L. L. Barton, and L. M. Dunkle. 1990. The spectrum of fusobacterial infections in children. Pediatr. Infect. Dis. J. 9:505-508. [DOI] [PubMed] [Google Scholar]
- 314.Razonable, R. R., A. E. Rahman, and W. R. Wilson. 2003. Lemierre syndrome variant: necrobacillosis associated with inferior vena cava thrombosis and pulmonary abscesses after trauma-induced leg abscess. Mayo Clin. Proc. 78:1153-1156. [DOI] [PubMed] [Google Scholar]
- 315.Reilly, S., and A. T. Willis. 1980. β-Lactamase-producing anaerobes. Lancet ii:970-971. (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Repanos, C., N. K. Chadha, and M. V. Griffiths. 2006. Sigmoid sinus thrombosis secondary to Lemierre's syndrome. Ear Nose Throat J. 85:98-101. [PubMed] [Google Scholar]
- 317.Reuben, M. S. 1933. Postanginal sepsis—report of nine cases. Arch. Pediatr. 52:152-185. [Google Scholar]
- 318.Reynolds, M. A., C. A. Hart, F. Harris, and L. S. Taitz. 1985. Anaerobes in acute otitis media. J. Infect. 10:262-264. [DOI] [PubMed] [Google Scholar]
- 319.Riordan, T., and M. Wilson. 2004. Lemierre's syndrome: more than a historical curiosa. Postgrad. Med. J. 80:328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Rist, E. 1898. Etudes bactériologiques sure les infections d'origine otique. Faculté de Médicine, Paris, France.
- 321.Roberts, D. S. 1967. The pathogenic synergy of Fusiformis necrophorus and Corynebacterium pyogenes. I. Influence of the leucocidal exotoxin of F. necrophorus. Br. J. Exp. Pathol. 48:665-673. [PMC free article] [PubMed] [Google Scholar]
- 322.Rosebury, T. 1966. Microrganisms indigenous to man. McGraw Hill, New York, NY.
- 323.Rubinstein, E., A. B. Onderdonk, and J. J. Rahal, Jr. 1974. Peritonsillar infection and bacteremia caused by Fusobacterium gonidiaformans. J. Pediatr. 85:673-675. [DOI] [PubMed] [Google Scholar]
- 324.Rustia, M., and P. Shubik. 1972. Induction of lung tumors and malignant lymphomas in mice by metronidazole. J. Natl. Cancer Inst. 48:721-726. [PubMed] [Google Scholar]
- 325.Saginala, S., T. G. Nagaraja, Z. L. Tan, K. F. Lechtenberg, M. M. Chengappa, K. E. Kemp, and P. M. Hine. 1996. Serum neutralizing antibody response and protection against experimentally induced liver abscesses in steers vaccinated with Fusobacterium necrophorum. Am. J. Vet. Res. 57:483-488. [PubMed] [Google Scholar]
- 326.Sánchez, E. N., I. W. De Cal, N. A. Bernal, M. D. M. Rodrigo, J. U. Jaeger, F. C. Del Pozo, A. J. Blanco, A. C. Valenciano, and M. C. Echevarría. 2000. Fusobacterium osteomyelitis of the pubic symphysis in a healthy soccer player. J. Rheumatol. 27:2047-2048. [PubMed] [Google Scholar]
- 327.Sanders, R. V., M. B. Kirkpatrick, C. C. Dacso, and J. B. Bass, Jr. 1986. Suppurative thrombophlebitis of the internal jugular vein. Alabama J. Med. Sci. 23:92-95. [PubMed] [Google Scholar]
- 328.Sastre, J., E. Casas, J. Sierra, J. G. Puig, and A. Gil. 1991. Splenic abscess due to Fusobacterium necrophorum. Rev. Infect. Dis. 13:1249-1250. (Letter.) [DOI] [PubMed] [Google Scholar]
- 329.Satchell, S. C., T. Riordan, and M. Beaman. 1998. An unusual case of an ‘acute abdomen.’ Postgrad. Med. J. 74:749-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Sayers, N. M., B. P. F. A. Gomes, D. B. Drucker, and A. S. Blinkhorn. 1997. Possible lethal enhancement of toxins from putative periodontopathogens by nicotine: implications for periodontal disease. J. Clin. Pathol. 50:245-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schmid, T., H. Miskin, Y. Schlesinger, Z. Aragaman, and D. Kleid. 2005. Respiratory failure and hypercoagulability in a toddler with Lemierre's syndrome. Pediatrics 115:620-622. [DOI] [PubMed] [Google Scholar]
- 332.Schmorl, G. 1891. Ueber ein pathogenes Fadenbacterium (Streptothrix cuniculi). Deutsch Z. Thiermed. 17:375-408. [Google Scholar]
- 333.Schottmüller, H. 1918. Ueber di Pathogenität anaërober Bazillen. Deutsche Med. Wochenschr. 44:1440. (Abstract.) [Google Scholar]
- 334.Schreckenberger, P. C., D. M. Celig, and W. M. Janda. 1988. Clinical evaluation of Vitek ANI Card for identification of anaerobic bacteria. J. Clin. Microbiol. 26:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Schwartz, B., S. M. Marcy, W. R. Phillips, M. A. Gerber, and S. F. Dowell. 1998. Pharyngitis—principles of judicious use of antimicrobial agents. Pediatrics 101:171-174. [Google Scholar]
- 336.Schwartz, H. C., and D. C. Nguyen. 1999. Postanginal septicaemia with external jugular venous thrombosis: case report. Br. J. Oral Max. Surg. 37:144-146. [DOI] [PubMed] [Google Scholar]
- 337.Screaton, N. J., J. G. Ravenel, P. J. Lehner, E. R. Heitzman, and C. D. R. Flower. 1999. Lemierre syndrome: forgotten but not extinct—report of four cases. Radiology 213:369-374. [DOI] [PubMed] [Google Scholar]
- 338.Seidenfeld, S. M., W. L. Sutker, and J. P. Luby. 1982. Fusobacterium necrophorum septicemia following oropharyngeal infection. JAMA 248:1348-1350. [PubMed] [Google Scholar]
- 339.Shadowen, R. D., and R. P. Trevor. 1989. Lemierre's postanginal septicaemia: internal jugular vein thrombosis related to pharyngeal infection. South. Med. J. 82:1583-1584. [PubMed] [Google Scholar]
- 340.Shannon, G. W., C. V. Ellis, and W. P. Stepp. 1983. Oropharyngeal Bacteroides melaninogenicus infection with septicemia: Lemierre's syndrome. J. Fam. Pract. 16:159-166. [PubMed] [Google Scholar]
- 341.Shapiro, J., M. P. Fried, and M. Strome. 1989. Postanginal sepsis. Head Neck 11:164-169. [DOI] [PubMed] [Google Scholar]
- 342.Sharland, M., H. Kendall, D. Yeates, A. Randall, G. Hughes, P. Glasziou, and D. Mant. 2005. Antibiotic prescribing in general practice and hospital admissions for peritonsillar abscess, mastoiditis, and rheumatic fever in children: time trend analysis. Br. Med. J. 331:328-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Shaw, F. W., and I. A. Bigger. 1934. Necrobacillosis of the lung. JAMA 102:688-689. [Google Scholar]
- 344.Shek, M., A. Karamali, N. McGuire, P. Seifert, and N. Alperin. 1988. Bacterial sepsis with Fusobacterium sp. Arch. Int. Med. 148:2303. [PubMed] [Google Scholar]
- 345.Shinjo, T., and H. Kiyoyama. 1986. Pathogenicity of a non-hemagglutinating mutant strain of Fusobacterium necrophorum biovar A in mice. Nippon Juigaku Zasshi 48:523-527. [DOI] [PubMed] [Google Scholar]
- 346.Shinjo, T., T. Fujisawa, and T. Mitsuoka. 1991. Proposal of two subspecies of Fusobacterium necrophorum (Flügge) Moore and Holdeman: Fusobacterium necrophorum subsp. necrophorum subsp. nov., nom. rev. (ex Flügge 1886), and Fusobacterium necrophorum subsp. funduliforme subsp. nov., nom. rev. (ex Hallé 1898). Int. J. Syst. Bacteriol. 41:395-397. [DOI] [PubMed] [Google Scholar]
- 347.Sibai, K., and F. Sarasin. 2004. Syndrome de Lemierre: un diagnostic à ne pas oublier. Rev. Méd. Suisse Romande 124:693-695. [PubMed] [Google Scholar]
- 348.Sinave, C. P., G. J. Hardy, and P. W. Fardy. 1989. The Lemierre syndrome: suppurative thrombophlebitis of the internal jugular vein secondary to oropharyngeal infection. Medicine (Baltimore) 68:85-94. [PubMed] [Google Scholar]
- 349.Singhal, A., and M. D. Kertsein. 2001. Lemierre's syndrome. South. Med. J. 94:886-887. [PubMed] [Google Scholar]
- 350.Smith, G. E., S. Smith, H. Heatlie, J. N. R. Bashford, J. Hawker, D. Ashcroft, D. Millson, N. Q. Verlander, and R. Warren. 2004. What has happened to antimicrobial usage in primary care in the United Kingdom since the SMAC report? Description of trends in antimicrobial usage using the General Practice Research Database. J. Public Health 26:359-364. [DOI] [PubMed] [Google Scholar]
- 351.Smith, G. R., and A. Turner. 1986. The adverse effect of dilution on the infectivity of Fusobacterium necrophorum culture. J. Hyg. (London) 96:199-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Smith, G. R., and E. A. Thornton. 1993. Pathogenicity of Fusobacterium necrophorum strains from man and animals. Epidemiol. Infect. 110:499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Smith, G. R., and E. A. Thornton. 1997. Classification of human and animal strains of Fusobacterium necrophorum by their pathogenic effects in mice. J. Med. Microbiol. 46:879-882. [DOI] [PubMed] [Google Scholar]
- 354.Smith, G. R., D. Till, L. M. Wallace, and D. E. Noakes. 1989. Enhancement of the infectivity of Fusobacterium necrophorum by other bacteria. Epidemiol. Infect. 102:447-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Smith, G. R., L. M. Wallace, and D. E. Noakes. 1990. Experimental observations on the pathogenesis of necrobacillosis. Epidemiol. Infect. 104:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Soave, R. L., and D. J. Kuchar. 2001. Bilateral forefoot gangrene secondary to Lemierre's disease. J. Am. Pod. Med. Assoc. 91:147-149. (Letter.) [DOI] [PubMed] [Google Scholar]
- 357.Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent, Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25:134-144. [DOI] [PubMed] [Google Scholar]
- 358.Socransky, S. S., and A. D. Haffajee. 1994. Evidence of bacterial etiology: a historical perspective. Periodontology 2000. 5:7-25. [DOI] [PubMed] [Google Scholar]
- 359.Socransky, S. S., and S. D. Manganiello. 1971. The oral microbiota of man from birth to senility. J. Periodontol. 42:485-496. [DOI] [PubMed] [Google Scholar]
- 360.Song, Y. 2005. PCR-based diagnostics for anaerobic infections. Anaerobe 11:79-91. [DOI] [PubMed] [Google Scholar]
- 361.Sonsale, P. D., M. R. Philipson, and J. Bowskill. 2004. Septic arthritis of the knee due to Fusobacterium necrophorum. J. Clin. Microbiol. 42:3369-3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Spencer, C. H., C. W. Slusher, C. V. Sanders, and K. E. Aldridge. 1989. Fusobacterium necrophorum sepsis with cerebral infarction. South. Med. J. 82:1040-1043. [DOI] [PubMed] [Google Scholar]
- 363.Spires, J. R., J. J. Owens, G. E. Woodson, and R. H. Miller. 1987. Treatment of peritonsillar abscess. Arch. Otolaryngol. Head Neck Surg. 113:984-986. [DOI] [PubMed] [Google Scholar]
- 364.Stahlman, G. C., D. K. DeBoer, and N. E. Green. 1996. Fusobacterium osteomyelitis and pyarthrosis: a classic case of Lemierre's syndrome. J. Pediatr. Ortho. 16:529-532. [DOI] [PubMed] [Google Scholar]
- 365.Stallworth, J. R., and J. M. Carroll. 1997. Lemierre's syndrome: new insights into an old disease. Clin. Pediatr. 36:715-718. [DOI] [PubMed] [Google Scholar]
- 366.Stam, J., S. F. De Bruijn, and G. DeVeber. 2002. Anticoagulation for cerebral sinus thrombosis. Cochrane Database Syst. Rev. 4:CD002005. [DOI] [PubMed] [Google Scholar]
- 367.Stavreas, N. P., C. D. Amanatidou, E. G. Hatzimanolis, I. Legakis, G. Naoum, E. Lakka-Papadodima, G. Georgoulias, P. Morfou, and S. Tsiodras. 2005. Thyroid abscess due to a mixed anaerobe infection with Fusobacterium mortiferum. J. Clin. Microbiol. 43:6202-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Stenfors, L.-E., H.-M. Bye, S. Räisänen, and R. Myklebust. 2000. Bacterial penetration into tonsillar surface epithelium during infectious mononucleosis. J. Laryngol. Otol. 114:848-852. [DOI] [PubMed] [Google Scholar]
- 369.Stenfors, L.-E., and S. Raisenen. 1996. Immunoglobulin-coated bacteria on the tonsillar surface during infectious mononucleosis. J. Laryngol. Otol. 110:339-342. [DOI] [PubMed] [Google Scholar]
- 370.Stokroos, R. J., J. J. Manni, J. R. de Kruijk, and E. R. Soudijn. 1999. Lemierre syndrome and acute mastoiditis. Arch. Otolaryngol. Head Neck Surg. 125:589-591. [DOI] [PubMed] [Google Scholar]
- 371.Stratton, C. W. 1996. Mechanisms of action for antimicrobial agents: general principles and mechanisms for selected classes of antibiotics, p. 591. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, MD.
- 372.Stuart, G., and C. Wren. 1992. Endocarditis with acute mitral regurgitation caused by Fusobacterium necrophorum. Pediatr. Cardiol. 13:230-232. [DOI] [PubMed] [Google Scholar]
- 373.Tan, Z. L., T. G. Nagaraja, and M. M. Chengappa. 1996. Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism and control measures. Vet. Res. Commun. 20:113-140. [DOI] [PubMed] [Google Scholar]
- 374.Tan, Z. L., T. G. Nagaraja, M. M. Chengappa, and J. S. Smith. 1994. Biological and biochemical characterisation of Fusobacterium necrophorum leukotoxin. Am. J. Vet. Res. 55:515-521. [PubMed] [Google Scholar]
- 375.Tanaka, I., K. Suzuki, E. Tanaka, and S. Baba. 1996. Investigation of normal bacterial flora in the upper respiratory tract. Acta Otolaryngol. Suppl. 525:44-50. [PubMed] [Google Scholar]
- 376.Tärnvik, A. 1986. Anaerobic meningitis in children. Eur. J. Clin. Microbiol. 5:271-274. [DOI] [PubMed] [Google Scholar]
- 377.Teissier, P., J. Reilly, E. Rivalier, and F. Layani. 1929. Contribution à l'étude des septicémies primitives à microbes anaérobies. Etude clinique et expérimentale d'un cas de septicémie due au Bacillus funduliformis. Paris Méd. 7:297-306. [Google Scholar]
- 378.Teissier, P., J. Reilly, E. Rivalier, and V. Stéfanesco. 1931. Mémoires originaux. Les septicémies primitives dues au Bacillus funduliformisétude clinique, bactériologique et expérimentale. Ann. Méd. 30:97-144. [Google Scholar]
- 379.Thatcher, P. 2003. Hepatic abscesses caused by Fusobacterium necrophorum as part of the Lemierre syndrome. J. Clin. Gastroenterol. 37:196-197. (Letter.) [DOI] [PubMed] [Google Scholar]
- 380.Trapp, C. M., J. Tamai, and M. R. Schleiss. 2005. Septic arthritis secondary to Fusobacterium necrophorum in a 4-year-old girl: case report and review of the literature. Pediatr. Infect. Dis. J. 24:846-847. [DOI] [PubMed] [Google Scholar]
- 381.Tsuneishi, M., T. Yamamoto, S. Kokeguchi, N. Tamaki, K. Fukui, and T. Watanabe. 2006. Composition of the bacterial flora in tonsilloliths. Microbes Infect. 8:2384-2389. [DOI] [PubMed] [Google Scholar]
- 382.Tunér, K., and C. E. Nord. 1983. Betalactamase-producing organisms in recurrent tonsillitis. Scand. J. Infect. Dis. Suppl. 39:83-85. [PubMed] [Google Scholar]
- 383.Uematsu, H., and E. Hoshino. 1992. Predominant obligate anaerobes in human periodontal pockets. J. Periodontal Res. 27:15-19. [DOI] [PubMed] [Google Scholar]
- 384.Uffenforde, W. 1925. Die verwicklungen der akuten halsentzündung unter besonderer berücksichtigung der beteiligung des spatium parapharyngeum. Curt Kabitzch, Leipzig, Germany.
- 385.Van den Akker, E. H., A. W. Hoes, M. J. Burton, and A. G. M. Schilder. 2004. Large international differences in (adeno)tonsillectomy rates. Clin. Otolaryngol. 29:161-164. [DOI] [PubMed] [Google Scholar]
- 386.Veillon, A., and A. Zuber. 1898. Sur quelques microbes strictement anaérobies et leur role en pathologie. Arch. Méd. Exp. 10:517-545. [Google Scholar]
- 387.Veldhoen, E. S., T. F. Wolfs, and A. J. van Vught. 2007. Two cases of fatal meningitis due to Fusobacterium necrophorum. Ped. Neurol. 36:261-263. [DOI] [PubMed] [Google Scholar]
- 388.Velez, M. R., C. Dorsett, Jr., H. W. Ferguson, and K. Hansen. 2003. Lemierre's syndrome: a case report. J. Oral Maxillofac. Surg. 61:968-971. [DOI] [PubMed] [Google Scholar]
- 389.Venkataraman, M. T., and M. Policar. 1997. Fever, sore throat and pulmonary infiltrates in a 20-year old-man. Chest 112:268-270. [DOI] [PubMed] [Google Scholar]
- 390.Venkateswaran, S., and F. K. H. Sze. 2005. A case of Lemierre's syndrome presenting with multiple pulmonary abscesses associated with a tension hydropneumothorax resulting in a mediastinal shift. Ann. Acad. Med. Singapore 34:450-453. [PubMed] [Google Scholar]
- 391.Vogel, L. C., and K. M. Boyer. 1980. Metastatic complications of Fusobacterium necrophorum sepsis. Am. J. Dis. Child. 134:356-358. [DOI] [PubMed] [Google Scholar]
- 392.Vohra, A., E. Saiz, and K. R. Ratzan. 1997. A young woman with a sore throat, septicaemia, and respiratory failure. Lancet 350:928. [DOI] [PubMed] [Google Scholar]
- 393.Wade, W. 2002. Unculturable bacteria—the unchararacterized organisms that cause oral infections. J. R. Soc. Med. 95:81-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Wardle, J. K., M. J. Connolly, H. R. Ingham, P. Hudgson, and M. H. Snow. 1984. Necrobacillosis. Br. Med. J. 288:1916. (Letter.)6234048 [Google Scholar]
- 395.Wennberg, J. E., L. Blowers, R. Parker, and A. M. Gittelsohn. 1977. Changes in tonsillectomy rates associated with feedback and review. Pediatrics 59:821-826. [PubMed] [Google Scholar]
- 396.Westendorp, R. G., J. J. Hottenga, and P. E. Slagboom. 1999. Variation in plasminogen-activator-inhibitor-1 gene and risk of meningococcal septic shock. Lancet 354:561-563. [DOI] [PubMed] [Google Scholar]
- 397.Wiedermann, C. J., and N. C. Kaneider. 2005. A meta-analysis of controlled trials of recombinant human activated protein C therapy in patients with sepsis. BMC Emerg. Med. http://www.biomedcenral.com/1471-227X/5/7. [DOI] [PMC free article] [PubMed]
- 398.Wolf, R. F. E., J. G. Konings, T. R. Prins, and J. Weits. 1991. Fusobacterium pyomyositis of the shoulder after tonsillitis. Acta Orthop. Scand. 62:595-596. [DOI] [PubMed] [Google Scholar]
- 399.Woywodt, A., S. Merkel, W. Buth, H. Haller, and A. Schwarz. 2002. A swollen neck. Lancet 360:1838. [DOI] [PubMed] [Google Scholar]
- 400.Yeung, P., and P. Noyce. 2001. Case report: Lemierre's syndrome following pharyngotonsillitis. Aust. J. Otolaryngol. http://www.findarticles.com/p/articles/mi_qa3868/is_200107/ai_n8961184.
- 401.Yousem, D. M., J. C. Scatarige, B. W. Gayler, and S. S. Siegelman. 1985. CT demonstration of postanginal sepsis. J. Otolaryngol. 14:333-335. [PubMed] [Google Scholar]
- 402.Yu, L., and J. B. Emans. 1988. Epidural abscess associated with spondylolysis. J. Bone Joint Surg. 70:444-447. [PubMed] [Google Scholar]
- 403.Zhang, F., T. G. Nagaraja, D. George, and G. C. Stewart. 2006. The two major subspecies of Fusobacterium necrophorum have distinct leukotoxin operon promoter regions. Vet. Microbiol. 112:73-78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.