Abstract
Type III secretion systems (T3SSs) are complex bacterial structures that provide gram-negative pathogens with a unique virulence mechanism enabling them to inject bacterial effector proteins directly into the host cell cytoplasm, bypassing the extracellular milieu. Although the effector proteins vary among different T3SS pathogens, common pathogenic mechanisms emerge, including interference with the host cell cytoskeleton to promote attachment and invasion, interference with cellular trafficking processes, cytotoxicity and barrier dysfunction, and immune system subversion. The activity of the T3SSs correlates closely with infection progression and outcome, both in animal models and in human infection. Therefore, to facilitate patient care and improve outcomes, it is important to understand the T3SS-mediated virulence processes and to target T3SSs in therapeutic and prophylactic development efforts.
INTRODUCTION
In 1994, Rosqvist and colleagues proposed a model for a specialized molecular machine in Yersinia closely related to the bacterial flagellar apparatus that has the capacity to secrete bacterial proteins into the extrabacterial environment (125). This bacterial type III secretion system (T3SS) is of special interest to those studying host-pathogen interactions because, by utilizing this system, bacteria are able to directly inject bacterial proteins called effectors into host cells across bacterial and host membranes, where they can manipulate host cell function.
Homologous T3SSs have been described for many gram-negative bacterial species, including pathogens (predominantly) and commensals of mammals, plants, and insects (for some examples, see Table 1) (reviewed in reference 149).
TABLE 1.
Pathogens with T3SSs and their effects on the host
| Pathogen | T3SS components
|
Hosts | Relationship with hosts | Diseases caused by agent | |
|---|---|---|---|---|---|
| Structural proteins/ translocators | Effectors | ||||
| Yersinia species (Y. pestis, Y. enterocolitica, Y. pseudotuberculosis) | Ysc injectisome, YopB, YopD, LcrV | YopH, -E, -T, and -O and YpkA, -P/J, and -M | Humans, cattle, rodents, fleas (Y. pestis) | Pathogen | Plague (bubonic, pneumonic, and septicemic) (Y. pestis), enterocolitis and mesenteric lymphadenitis (Y. enterocolitica and Y. pseudotuberculosis) |
| Salmonella enterica serovars (Typhimurium, Typhi, Paratyphi, Sendai, Dublin, and Choleraesuis) | SPI1, PrgK, PrgH, InvG (ring base), PrgI (needle), and SipB/C/D (putative translocators); SPI2, Ssa proteins (apparatus), Ssc proteins (effector chaperones), SsrAB (regulators), and SseB/C/D (translocators) | SPI1, AvrA, SipA/B/C/D, SlrP, SseK, SopA/B/D/E/E, and SptP; SPI2, SpiC, SseF/G/I/J, SlrP, SspH1/H2, SifA, SifB, PipB/B2, SseK1/K2, GogB, and SopD2 | Humans, rodents, chickens, cows, and pigs | Pathogen (in humans, rodents, cows, and pigs), innocuous carriage (in chickens and some human cases) | Enterocolitis in humans and typhlitis and typhoid-like disease in mice (serovar Typhimurium), enteric fever in humans (serovars Typhi, Paratyphi, and Sendai), intestinal inflammation and bacteremia in cows (serovar Dublin), septicemia in pigs (serovar Choleraesuis) |
| EPEC/EHEC | EspA/B/D (translocators) | Tir, Map, Nle's, EspF/G, Cif, Orf3 | Humans, cows, calves | Pathogen (in humans and calves), innocuous carriage (in cows) | Intestinal inflammation and bloody diarrhea (EPEC/EHEC), possibility of renal failure and septic shock (EHEC) |
| Shigella species (S. dysenteriae, S. flexneri, S. boydii, and S. sonnei [multiple serotypes]) | Mxi/Spa (apparatus), IpaB/C (translocators), IpgC (IpaB/C chaperone) | IpaA/B, C terminus of IpaC, VirA, IpaH, Osp's, IpgB1 | Humans (only known reservoir) | Pathogen | Bacillary dysentery (shigellosis), sporadic dysentery pandemics (S. dysenteriae) |
| Bordetella species (B. pertussis, B. parapertussis, and B. bronchiseptica)a | BopB, BopD (potential translocators) | BopC | Humans, dogs, pigs | Pathogen | Whooping cough (B. pertussis and B. parapertussis [milder with B. parapertussis]), kennel cough in dogs, atrophic rhinitis in swine, possible respiratory illness in humans (B. bronchiseptica) |
| Pseudomonas aeruginosa | PopB and PopD (translocators), PcrV, SpcU (chaperon for ExoU) | ExoS, ExoT, ExoU, ExoY | Part of normal flora in up to 20% of humans, common in the environment | Opportunistic and nosocomial pathogen | Pneumonia (common cause of hospital-acquired pneumonia and occasionally of community-acquired pneumonia), chronic airway infection in cystic fibrosis, urinary tract infections in long-term care facilities, various other clinical infections (e.g., endocarditis) in immunocompromised patients |
| Burkholderia pseudomallei | T3SS-1, T3SS-2, T3SS-3 (Bsa) | BopAB (putative), BopE (T3SS-3) | Environmental isolate, humans | Human pathogen | Melioidosis, community-acquired bacteremias and pneumonias |
| Vibrio parahaemolyticus, V. cholerae | T3SS1 (V. parahaemolyticus), T3SS2 (V. parahaemolyticus and V. cholerae) | VP1680 (T3SS1), VopA (T3SS2) | Aquatic isolate, humans | Human pathogens | Noninflammatory secretory diarrhea (V. cholerae), inflammatory diarrhea with potential systemic spread (V. parahaemolyticus) |
| Chlamydia species | YscN (ATPase), LcrH1 and -2 and SycE (chaperones), LcrE (structural “lid”) | IncA and additional Inc proteins, Cpn0909, Cpn1020 | Obligate intracellular pathogens, infectious bodies found in the environment | Human pathogens (C. trachomatis and C. pneumoniae), bird pathogen (C. psittaci) | Sexually transmitted infection (C. trachomatis), pneumonia (C. pneumoniae), psittacosis in birds (C. psittaci) |
Also carry the T3SS components BopN and Bsp22 (whose functions are unknown).
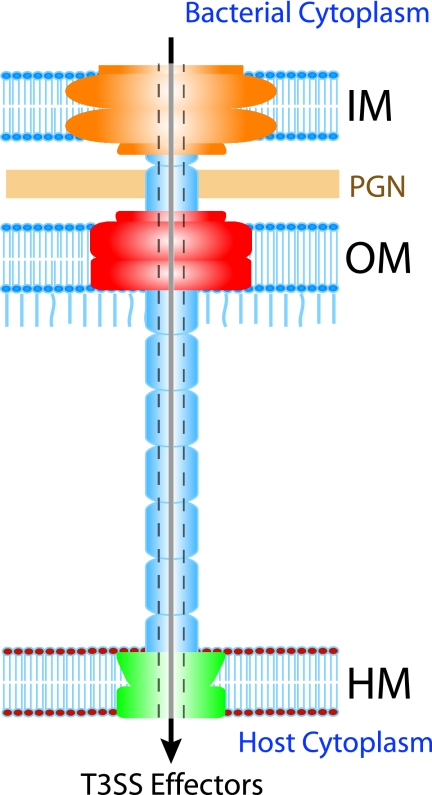
The T3SS is a complex structure composed of several subunits, which in turn are made up of approximately 20 bacterial proteins (Fig. 1). The proteins that make up the T3SS apparatus are termed structural proteins. Additional proteins called “translocators” serve the function of translocating another set of proteins into the host cell cytoplasm. The translocated proteins are termed “effectors,” since they are the virulence factors that effect the changes in the host cells, allowing the invading pathogen to colonize, multiply, and in some cases chronically persist in the host. The structural components of the T3SS and the process of translocation are expertly reviewed by Ghosh (40). Briefly, the T3SS apparatus consists of two rings that provide a continuous path across the inner and outer membranes, including the peptidoglycan layer. The inner membrane ring is the larger of the two coaxial rings, and protein components that make up the inner ring have been identified for a number of bacteria. The outer membrane ring is composed of the secretin protein family, which is also known to be involved in type 2 secretion and in the assembly of type IV bacterial pili. A needle-like structure associates with the outer membrane ring and projects from the bacterial surface. It varies in length among the different pathogens and, in the case of pathogenic Escherichia coli, is extended by the addition of filaments that are thought to facilitate attachment to the host cells through the thick glycocalyx layer. Effectors are thought to be transported through the hollow tube-like needle into the host cell through the pores formed in the host cell membrane by the translocator proteins. Translocators are usually conserved among the different pathogens possessing a T3SS and show functional complementarity for secretion and translocation, whereas the effectors are most often distinct, having unique functions suited to a particular pathogen's virulence strategy. However, effector homologues also exist among different T3SS-possessing bacteria.
FIG. 1.
The T3SS apparatus consists of rings that provide a continuous path across the inner (IM) and outer (OM) bacterial membranes, including the peptidoglycan layer (PGN). A needle-like structure associates with the outer membrane ring and projects from the bacterial surface. Considering the hollow tube-like morphology of the needle, an attractive theory is that the effector proteins are transported through the hollow center of this structure.
The clinical spectrum of disease caused by T3SS-containing pathogens is remarkably broad. Infection with enteropathogenic and enterohemorrhagic E. coli (EPEC and EHEC, respectively), Shigella, Salmonella, and Yersinia species results in intestinal disease. Yersinia pestis is the causative agent of plague. Salmonella serovar Typhi causes enteric fever. Bordetella causes whooping cough, while the opportunistic pathogen Pseudomonas aeruginosa can cause a variety of problems, including pneumonia, urinary tract infection, wound infection, septicemia, and endocarditis. Chlamydia trachomatis is a common sexually transmitted organism, and Chlamydia pneumoniae causes pneumonia and has been implicated in atherosclerotic disease of blood vessels. Burkholderia pseudomallei causes community-acquired bacteremia and pneumonia. Whether by a direct toxic mechanism or through induction of self-damaging host responses, the virulence of all of these bacteria utilizes T3SSs. Clearly, T3SSs are not restricted to a specific pathogen, tissue, host environment, clinical disease spectrum, or patient population. Here we explore how the activity of the T3SS has been adapted by these human pathogens to execute their virulence programs.
ADHERENCE, INVASION, AND COLONIZATION
To promote adherence, invasion, and colonization, many known bacterial, viral, and eukaryotic pathogens establish intimate interactions with the cytoskeletons of infected host cells. The maintenance of the host cell cytoskeleton is crucial to many aspects of normal host physiology, such as the preservation of cellular architecture, highly selective epithelial permeability, vesicular transport, and phagocytosis. Infections with T3SS-containing pathogens often disrupt these structures and processes.
A common theme of T3SS-dependent virulence is the subversion of the host cytoskeleton through direct or indirect manipulation of small Rho GTPases or direct interactions with filamentous (F-actin) or globular (G-actin) actin. Rho GTPases are central in regulating cytoskeletal functions (121). They are activated by guanine nucleotide exchange factors (GEFs) and down-regulated by GTPase-activated proteins (GAPs). Activated Rho GTPases recruit proteins, such as N-WASP and WAVE-2, which drive actin nucleation through the activity of the Arp2/3 complex. T3SS-containing pathogens also manipulate the levels of cellular phosphoinositides that anchor the actin cytoskeleton to the plasma membrane and activate GEFs.
Adherence to and Invasion of Host Cells
The hallmark of EPEC- and EHEC-induced intestinal pathology is the attaching and effacing (A/E) lesion, whose formation depends on a T3SS encoded within the loci of enterocyte effacement (LEE) and the interplay of many T3SS effectors.
Following intimate attachment of the bacteria to the intestinal epithelium, the brush border microvilli are disrupted (effacement), and the bacteria promote formation of actin pedestals that elevate the pathogen above the intestinal epithelium (75). To attach to the enterocytes, EPEC and EHEC utilize their T3SSs to inject the translocated intimin receptor (Tir) into the host cell (33), where it inserts into the host cell membrane and binds to the bacterial outer membrane protein intimin. Binding of intimin to Tir induces Tir clustering, initiating a cascade of signaling events that leads to actin polymerization and pedestal formation (13). This ultimately results in the formation of the A/E lesion. EPEC Tir is tyrosine phosphorylated to recruit the Arp2/3 complex and drive actin polymerization (12, 117), whereas EHEC Tir is not phosphorylated but, rather, relies on an additional T3SS effector, TccP/EspFU, for Arp2/3 recruitment (11, 37). Successful pedestal formation requires down-regulation of filopodia, which form in response to EPEC/EHEC infection, as well as disruption of the host microtubule network. The T3SS effectors Map (mitochondrion-associated protein) (69), Tir (64), EspH (153), EspG, and EspG2 (14) mediate these processes. This multifaceted approach allows A/E pathogens to coordinate the formation of A/E lesions and actin pedestals, providing them with a unique niche in the intestine of the infected host.
Salmonella and Shigella promote their own uptake into nonphagocytic cells by targeting several signaling pathways that converge to induce the formation of transient actin-rich membrane ruffles that engulf the infecting bacterium (44, 134). This allows them to reside and replicate in a privileged site, minimizing exposure to the immune system of the host.
Salmonella encodes two T3SSs on two Salmonella pathogenicity islands (SPI1 and -2). SPI1 is necessary for invasion into the nonphagocytic cells of the intestinal epithelium. Two SPI1-encoded T3SS effectors, SipA (95, 169) and SipC (17, 51), cooperate to stabilize and nucleate actin, respectively, but are not sufficient to induce formation of the membrane ruffles necessary for Salmonella uptake into nonphagocytic cells (134). Additional SPI1 effectors activate Cdc42, Rac, and RhoG (Rho GTPases) by either mimicking the function of their cognate GEFs (SopE and SopE2) (168) or generating activating second messengers (SopB/SigD, a phosphatidylinositol phosphatase) (134). Another effect of SopB/SigD-mediated depletion of phosphatidylinositol 4,5-bisphosphate in the invaginating regions of the host cell membrane is increased malleability of the host cell membrane, which facilitates remodeling associated with Salmonella entry (145). Modulation of vesicular trafficking seems to involve SopB modulation of phosphoinositide metabolism (54). Once Salmonella is engulfed, the host cells need to return to a somewhat normal state. The normalization process is facilitated by SptP, a T3SS-1 effector with GAP activity that inactivates Rac and Cdc42 (44). The concerted efforts of the SPI1 effectors SipA, SipC, SopB/SigD, SopE, SopE2, and SptP result in the engulfment of Salmonella by the host cell.
Unlike Salmonella, Shigella does not infect the apical surface of the intestinal epithelium but, rather, gains entry into the submucosa by infecting M cells and macrophages. After inducing apoptosis in infected macrophages, it is released at the basolateral surface of the intestinal epithelium, where it invades and ultimately spreads intra- and intercellularly (58). Entry into epithelial cells is followed by escape from the phagosome; both are dependent on a T3SS invasion complex comprised of IpaB and IpaC (96), which translocates T3SS effectors into the host cell. In a process similar to Salmonella invasion, Shigella T3SS effectors act in concert to activate or mimic the action of Rho GTPases (IpaC [148], IpgB1 [109], and IpgB2 [3]), induce inositol fluxes (IpgD [92]), and destabilize microtubules (VirA [163]). These actions culminate in Shigella entry into the host cell. Following entry, the normal cellular architecture is restored, likely through the action of IpaA, which promotes F-actin depolymerization (9).
Hence, Shigella invasion of epithelial cells proceeds through the basolateral side of the epithelium, is mediated by the Ipa complex, IpaD, IpgB1, and IpgB2, and is made more efficient by IpaA and VirA. Once inside the host cell, Shigella escapes from the phagolysosome through the action of T3SS effectors IpaB (57) and IpaH7.8 (28). Although Shigella is a nonmotile pathogen, it utilizes the host cytoskeleton to induce the formation of actin tails that propel it within and between the cells. The T3SS effector OspE2 appears to preserve cellular architecture and, in this manner, to facilitate Shigella intercellular spread. In its absence, Shigella-infected cells exhibit rounding, which reduces the efficiency of actin-based motility (99). VirA was recently demonstrated to have an additional function as a cysteine protease digesting alpha-tubulin, thus degrading the microtubule network, to promote Shigella motility (162). The microtubule network in the host cell cytosol inhibits Shigella movement, and VirA cysteine protease activity creates clear paths in the microtubule jungle along which Shigella can move. Consequently, the concerted actions of multiple Shigella T3SS effectors facilitate its invasion of the host cell and promote its further dissemination.
Intracellular Survival and Subversion of Cellular Trafficking Processes
Once Salmonella is engulfed, the SPI2-encoded T3SS is activated and interferes with phagosome maturation, resulting in the formation of a specialized Salmonella-containing vacuole (SCV), where Salmonella resides during intracellular survival and replication (reviewed in reference 80). SCV formation proceeds through a number of steps, including interference with the endocytic pathway, which prevents the delivery of hydrolytic enzymes to the SCV; transport of the SCV to the perinuclear region; and development of Salmonella-induced filaments (SIFs) (80). All of these events proceed through Salmonella interference with many cellular processes and culminate in the survival, multiplication, and spread of Salmonella. The SPI2-encoded T3SS is essential to orchestrate SCV biogenesis and Salmonella's intracellular survival.
One of the features of intracellular Salmonella pathogenesis is the assembly of an actin coat around intracellular bacteria, which may promote SCV fusion with actin-containing or actin-propelled vesicles and may also exert a protective effect on the SCV by preventing its fusion with unfavorable compartments (44). The assembly of the F-actin coat is dependent on the SPI2-encoded T3SS, specifically the effectors SseB, -C, and D as well as SpiC (164).
SCVs are transported along the microtubules to the microtubule organizing center located in the perinuclear region (80). From this position, Salmonella modulates cellular trafficking processes in a SPI2 T3SS-dependent manner, thus gaining access to a supply of nutrients and membrane materials for maintenance of SCVs and formation of a tubular network of membrane structures called SIFs that connect the individual SCVs in the infected cell. Dependent on SseF and SseG, intracellular Salmonella redirects the Golgi apparatus-derived exocytic transport vehicles—which are normally targeted to the cytoplasmic membrane—to the SCV instead (81). The subversion of Golgi apparatus-derived vesicles is crucial for efficient replication of SCV-bound Salmonella, as disruption of the Golgi network was shown to strongly inhibit Salmonella intracellular growth (80).
A number of Salmonella SPI2 effectors are involved in the proper positioning and maintenance of the SCV. SifA modulates the association of SCV with the kinesin motor protein, potentially preventing excessive formation of outgoing vesicles and resultant SCV disruption (8). SseG and SseF are additional SPI2 effectors required for SCV biogenesis and potentially for perinuclear localization of SCV (1, 82). Salmonella lacking SseF is deficient in the ability to recruit the motor protein dynein to the SCV, and SseF appears to partner with SseG to promote dynein recruitment and SCV transport along the microtubules. The concerted function of SifA, SseF, and SseG has the effect of differentially recruiting the motor proteins of the microtubules to the SCV to promote proper SCV positioning.
SifA is required for the formation of SIFs, although the exact mechanism involved in SIF biogenesis is still not known (74). SPI2 effectors SseF and -G (82, 83), as well as PipB2 (73) and SopD2 (65), have also been shown to have a role in SIF formation.
In sum, by interfering with multiple cellular processes via its SPI1- and SPI2-encoded T3SSs, Salmonella provides itself with a convenient niche for intracellular survival, replication, and subsequent spread.
T3SS-assisted colonization strategies of various pathogens demonstrate that T3SSs are central to their virulence. Using T3SSs, pathogens undertake the first step of pathogenesis: they establish a convenient and safe niche inside the host from where they can interfere with various physiological processes, as described below.
TYPE III SECRETION IN CYTOTOXICITY
The T3SS-dependent invasion, survival, and persistence of pathogens within the host are invariably associated with damage to host tissues. T3SS-associated damage takes a number of forms, including direct cytotoxicity associated with the induction of apoptosis or necrosis or tissue damage associated with the disruption of tissue barriers. These mechanisms promote pathological outcomes, such as diarrhea or inflammation.
Direct Cytotoxicity
Cell death is an important mediator of tissue destruction and pathology in a variety of diseases. The destruction of cells may be sufficient to cause tissue/organ dysfunction alone, it may enable pathogens to disseminate or breach barriers, or it may induce an inflammatory response which is the root of disease production. T3SSs have been implicated in a variety of these pathogenic mechanisms.
One of the hallmarks of Pseudomonas infection is epithelial injury in infected lungs. Lung pathology in murine infection models requires a functional cytotoxin, ExoU, which is translocated by the Pseudomonas T3SS (30). Cytotoxicity induced by ExoU occurs independently of other T3SS effectors and appears to be due to phospholipase activity. The translocation of active ExoU results in destabilization and destruction of intracellular membranes. This destabilization results in necrosis and correlates with a variety of clinical parameters of disease in infection models, including transudation into alveoli, interstitial pathology, and mortality (29, 30, 133).
In 2003, following genome sequencing of Vibrio parahaemolyticus, two T3SSs were identified (91). Each of the two V. parahaemolyticus chromosomes encodes one T3SS, which were therefore designated T3SS1 and T3SS2. This finding was thought to account for the differences in clinical manifestations of V. parahaemolyticus and Vibrio cholerae infections: V. cholerae generally causes noninflammatory secretory diarrhea, whereas the clinical features of V. parahaemolyticus infection commonly include inflammatory diarrhea and even systemic spread. Vibrio cholerae is subdivided into a number of serotypes, among which O1 and O139 are the most common clinical isolates and are also those responsible for the seven cholera pandemics documented since 1861 (25). However, some of the non-O1, non-O139 V. cholerae species are clearly pathogenic and have been implicated in several outbreaks and sporadic cases of diarrhea and extraintestinal infections (22, 112). In 2005, Dziejman et al. (25) demonstrated that clinically important non-O1, non-O139 V. cholerae isolates are very likely to encode a T3SS homologous to the T3SS2 of V. parahaemolyticus. Hence, the pathogenic potential and fitness of Vibrio species might be much more dependent on the T3SS and in this way related to those of other T3SS-carrying enteric pathogens than was previously thought.
The genome organization of V. parahaemolyticus is very similar to that observed for Yersinia species, and a number of genes homologous to Yersinia T3SS effectors have been identified (114). When the functional contribution of each T3SS to the manifestations of V. parahaemolyticus infection was analyzed, it was observed that T3SS1 was key for the in vitro cytotoxicity of V. parahaemolyticus, whereas T3SS2 was dispensable for this function (114). Recently, Ono et al. (110) demonstrated that T3SS1-dependent cell death was by apoptosis and identified VP1680 as a T3SS1-translocated protein central to T3SS1-induced cytotoxicity. In contrast to cytotoxicity, enterotoxicity induced by V. parahaemolyticus appeared to be due mainly to the presence of T3SS2, as demonstrated by infections in rabbit ileal loops. Ileal loops infected with wild-type V. parahaemolyticus or T3SS1 mutant bacteria exhibited massive hemorrhage and inflammatory changes, while infection with T3SS2 mutant bacteria failed to induce these pathological changes. The differential functions of T3SS1 and T3SS2 are compatible with the observation that different effector proteins are selectively targeted for secretion by each of these secretion systems (114).
Disruption of Epithelial TJs
Disruption of tight junctions (TJs) is a common mechanism utilized by pathogens to promote their virulence. TJs are composed of transmembrane proteins (occludin, claudins, and junctional adhesion molecule) and cytoplasmic proteins, among them zona occludens (ZO) proteins. ZO proteins act as adaptors linking the TJ-associated proteins to each other and to the actin cytoskeleton, including the perijunctional actomyosin ring that regulates TJ permeability. Several signaling molecules localize at TJs (151), and Rho family proteins are also implicated in their assembly and maintenance (152). Diarrhea is one of the most frequent manifestations of infectious intestinal diseases, promoting the spread of the pathogen to new hosts. The disruption of TJs is an important contributor to diarrheagenesis.
T3SS-facilitated disruption of TJs was shown to be an important factor contributing to diarrhea initiation during Citrobacter rodentium infection of mice, with the T3SS effector EspF being central to the process of TJ disruption (46). Murine C. rodentium infection is a well-established model of EPEC/EHEC infections (88). EHEC, although closely related to EPEC, does not induce epithelial barrier disruption as quickly or severely as that induced by EPEC. This difference is possibly due to the fact that EHEC-induced TJ disruption is mainly regulated by U-EspF, an EspF homologue, with EspF itself playing only a minor role (158). Map and EspG, together with EspG2, are additional EPEC T3SS effectors implicated in the disruption of the intestinal epithelial barrier (23, 90, 147).
An additional mechanism employed by C. rodentium to induce diarrhea in infected mice is mislocalization of aquaporin water channels (45). Aquaporins contribute to stool dehydration, and during C. rodentium infection, they move from their normal location along the apical cell membrane to the cytoplasm, an effect which is partially dependent on EspF and EspG T3SS effectors. Restoration of aquaporin localization to the cell membrane is observed following recovery from infection and cessation of diarrhea.
Many cellular mechanisms underlie EPEC-mediated disruption of epithelial integrity. A cause-effect relationship can be observed in phosphorylation of myosin light chain, which results in contraction of the actomyosin ring, opening up the TJs and decreasing the transepithelial resistance.
Similar to the case with A/E pathogens, Salmonella-induced diarrhea is dependent on disruption of the intestinal epithelial barrier via the T3SS. Salmonella enterica serovar Typhimurium SPI1 T3SS effectors SipA and SopA, -B, -D, and -E2 were shown together to induce fluid accumulation in intestinal bovine loops and diarrhea in vitro and in vivo in calves (10, 120, 167). The SPI1 T3SS effector SopB/SigD is involved in modulating chloride secretory responses in infected epithelial cells (5), a possible mechanism in the induction of diarrhea by S. enterica serovar Typhimurium. Thus, SPI1, in addition to mediating Salmonella invasion of host cells, also promotes the induction of diarrhea as a consequence of disruption of the epithelial barrier.
Yersinia pseudotuberculosis was shown to bind to β1-integrins via the T3SS effector YopE, disturbing ZO-1 and occludin localization, promoting actin rearrangement, and disrupting the barrier properties of the epithelium, thereby promoting paracellular translocation of bacteria (143). It appears that disruption of TJs by Y. pseudotuberculosis is an important mechanism of transport to the basolateral side of the epithelium, from which invasion of Y. pseudotuberculosis into the epithelial layer occurs.
T3SS-mediated cytotoxicity induces multiple results, such as cell death-associated organ dysfunction, inflammatory changes, and diarrhea. All of these are important manifestations of the disease process that mediate both morbidity and mortality.
T3SS AND HOST IMMUNITY
In addition to physical barriers such as the intestinal epithelium, an important biological barrier to disease-causing microbial infection is the defense of the host. The evolution of the T3SS is associated almost exclusively with pathogenic organisms, and it is not surprising that T3SSs are involved in interactions with host immune responses. T3SSs have been adapted by pathogens to provoke or evade host innate immunity in a variety of ways, including the direct activation of host signaling cascades, triggering of host pattern recognition through extracellular and intracellular pattern recognition receptors (PRRs), and the suppression or evasion of innate and adaptive defenses.
T3SSs and Pathogen Recognition by the Host
Upon arrival in the host environment, bacteria encounter an array of PRRs that have evolved to sense pathogenic motifs (called pathogen-associated molecular patterns) and to trigger host defenses. These PRRs include extracellular receptors of the Toll-like receptor (TLR) family and the intracellular leucine-rich repeat-containing proteins of the Nod-like receptor family. These important host innate immune receptors signal the presence of bacterial motifs in privileged host environments. T3SSs are required for many pathogens to occupy or establish their host niche and are often required for indirect PRR activation (or avoidance). Agonism of these receptors also seems to occur directly by T3SS-dependent mechanisms.
Delivery of Salmonella flagellin to the basolateral intestinal epithelium is a potent inducer of epithelial inflammatory interleukin-8 (IL-8) production through the activation of the flagellin PRR TLR5 (39, 165, 166). Although flagellin is not normally secreted through the T3SS, delivery of flagellin to the basolateral membrane depends in some way on the Salmonella SPI2-encoded T3SS (89). Similarly, intracellular monomeric flagellin from Salmonella induces the activation of caspase-1 and the production of active IL-1β and IL-18 through activation of Ipaf signaling independently of TLR5 (31, 98). Either directly via the translocation of flagellin or indirectly via another mechanism, this process requires a functional SPI1 T3SS (98).
Pseudomonas more directly activates PRRs in a T3SS-dependent manner. The activation of both TLR2 and TLR4 is necessary for the production of a complete inflammatory response in the lungs of mice infected with P. aeruginosa (119). The T3SS effector ExoS activates TLR2 and TLR4/MD-2/CD14 signaling through its C and N termini, respectively (26). This activation could be induced in cultured monocytes by exogenously administered ExoS in a manner that required neither effector translocation nor ExoS internalization, suggesting that this effector can act both as a modifier of intracellular signaling (discussed below) and as a soluble secreted protein (26).
Direct Manipulation of Host Innate Immune Signaling
The defining feature of T3SSs is the ability to directly inject bacterial proteins into the cytosol of a host cell. Yersinia, Salmonella, and Shigella species have exploited this ability to manipulate the inflammatory signaling of the host. This manipulation can result in both the induction and inhibition of host innate immune signaling. Innate immune signaling in mammals involves an intricate synthesis of signaling molecules triggered by a variety of intracellular and extracellular triggers. Of particular interest for this review are two critical signaling “end points,” namely, the nuclear translocation of the cytosolic transcription factor NF-κB (nuclear factor kappa B) with the activation of inflammatory gene expression and the proteolytic cleavage and activation of the inflammatory cytokines IL-1β and IL-18 by caspase-1 (also called IL-1-converting enzyme). Both of these are potent inducers of inflammation that reflect the culmination of a variety of signaling pathways in the host. Both are also the targets of T3SS effectors.
NF-κB
Following the activation of a number of kinase cascades, NF-κB is liberated from its cytosolic binding partners (the IκBs), at which point it is freely translocated to the nucleus and activates the expression of inflammatory genes (reviewed in reference 66). The T3SS effectors of Salmonella, Yersinia, and Shigella target these signaling cascades to promote or prevent inflammatory gene expression.
The SPI1 T3SS effector SopE activates NF-κB translocation by triggering Rho GTPases (48). The result of this activation is a potent inflammatory response in epithelial cells. The initial surge of NF-κB activity initiated by SopE is subsequently dampened by another SPI1 T3SS effector, SptP. This effector antagonizes Rac-1 and Rho GTPases, resulting in the delayed diminution of inflammatory signaling (34). Notably, SPI1 T3SS effectors are also responsible for the invasive phenotype of Salmonella, with a consequent overlap between the proinflammatory and invasive phenotypes associated with this pathogen in some in vivo models. Furthermore, as discussed above, SPI1 T3SS effectors are somehow required for the delivery of monomeric flagellin to intracellular PRRs.
The Shigella T3SS effector OspG also directly inhibits NF-κB activation upon infection of host cells. OspG of Shigella flexneri targets the ubiquitination of IκBα, preventing it from being degraded and thereby sequestering NF-κB in the cytosol and preventing inflammatory gene induction in response to tumor necrosis factor alpha. The phenotypic result of this inhibition is decreased inflammatory severity in rabbit ileal loops that requires the OspG effector (71). An additional T3SS effector, OspF, demonstrates a highly selective inhibition of NF-κB function: OspF is a phosphatase that dephosphorylates mitogen-activated protein kinases (MAPKs), preventing epigenetic modification of histone H3 and ultimately blocking the activation of a subset of NF-κB-responsive genes (4). This compromises the proinflammatory response and leads to a reduced recruitment of inflammatory cells to the site of infection. The Yersinia T3SS effector YopP/YopJ inhibits NF-κB translocation to the nucleus by inhibiting the degradation of IκBβ as well as MAPK signaling (127). YopP/YopJ was recently demonstrated to function as an acetyltransferase (102). It uses acetyl-coenzyme A to covalently modify MAPK and in this manner prevent the phosphorylation and modification of the modified protein. The Y. pseudotuberculosis effector YopE also inhibits NF-κB and caspase-1 activation by inactivating Rho GTPases.
In addition to the anti-inflammatory effect discussed above, inhibition of NF-κB may have a proapoptotic effect. The Yersinia T3SS effector YopJ and the V. parahaemolyticus T3SS1 effector VP1686 suppress NF-κB and, via this suppression, induce a conserved apoptotic pathway in macrophages (6, 100).
Caspase-1
Following transcriptional activation by NF-κB, the proinflammatory cytokines IL-1β and IL-18 are activated by caspase-1-mediated cleavage. Parallel to the induction of inflammation by hijacking NF-κB signaling, T3SSs have evolved to induce caspase-1-mediated cleavage of IL-1β and IL-18, as well as caspase-1-mediated apoptosis. The Shigella T3SS effector IpaB activates caspase-1-mediated apoptosis and IL-1β release necessary for inflammation (132, 170, 171). It does so by directly binding and activating caspase-1 (59). Similarly, the SPI1 T3SS effector SipB of Salmonella directly binds and activates caspase-1, resulting in IL-1β and IL-18 release (56). In the reverse of this process, the Yersinia T3SS effector YopE inhibits the production of proteolytically active caspase-1, likely through its inhibition of host GTPases (135).
Manipulation of host immune signaling pathways via the T3SSs enables pathogens to modify the cellular and organ environment to their advantage, promoting survival. At the same time, hijacking of the signaling pathways provokes such pathological manifestations as inflammation and apoptosis.
T3SS and Cells of the Immune System
A consequence of breaching an anatomical barrier, damaging tissues, or triggering host inflammatory signaling is the recruitment and activation of a variety of host immune cells. Resident phagocytes, circulating inflammatory cells, antigen-presenting cells, and lymphocytes have the combined function of identifying, sequestering, and neutralizing invading pathogens. Above, we described some avoidance and resistance strategies used by pathogens to avoid cellular killing by creating specialized environmental niches (A/E lesions and specialized intracellular compartments). In addition to these behaviors, T3SSs are involved in the perturbation of host cellular immune responses. These pathogens exploit T3SS function to select which cells are recruited/produced by the host to combat infection. They also sabotage the function of the recruited cells to pathogenic advantage.
Host immune cells have diverse functions in the control of infection, and their recruitment and coordinated activation are critical for infection control. Pathogens exploit T3SSs to alter this coordination in a variety of ways. Cells responsible for pathogen destruction and processing (antigen-presenting cells), such as neutrophils, macrophages, and dendritic cells (DCs), are critical antibacterial effector cells of the innate immune system.
Immune cell recruitment.
Yersinia pestis selectively targets neutrophils, macrophages, and DCs for the injection of T3SS effectors (93). Through the actions of the effector YopM, Y. pestis also depletes the host natural killer (NK) cell population (70). In an analogous way, Phalipon and Sansonetti reported that Shigella skews T-cell responses to the Th2 phenotype by down-regulating the production of IL-12 in a T3SS-dependent fashion (116). Salmonella is able to overcome the host defense mechanism that immobilizes infected cells and to promote the motility of the infected phagocytes to accelerate its systemic spread from the intestinal lumen (160). This effect is dependent on the interaction of a SPI2 T3SS effector, SrfH/SseI, with the host cell protein TRIP6, which is an adaptor protein in a pathway that stimulates cellular motility. Additional SPI2 effectors are likely to also contribute to this effect. The exploitation of the T3SS to select specific immune cells for destruction or to skew immune cell activation and migration is another mechanism by which T3SS-containing pathogens subvert the host defenses.
Autophagy and phagocytosis.
If T3SS-containing pathogens are unable to avoid specific host immune cells by preventing their production or recruitment or causing their destruction, they often directly interfere with the antibacterial functions of the cells they encounter. T3SSs are used to interfere with a variety of host cell defenses, such as autophagy and phagolysosomal degradation.
Autophagy was originally described as a mechanism activated in response to nutrient depletion but has since been demonstrated to play an important role as a host innate immune defense (141). This process enables eukaryotic cells to isolate cytoplasmic materials within a membranous compartment and target them for degradation within lysosomes. Autophagy was also shown to promote the presentation of cytosolic antigens via major histocompatibility complex class II and to correlate with the activation of inflammasomes that induce pyroptosis, or inflammatory cell death (24).
Shigella actin-based motility is mediated by VirG, which is essential for Shigella virulence and dissemination to new hosts. Unfortunately for Shigella, however, VirG induces autophagy by binding to Atg5, a host cell autophagy protein. To avoid this host cell detection and elimination mechanism, Shigella secretes IcsB, a T3SS effector, which binds to VirG, thus preventing its interaction with Atg5, avoiding autophagy and promoting Shigella survival (108).
In contrast to Shigella, Salmonella stimulates autophagy in infected macrophages through the action of SipB (55). The advantage of this behavior to Salmonella is not well understood, but a striking observation is that all of the pathogens known to trigger pyroptosis also trigger autophagy (141). Regardless of the advantage to the pathogen, however, the cells can retaliate: it was demonstrated that when Salmonella is released into the cytosol following the disruption of SCV in a SPI1 T3SS-dependent fashion, host cells can degrade it by autophagy as a means to control infection (7).
Phagocytosis is normally thought of as a host defense mechanism, but this process may provide unintended benefits to the invading pathogen, which can disrupt the phagocytic process and set up residence in the phagocyte, taking advantage of this location as a safe niche and a dissemination mechanism. The ability of Salmonella and Shigella to induce phagocytosis by nonphagocytic cells and the contribution of this process to virulence are discussed above. Here we review the mechanisms that T3SS-containing pathogens utilize to either avoid phagocytosis by professional phagocytes or infect professional phagocytes by disrupting the normal phagocytic process.
Salmonella not only has the capacity to survive in the phagosomes of epithelial cells but is also able to subvert the macrophage phagocytic process by preventing delivery of NADPH oxidase and nitric oxide synthase to the phagosome, thus protecting the resident pathogen from damage by reactive oxygen (36, 156) and nitrogen (16) intermediates. These pathways are dependent on the SPI2 T3SS, consistent with its role in promoting systemic disease.
Yersinia, EPEC, and P. aeruginosa, on the other hand, have evolved to avoid the harsh environment of the phagosome by interfering with phagocytic uptake (123). Enteric Yersinia pathogens and Y. pestis resist phagocytosis by both macrophages and neutrophils (124, 157). YopH inhibits the process of phagocytosis by inhibiting the β1-integrin pathway. In neutrophils, it also blocks calcium signaling, which is essential for neutrophil degranulation (115). In macrophages, YopH targets a number of focal adhesion proteins (157). The small GTPases RhoA, Rac, and Cdc42 are inactivated by YopE of Yersinia, preventing actin polymerization (2), while the cysteine protease YopT cleaves the isoprenyl lipid moiety of RhoA to depolymerize actin filaments (136). YopO is a serine/threonine kinase that can bind host actin and Rho GTPases, but its contribution to the phagocytic process is still debated (157). The importance of Yersinia avoidance of phagocytosis is demonstrated by the decreased virulence in murine models of mutants lacking various Yop effectors involved in this process (157).
EPEC has a different strategy for blocking phagocytosis: it inhibits phosphatidylinositol 3-kinase function (which is initially activated in response to EPEC infection) in a T3SS-dependent manner, in this way preventing actin polymerization (15).
Pseudomonas aeruginosa inhibits phagocytosis by macrophages in vitro via the GAP activity of ExoS, which promotes the inactivation of Rac1 (122). Furthermore, P. aeruginosa is able to induce both necrosis and apoptosis in macrophages in a T3SS-dependent manner (50) and to cause neutrophil cytotoxicity, which was also suggested to be mediated by the T3SS (124). Evasion of phagocytosis and cytotoxicity towards professional phagocytes likely contribute to P. aeruginosa persistence and dissemination in infected patients.
Overall, the incredibly diverse mechanisms that many pathogens utilize to subvert the host phagocytic process highlight its importance in limiting infection, as well as the importance of phagocytosis subversion as a virulence strategy.
T3SS pathogens manipulate the cells of the immune system in various ways, which ultimately lead to the evasion of clearance and promote persistence and dissemination. The T3SS is vital to all these aspects of pathogenesis, highlighting its central role in the virulence strategy of T3SS pathogens.
PATHOGENESIS IN SUM: T3SSs IN VIVO
We have described a series of important T3SS-dependent pathogenic behaviors established in various model infections and cell culture systems. Clearly, T3SSs do not act alone during the pathogenic process and a full complement of virulence factors is required for disease genesis. What, then, is the impact of the T3SS on the infectious process? Moreover, what clinical impact might therapeutics developed to inhibit T3SS function yield? Experimental and bacterial genetic and epidemiological studies provide substantial direct and supportive evidence that T3SSs have become critical to the pathogenic efficiency of the pathogens harboring them.
Although T3SSs do not act in isolation, the impact of their absence on disease pathogenesis is dramatic. The absence of the SPI2-encoded T3SS of S. enterica serovar Typhimurium completely abrogates the severity of systemic infection in mice (53, 137) and decreases the severity of intestinal inflammation in mice and cows. In the absence of the proinflammatory stimulus of its two T3SSs, Salmonella serovar Typhimurium is totally attenuated in murine and bovine infectious inflammation disease models (19, 20, 47). Yersinia species lacking the effectors YopH, YopM, and YopQ are highly attenuated in murine infection (150). Yersinia enterocolitica, in which the T3SS component LcrV is unable to activate TLR2, is attenuated during murine infection (139). Mice infected with T3SS-deficient C. rodentium, an A/E pathogen, do not manifest any pathological symptoms caused by wild-type C. rodentium infection (46), and deletions of various T3SS effectors of A/E pathogens have been demonstrated to diminish distinct aspects of disease, such as formation of the A/E lesion and disruption of the epithelial barrier (14).
Correlations between the functions of T3SS in vitro and in animal models are predictably recapitulated in human disease. In a retrospective pilot cohort study of endotracheally intubated patients with ventilator-associated Pseudomonas pneumonia, severe (e.g., fatal) illness was associated with the ability of the Pseudomonas isolates to secrete T3SS effectors, in particular the effector ExoU (49). Cystic fibrosis patients were also more likely to have severe acute Pseudomonas infection than chronic infection if they were infected with a T3SS-bearing strain (126). The importance of T3SSs in various in vitro and in vivo models of virulence and their association with severe infection in humans make T3SSs a promising therapeutic target.
THERAPEUTICS: INTERFERENCE WITH T3SS AND VIRULENCE
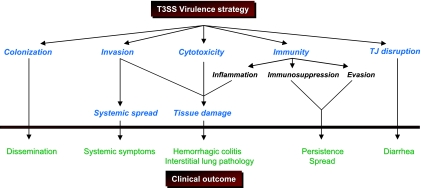
The T3SS can be viewed as a bacterial organelle that manipulates cell signaling and trafficking, alters cellular morphology, and interferes with the generation of the host immune response (Fig. 2). On the other hand, the T3SS can also be exploited to the host's advantage. Being unique to the pathogen, it provides a convenient target that can be exploited in designing both therapeutic and prophylactic interventions. Therapeutics specifically targeting the T3SS have special appeal. Unlike the case with conventional antibiotics, targeting a virulence mechanism associated almost exclusively with pathogenic organisms would not perturb the balance of normal flora, resulting in opportunistic infections (e.g., clostridial pseudomembraneous colitis). Although resistance to therapeutic interventions may be possible (depending on the mechanism of T3SS inhibition), this would not be a resistance mechanism that would be transferred to non-T3SS-bearing genera of bacteria. Of particular advantage would be the disruption of bacterial virulence mechanisms that are at the core of disease formation, i.e., at the interface of the host and pathogen.
FIG. 2.
T3SS effector functions of pathophysiologic importance. Type III secretion effectors have been implicated in a variety of critical pathogenic behaviors. These virulence strategies have specific consequences in disease pathogenesis in the infected host.
Antibodies against T3SS Proteins
In recent years, we have seen an expansion in the use of antibodies for therapeutic purposes. Passive immunization has been used to counteract the action of various toxins and venoms. It might also prove feasible to use antibodies to treat infection with T3SS-containing pathogens, particularly those such as EHEC, where the use of antibiotics can worsen clinical outcomes.
Monoclonal and polyclonal antibodies raised against T3SS structural proteins have been shown, in vitro and in vivo, to prevent important T3SS-mediated virulence behaviors. In vitro, antibodies raised against the EHEC T3SS structural protein EspA prevent the actin cytoskeletal rearrangements required for formation of the A/E lesion characteristic of T3SS-associated virulence (84). Antibody treatment prevents A/E lesion formation but not filament formation, suggesting that antibodies interfere with effector translocation. Although this inhibition was limited to specific serotypes of EHEC/EPEC, it does suggest that targeting the T3SS apparatus is an appropriate therapeutic intervention to interfere with T3SS-mediated virulence in a broader range of pathogenic E. coli strains.
The IpaD effector of Shigella localizes to the tip of the T3SS needle (27, 131). Although a concrete function for IpaD has not been established, deletions in the C-terminal two-thirds of IpaD have been shown to result in the complete failure of bacterial invasion. In vitro, antibodies to IpaD prevent translocon insertion, Shigella contact-mediated hemolysis of erythrocytes, and Shigella entry into epithelial cells (27, 131). Thus, IpaD has a crucial role in Shigella pathogenesis, and inhibition of its function will likely be very effective in reducing or abolishing Shigella virulence. Moreover, there is significant functional homology between IpaD of Shigella, LcrV of Yersinia, and PcrV of Pseudomonas. In concordance with the observed requirement for IpaD for in vitro T3SS function, immunization against LcrV and PcrV impairs Yersinia and Pseudomonas virulence in vivo.
It has been shown that antibodies to Yersinia LcrV (43) and Pseudomonas PcrV (32, 43) also prevent translocon insertion, while LcrV was recently shown to localize to the T3SS needle tip (101). Anti-PcrV antibodies have been shown to be protective in animal models of septic shock. Antibody treatment improved hemodynamic parameters, minimized the extent of tissue injury, and increased host survival (138). Neely et al. (104) also recently demonstrated that intraperitoneal treatment of mice with anti-PcrV antibodies provided protection against a lethal challenge with Pseudomonas in the setting of a third-degree burn. The improvements were shown to be independent of the Fc receptor, as the administration of the Fab fragment had a protective effect comparable to that of whole immunoglobulin G, indicating that it is the inhibition of T3SS function rather than immunization that likely provides this protection. Antibodies to LcrV have been shown to have a protective effect in animal models of Y. pestis infection. All mice treated with monoclonal antibodies to LcrV and challenged with Y. pestis survived (61). More recently, monoclonal anti-LcrV antibodies were shown to be protective when administered up to 48 h following Y. pestis challenge in both the bubonic and pneumonic plague models, significantly reducing the mortality of mice and prolonging survival (60). Additionally, simultaneous administration of anti-LcrV antibody with an antibody to F1 capsular antigen following Y. pestis challenge in a bubonic plague model had a synergistic protective effect compared to administration of either antibody alone.
The therapeutic potential of these antibodies in human infection is clearly worth exploring. Furthermore, the conceptual approach—passive immunization with antibodies targeting T3SS structural proteins—is one that has the potential for broad application to other T3SS-containing pathogens.
Secreted Effectors as Vaccines
Attempts are being made to design cheap and effective vaccines against T3SS-containing pathogens, especially because many are endemic to various parts of the world and have tendencies to cause sporadic outbreaks.
LcrV not only is protective against Y. pestis during passive immunization but also confers protection to mice immunized with LcrV in models of bubonic and pneumonic plague (52, 85). This may have limited prophylactic potential, however, due to concerns that LcrV is an immunosuppressant in infected hosts (94). Immunization with an alternative T3SS protein, YscF, which facilitates Yop delivery into host cells, was protective in a murine bubonic plague model, suggesting that this type of immunization is experimentally robust for Yersinia infection prophylaxis (94, 142).
Because most EHEC outbreaks result from consumption of meat, water, or dairy products contaminated by cattle fecal material, a logical approach to curb the spread of EHEC is to immunize cattle, thus preventing colonization of cattle by EHEC, fecal shedding, and human infections. Some positive results were obtained with the use of secreted proteins of EHEC O157:H7 to immunize cattle and prevent colonization and shedding (118, 155). Therefore, the potential of EHEC secreted proteins as vaccines needs to be evaluated further and specifics that determine the induction of a protective response or the lack thereof determined.
Proteins associated with inclusion membranes of Chlamydia have the potential to be presented to the host's immune system via the major histocompatibility complex class I pathway. The T3SS component LcrE (146) localizes to the inclusion membrane and body (87). Immunization with LcrE was protective in a number of C. pneumoniae animal models (130, 146). CopN, another type III secreted Chlamydia inclusion membrane protein secreted via the T3SS, was shown to be both immunogenic and protective in mice when administered with E. coli heat-labile toxin as an adjuvant (144). However, the extent of protection following immunization with CopN was not as great as that following immunization with LcrE.
Efforts are being made to create a subcellular vaccine that will confer immunity to multiple Shigella species to cover a wide range of causative agents of shigellosis. A subunit vaccine consisting of single Shigella species lipopolysaccharide and the invasin complex IpaB/C was shown to be protective in homologous challenges in various animal models (154). Oaks and Turbyfill report that a bivalent vaccine incorporating antigens from S. flexneri and Shigella sonnei was as effective in animal models against challenge with either S. flexneri or S. sonnei as a monovalent vaccine followed by homologous challenge (107). Antigenic determinants of different Shigella species can be combined in this fashion to produce multivalent vaccines conferring immunity to all agents of shigellosis that are clinically prevalent in a particular area.
T3SS Mutants as Vaccine Strains
The generation of effective protective immunity to intracellular pathogens often necessitates the presentation of antigens from their unique environmental niches, such as an intracellular compartment, which is best accomplished by the use of live attenuated vaccines. Strains used as live vaccines should have the capacity for intracellular invasion, but at the same time, they should be sufficiently attenuated to prevent tissue damage and long-term persistence. Since T3SSs endow pathogens with the capacity for intracellular invasion and persistence, live vaccines for T3SS-containing pathogens need to be constructed carefully to elicit an appropriate protective immune response while avoiding T3SS-associated damage.
The currently approved live Salmonella serovar Typhi vaccine requires the administration of three or four separate doses given every other day, which is a significant practical drawback, especially in developing countries with limited access to medical care (63). An alternative approach that has been explored is to halt Salmonella's persistence by disabling the SPI2 T3SS and limiting its growth in vivo by introducing mutations in essential metabolic pathways. Although it is ineffective as a vaccine for Salmonella serovar Typhimurium, a Salmonella serovar Typhi strain with mutations in aroC (required for aromatic amino acid biosynthesis) and ssaV (a structural component of the SPI2 T3SS) was found to be immunogenic in adult volunteers while eliciting only few and mild adverse effects (62, 72).
Shigella strains with mutations in various T3SS effectors are potential candidates for vaccine development. A live virG mutant (defective for intercellular spreading) and strains that have the virG mutation coupled to a mutation in a gene essential for metabolism have been shown to be effective in human volunteers in terms of both immunogenicity (67, 76-78, 111) and protection (21), although undesired residual cytotoxicity was still observed, prompting further adjustments. To address the residual pathology associated with virG mutant strains while still eliciting an immune response protective against an intracellular pathogen, an alternative strategy was developed by Suzuki et al. (140). A noninvasive Shigella strain with a mutation in ipaB was complemented with inv, an invasin gene of Yersinia (ΔipaB inv strain). This rendered the ΔipaB inv strain invasion competent and immunogenic, yet it remained confined to the phagosome and did not induce clinically evident pathology in an animal model (140). As a result, it provides a very attractive alternative to virG mutant vaccine strains as well as a generally interesting paradigm for developing vaccines against intracellular pathogens.
Recombinant antigens of other pathogens and even tumor-associated antigens can be introduced into live attenuated T3SS-expressing carrier strains (by creating effector-antigen fusions) for delivery into the host cell cytosol and efficient induction of both humoral- and cell-mediated immune responses as well as mucosal immunity. Many heterologous antigens have been incorporated into live Salmonella, Shigella, Y. enterocolitica, and Y. pseudotuberculosis carrier strains, often with good success in inducing a heterologous antigen-specific protective immune response (18, 105, 113, 128, 129, 130, 161). However, the nature of the antigen and the specifics of the presenting strain need to be evaluated in detail and optimized on a case-by-case basis, as Kotton et al. (79) had very limited success in using a recombinant Salmonella strain to induce an immune response to the human immunodeficiency virus Gag antigen.
Inhibitors of T3SSs
In addition to the exploitation of T3SSs as passive or active immunization targets and for live attenuated vaccines, direct interference with T3SS function has recently become experimentally feasible. Reports of T3SS inhibitors in models of Yersinia and Chlamydia infections have shown promise. Previously identified compounds have been shown to inhibit T3SS function and pathogenesis. Lactoferrin inhibits T3SS-dependent invasion by Shigella by causing degradation of IpaA and IpaB (41, 42). Other novel inhibitors of T3SS function have also been shown to inhibit bacterial virulence. Kauppi and colleagues (68) recently identified a small-molecule inhibitor of T3SS called INP0400. This class of salicylaldehydes inhibits the production of an effective Yersinia T3SS in vitro (106). A set of compounds that function as transcriptional inhibitors of the EPEC T3SS was also identified (38). The therapeutic potential of these compounds against other T3SS-containing pathogens was highlighted by their use in in vitro models of Chlamydia infection. When given at the time of infection, INP0400 inhibited the growth of Chlamydia in infected cells, limiting reticulate body formation and chlamydial fitness (103).
The potential development of these and other T3SS inhibitor compounds will be an area of exciting development in coming years.
T3SSs and Diagnostics
T3SSs are important virulence determinants for all T3SS-possessing pathogens, and even the presence or absence of particular T3SS effectors was shown to correlate with the progression and outcome of infection in a C. rodentium animal model (159). Consequently, detection of T3SSs in clinical isolates may provide invaluable indications for the management of infected patients.
Detection of the Burkholderia pseudomallei T3SS in clinical specimens by PCR shows promise as a quick diagnostic tool that will enable rapid commencement of appropriate therapy and potentially enhance patient outcomes (35, 97). Similarly, enzyme-linked immunosorbent assay identification of the Pseudomonas T3SS effectors ExoU and ExoT and the T3SS structural components PopD and PcrV can provide fast clues about the type of P. aeruginosa isolate and assist in patient management (86).
The development of similar diagnostic methods for other T3SS-carrying pathogens has the potential to provide significant improvements to patient care and outcomes.
DISCUSSION
It is clear that T3SSs have been adapted to a variety of purposes by bacteria, resulting in the production of disease in infected hosts. The exploitation of T3SSs to establish environmental niches in a variety of host organs, to penetrate barrier defenses, and to provoke or prevent immune responses in the host gives an example of successful T3SS-dependent virulence strategies. It is interesting that opposing strategies (e.g., avoiding or inducing phagocytosis or inducing or inhibiting host immunity) have been employed successfully by pathogens with T3SSs to promote virulence. To debate the relative advantages of the induction of host defenses, such as inflammation, to the host and the T3SS pathogen is a complex subject. It is clear not only that these diverse strategies are successfully executed by these pathogens but also that clinical outcomes associated with infection with these pathogens are worsened by the pathogenic exploitation of T3SSs and that the absence of T3SSs results in decreased disease severity.
Interference with T3SS function by chemical or biological inhibition and vaccination has great potential to reduce the morbidity and mortality associated with infection with these pathogens.
Acknowledgments
We thank W. Deng, J. Guttman, A. Menendez, E. Arena, and S. Shames for critically reading the manuscript.
This work was supported by operating grants to B.B.F. from the Canadian Institutes of Health Research (CIHR), the Howard Hughes Medical Institute (HHMI), and the Foundation for the NIH as part of the Bill and Melinda Gates Grand Challenge program. B.C. is supported by CIHR and MSFHR studentships. I.S. is supported by a TRID studentship. B.B.F. is a CIHR Distinguished Investigator, an HHMI International Research Scholar, and the University of British Columbia Peter Wall Distinguished Professor.
REFERENCES
- 1.Abrahams, G. L., P. Muller, and M. Hensel. 2006. Functional dissection of SseF, a type III effector protein involved in positioning the salmonella-containing vacuole. Traffic 7:950-965. [DOI] [PubMed] [Google Scholar]
- 2.Aepfelbacher, M. 2004. Modulation of Rho GTPases by type III secretion system translocated effectors of Yersinia. Rev. Physiol. Biochem. Pharmacol. 152:65-77. [DOI] [PubMed] [Google Scholar]
- 3.Alto, N. M., F. Shao, C. Lazar, R. Brost, G. Chua, S. Mattoo, S. McMahon, P. Ghosh, T. Hughes, C. Boone, and J. Dixon. 2006. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124:133-145. [DOI] [PubMed] [Google Scholar]
- 4.Arbibe, L., D. Kim, E. Batsche, T. Pedron, B. Mateescu, C. Muchardt, C. Parsot, and P. Sansonetti. 2007. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 8:47-56. [DOI] [PubMed] [Google Scholar]
- 5.Bertelsen, L., G. Paesold, S. Marcus, B. Finlay, L. Eckmann, and K. Barrett. 2004. Modulation of chloride secretory responses and barrier function of intestinal epithelial cells by the Salmonella effector protein SigD. Am. J. Physiol. Cell Physiol. 287:C939-C948. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee, R., K. Park, Y. Kumagai, K. Okada, M. Yamamoto, S. Uematsu, K. Matsui, H. Kumar, T. Kawai, T. Iida, T. Honda, O. Takeuchi, and S. Akira. 2006. VP1686, a Vibrio type III secretion protein, induces Toll-like receptor-independent apoptosis in macrophage through NF- kappaB inhibition. J. Biol. Chem. 281:36897-36904. [DOI] [PubMed] [Google Scholar]
- 7.Birmingham, C., A. Smith, M. Bakowski, T. Yoshimori, and J. Brumell. 2006. Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281:11374-11383. [DOI] [PubMed] [Google Scholar]
- 8.Boucrot, E., T. Henry, J. Borg, J. Gorvel, and S. Meresse. 2005. The intracellular fate of Salmonella depends on the recruitment of kinesin. Science 308:1174-1178. [DOI] [PubMed] [Google Scholar]
- 9.Bourdet-Sicard, R., M. Rudiger, B. Jockusch, P. Gounon, P. Sansonetti, and G. Nhieu. 1999. Binding of the Shigella protein IpaA to vinculin induces F-actin depolymerization. EMBO J. 18:5853-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle, E., N. Brown, and B. Finlay. 2006. Salmonella enterica serovar Typhimurium effectors SopB, SopE, SopE2 and SipA disrupt tight junction structure and function. Cell. Microbiol. 8:1946-1957. [DOI] [PubMed] [Google Scholar]
- 11.Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7:217-228. [DOI] [PubMed] [Google Scholar]
- 12.Campellone, K., and J. Leong. 2005. Nck-independent actin assembly is mediated by two phosphorylated tyrosines within enteropathogenic Escherichia coli Tir. Mol. Microbiol. 56:416-432. [DOI] [PubMed] [Google Scholar]
- 13.Campellone, K., S. Rankin, T. Pawson, M. Kirschner, D. Tipper, and J. Leong. 2004. Clustering of Nck by a 12-residue Tir phosphopeptide is sufficient to trigger localized actin assembly. J. Cell Biol. 164:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caron, E., V. F. Crepin, N. Simpson, S. Knutton, J. Garmendia, and G. Frankel. 2006. Subversion of actin dynamics by EPEC and EHEC. Curr. Opin. Microbiol. 9:40-45. [DOI] [PubMed] [Google Scholar]
- 15.Celli, J., M. Olivier, and B. Finlay. 2001. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 20:1245-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakravortty, D., I. Hansen-Wester, and M. Hensel. 2002. Salmonella pathogenicity island 2 mediates protection of intracellular Salmonella from reactive nitrogen intermediates. J. Exp. Med. 195:1155-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang, J., J. Chen, and D. Zhou. 2005. Delineation and characterization of the actin nucleation and effector translocation activities of Salmonella SipC. Mol. Microbiol. 55:1379-1389. [DOI] [PubMed] [Google Scholar]
- 18.Chen, H., and D. Schifferli. 2000. Mucosal and systemic immune responses to chimeric fimbriae expressed by Salmonella enterica serovar Typhimurium vaccine strains. Infect. Immun. 68:3129-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coburn, B., Y. Li, D. Owen, B. A. Vallance, and B. B. Finlay. 2005. Salmonella enterica serovar Typhimurium pathogenicity island 2 is necessary for complete virulence in a mouse model of infectious enterocolitis. Infect. Immun. 73:3219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coombes, B. K., B. A. Coburn, A. A. Potter, S. Gomis, K. Mirakhur, Y. Li, and B. B. Finlay. 2005. Analysis of the contribution of Salmonella pathogenicity islands 1 and 2 to enteric disease progression using a novel bovine ileal loop model and a murine model of infectious enterocolitis. Infect. Immun. 73:7161-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coster, T. S., C. W. Hoge, L. VanDeVerg, A. B. Hartman, E. V. Oaks, M. M. Venkatesan, D. Cohen, G. Robin, A. Fontaine-Thompson, P. J. Sansonetti, and T. Hale. 1999. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect. Immun. 67:3437-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalsgaard, A., O. Serichantalergs, A. Forslund, W. Lin, J. Mekalanos, E. Mintz, T. Shimada, and J. Wells. 2001. Clinical and environmental isolates of Vibrio cholerae serogroup O141 carry the CTX phage and the genes encoding the toxin-coregulated pili. J. Clin. Microbiol. 39:4086-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dean, P., and B. Kenny. 2004. Intestinal barrier dysfunction by enteropathogenic Escherichia coli is mediated by two effector molecules and a bacterial surface protein. Mol. Microbiol. 54:665-675. [DOI] [PubMed] [Google Scholar]
- 24.Dengjel, J., O. Schoor, R. Fischer, M. Reich, M. Kraus, M. Muller, K. Kreymborg, F. Altenberend, J. Brandenburg, H. Kalbacher, R. Brock, C. Driessen, H. G. Rammensee, and S. Stevanovic. 2005. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA 102:7922-7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dziejman, M., D. Serruto, V. C. Tam, D. Sturtevant, P. Diraphat, S. M. Faruque, M. H. Rahman, J. F. Heidelberg, J. Decker, L. Li, K. T. Montgomery, G. Grills, R. Kucherlapati, and J. Mekalanos. 2005. Genomic characterization of non-O1, non-O139 Vibrio cholerae reveals genes for a type III secretion system. Proc. Natl. Acad. Sci. USA 102:3465-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epelman, S., D. Stack, C. Bell, E. Wong, G. G. Neely, S. Krutzik, K. Miyake, P. Kubes, L. D. Zbytnuik, L. L. Ma, X. Xie, D. E. Woods, and C. H. Mody. 2004. Different domains of Pseudomonas aeruginosa exoenzyme S activate distinct TLRs. J. Immunol. 173:2031-2040. [DOI] [PubMed] [Google Scholar]
- 27.Espina, M., A. J. Olive, R. Kenjale, D. S. Moore, S. F. Ausar, R. W. Kaminski, E. V. Oaks, C. R. Middaugh, W. D. Picking, and W. Picking. 2006. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect. Immun. 74:4391-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Prada, C. M., D. L. Hoover, B. D. Tall, A. Hartman, J. Kopelowitz, and M. Venkatesan. 2000. Shigella flexneri IpaH(7.8) facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human macrophages. Infect. Immun. 68:3608-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finck-Barbancon, V., and D. W. Frank. 2001. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J. Bacteriol. 183:4330-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finck-Barbancon, V., J. Goranson, L. Zhu, T. Sawa, J. P. Wiener-Kronish, S. M. Fleiszig, C. Wu, L. Mende-Mueller, and D. W. Frank. 1997. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 25:547-557. [DOI] [PubMed] [Google Scholar]
- 31.Franchi, L., A. Amer, M. Body-Malapel, T. D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat. Immunol. 7:576-582. [DOI] [PubMed] [Google Scholar]
- 32.Frank, D. W., A. Vallis, J. Wiener-Kronish, A. Roy-Burman, E. Spack, B. Mullaney, M. Megdoud, J. Marks, R. Fritz, and T. Sawa. 2002. Generation and characterization of a protective monoclonal antibody to Pseudomonas aeruginosa PcrV. J. Infect. Dis. 186:64-73. [DOI] [PubMed] [Google Scholar]
- 33.Frankel, G., A. Phillips, L. Trabulsi, S. Knutton, G. Dougan, and S. Matthews. 2001. Intimin and the host cell—is it bound to end in Tir(s)? Trends Microbiol. 9:214-218. [DOI] [PubMed] [Google Scholar]
- 34.Fu, Y., and J. E. Galan. 1999. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401:293-297. [DOI] [PubMed] [Google Scholar]
- 35.Gal, D., M. Mayo, E. Spencer, A. Cheng, and B. Currie. 2005. Short report: application of a polymerase chain reaction to detect Burkholderia pseudomallei in clinical specimens from patients with suspected melioidosis. Am. J. Trop. Med. Hyg. 73:1162-1164. [PubMed] [Google Scholar]
- 36.Gallois, A., J. Klein, L. Allen, B. Jones, and W. Nauseef. 2001. Salmonella pathogenicity island 2-encoded type III secretion system mediates exclusion of NADPH oxidase assembly from the phagosomal membrane. J. Immunol. 166:5741-5748. [DOI] [PubMed] [Google Scholar]
- 37.Garmendia, J., A. Phillips, M. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 6:1167-1183. [DOI] [PubMed] [Google Scholar]
- 38.Gauthier, A., M. Robertson, M. Lowden, J. Ibarra, J. Puente, and B. Finlay. 2005. Transcriptional inhibitor of virulence factors in enteropathogenic Escherichia coli. Antimicrob. Agents Chemother. 49:4101-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh, P. 2004. Process of protein transport by the type III secretion system. Microbiol. Mol. Biol. Rev. 68:771-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomez, H. F., I. Herrera-Insua, M. M. Siddiqui, V. A. Diaz-Gonzalez, E. Caceres, D. S. Newburg, and T. G. Cleary. 2001. Protective role of human lactoferrin against invasion of Shigella flexneri M90T. Adv. Exp. Med. Biol. 501:457-467. [DOI] [PubMed] [Google Scholar]
- 42.Gomez, H. F., T. J. Ochoa, L. G. Carlin, and T. G. Cleary. 2003. Human lactoferrin impairs virulence of Shigella flexneri. J. Infect. Dis. 187:87-95. [DOI] [PubMed] [Google Scholar]
- 43.Goure, J., P. Broz, O. Attree, G. Cornelis, and I. Attree. 2005. Protective anti-V antibodies inhibit Pseudomonas and Yersinia translocon assembly within host membranes. J. Infect. Dis. 192:218-225. [DOI] [PubMed] [Google Scholar]
- 44.Gruenheid, S., and B. Finlay. 2003. Microbial pathogenesis and cytoskeletal function. Nature 422:775-781. [DOI] [PubMed] [Google Scholar]
- 45.Guttman, J. A., F. N. Samji, Y. Li, W. Deng, A. Lin, and B. B. Finlay. 2007. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell. Microbiol. 9:131-141. [DOI] [PubMed] [Google Scholar]
- 46.Guttman, J. A., Y. Li, M. Wickham, W. Deng, A. Vogl, and B. Finlay. 2006. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 8:634-645. [DOI] [PubMed] [Google Scholar]
- 47.Hapfelmeier, S., B. Stecher, M. Barthel, M. Kremer, A. J. Muller, M. Heikenwalder, T. Stallmach, M. Hensel, K. Pfeffer, S. Akira, and W. D. Hardt. 2005. The Salmonella pathogenicity island (SPI)-2 and SPI-1 type III secretion systems allow Salmonella serovar Typhimurium to trigger colitis via MyD88-dependent and MyD88-independent mechanisms. J. Immunol. 174:1675-1685. [DOI] [PubMed] [Google Scholar]
- 48.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 49.Hauser, A. R., E. Cobb, M. Bodi, D. Mariscal, J. Valles, J. N. Engel, and J. Rello. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit. Care Med. 30:521-528. [DOI] [PubMed] [Google Scholar]
- 50.Hauser, A. R., and S. Epelman. 1999. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect. Immun. 67:5530-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayward, R. D., and V. Koronakis. 1999. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 18:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heath, D. G., G. J. Anderson, J. Mauro, S. Welkos, G. Andrews, J. Adamovicz, and A. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16:1131-1137. [DOI] [PubMed] [Google Scholar]
- 53.Hensel, M., J. E. Shea, S. R. Waterman, R. Mundy, T. Nikolaus, G. Banks, A. Vazquez-Torres, C. Gleeson, F. C. Fang, and D. W. Holden. 1998. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol. Microbiol. 30:163-174. [DOI] [PubMed] [Google Scholar]
- 54.Hernandez, L. D., K. Hueffer, M. R. Wenk, and J. E. Galan. 2004. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science 304:1805-1807. [DOI] [PubMed] [Google Scholar]
- 55.Hernandez, L. D., M. Pypaert, R. Flavell, and J. Galan. 2003. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 163:1123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hersh, D., D. M. Monack, M. R. Smith, N. Ghori, S. Falkow, and A. Zychlinsky. 1999. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 96:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.High, N., J. Mounier, M. Prevost, and P. Sansonetti. 1992. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 11:1991-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilbi, H. 1999. Host responses to secreted Shigella virulence factors. Curr. Opin. Infect. Dis. 12:221-228. [DOI] [PubMed] [Google Scholar]
- 59.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 60.Hill, J., C. Copse, S. Leary, A. Stagg, E. Williamson, and R. Titball. 2003. Synergistic protection of mice against plague with monoclonal antibodies specific for the F1 and V antigens of Yersinia pestis. Infect. Immun. 71:2234-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill, J., S. Leary, K. Griffin, E. Williamson, and R. Titball. 1997. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 65:4476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hindle, Z., S. Chatfield, J. Phillimore, M. Bentley, J. Johnson, C. Cosgrove, M. Ghaem-Maghami, A. Sexton, M. Khan, F. Brennan, P. Everest, T. Wu, D. Pickard, D. Holden, G. Dougan, G. Griffin, D. House, J. Santangelo, S. Khan, J. Shea, R. Feldman, and D. Lewis. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect. Immun. 70:3457-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ivanoff, B., M. Levine, and P. Lambert. 1994. Vaccination against typhoid fever: present status. Bull. W. H. O. 72:957-971. [PMC free article] [PubMed] [Google Scholar]
- 64.Jepson, M. A., S. Pellegrin, L. Peto, D. Banbury, A. Leard, H. Mellor, and B. Kenny. 2003. Synergistic roles for the Map and Tir effector molecules in mediating uptake of enteropathogenic Escherichia coli (EPEC) into non-phagocytic cells. Cell. Microbiol. 5:773-783. [DOI] [PubMed] [Google Scholar]
- 65.Jiang, X., O. Rossanese, N. Brown, S. Kujat-Choy, J. Galan, B. Finlay, and J. Brumell. 2004. The related effector proteins SopD and SopD2 from Salmonella enterica serovar Typhimurium contribute to virulence during systemic infection of mice. Mol. Microbiol. 54:1186-1198. [DOI] [PubMed] [Google Scholar]
- 66.Karin, M., and Y. Ben Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 67.Katz, D. E., T. Coster, M. Wolf, F. Trespalacios, D. Cohen, G. Robins, A. Hartman, M. Venkatesan, D. Taylor, and T. Hale. 2004. Two studies evaluating the safety and immunogenicity of a live, attenuated Shigella flexneri 2a vaccine (SC602) and excretion of vaccine organisms in North American volunteers. Infect. Immun. 72:923-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kauppi, A. M., R. Nordfelth, H. Uvell, H. Wolf-Watz, and M. Elofsson. 2003. Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem. Biol. 10:241-249. [DOI] [PubMed] [Google Scholar]
- 69.Kenny, B., S. Ellis, A. Leard, J. Warawa, H. Mellor, and M. Jepson. 2002. Co-ordinate regulation of distinct host cell signalling pathways by multifunctional enteropathogenic Escherichia coli effector molecules. Mol. Microbiol. 44:1095-1107. [DOI] [PubMed] [Google Scholar]
- 70.Kerschen, E. J., D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 72:4589-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim, D. W., G. Lenzen, A. L. Page, P. Legrain, P. J. Sansonetti, and C. Parsot. 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 102:14046-14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirkpatrick, B., R. McKenzie, J. O'Neill, C. Larsson, A. Bourgeois, J. Shimko, M. Bentley, J. Makin, S. Chatfield, Z. Hindle, C. Fidler, B. Robinson, C. Ventrone, N. Bansal, C. Carpenter, D. Kutzko, S. Hamlet, C. LaPointe, and D. Taylor. 2006. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC−ssaV−) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine 24:116-123. [DOI] [PubMed] [Google Scholar]
- 73.Knodler, L. A., and O. Steele-Mortimer. 2005. The Salmonella effector PipB2 affects late endosome/lysosome distribution to mediate Sif extension. Mol. Biol. Cell 16:4108-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knodler, L., and O. Steele-Mortimer. 2003. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic 4:587-599. [DOI] [PubMed] [Google Scholar]
- 75.Knutton, S., D. Lloyd, and A. McNeish. 1987. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect. Immun. 55:69-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kotloff, K. L., F. Noriega, T. Samandari, M. Sztein, G. Losonsky, J. Nataro, W. Picking, E. Barry, and M. M. Levine. 2000. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect. Immun. 68:1034-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kotloff, K., F. Noriega, G. Losonsky, M. Sztein, S. Wasserman, J. Nataro, and M. Levine. 1996. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect. Immun. 64:4542-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kotloff, K., D. Taylor, M. Sztein, S. Wasserman, G. Losonsky, J. Nataro, M. Venkatesan, A. Hartman, W. Picking, D. Katz, J. Campbell, M. Levine, and T. Hale. 2002. Phase I evaluation of ΔvirG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect. Immun. 70:2016-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kotton, C., A. Lankowski, N. Scott, D. Sisul, L. Chen, K. Raschke, G. Borders, M. Boaz, A. Spentzou, J. Galan, and E. Hohmann. 2006. Safety and immunogenicity of attenuated Salmonella enterica serovar Typhimurium delivering an HIV-1 Gag antigen via the Salmonella type III secretion system. Vaccine 24:6216-6224. [DOI] [PubMed] [Google Scholar]
- 80.Kuhle, V., and M. Hensel. 2004. Cellular microbiology of intracellular Salmonella enterica: functions of the type III secretion system encoded by Salmonella pathogenicity island 2. Cell. Mol. Life Sci. 61:2812-2826. [DOI] [PubMed] [Google Scholar]
- 81.Kuhle, V., G. Abrahams, and M. Hensel. 2006. Intracellular Salmonella enterica redirect exocytic transport processes in a Salmonella pathogenicity island 2-dependent manner. Traffic 7:716-730. [DOI] [PubMed] [Google Scholar]
- 82.Kuhle, V., D. Jackel, and M. Hensel. 2004. Effector proteins encoded by Salmonella pathogenicity island 2 interfere with the microtubule cytoskeleton after translocation into host cells. Traffic 5:356-370. [DOI] [PubMed] [Google Scholar]
- 83.Kuhle, V., and M. Hensel. 2002. SseF and SseG are translocated effectors of the type III secretion system of Salmonella pathogenicity island 2 that modulate aggregation of endosomal compartments. Cell. Microbiol. 4:813-824. [DOI] [PubMed] [Google Scholar]
- 84.La Ragione, R., S. Patel, B. Maddison, M. Woodward, A. Best, G. Whitelam, and K. Gough. 2006. Recombinant anti-EspA antibodies block Escherichia coli O157:H7-induced attaching and effacing lesions in vitro. Microbes Infect. 8:426-433. [DOI] [PubMed] [Google Scholar]
- 85.Leary, S., K. Griffin, E. Galyov, J. Hewer, E. Williamson, A. Holmstrom, A. Forsberg, and R. Titball. 1999. Yersinia outer proteins (YOPS) E, K and N are antigenic but non-protective compared to V antigen, in a murine model of bubonic plague. Microb. Pathog. 26:159-169. [DOI] [PubMed] [Google Scholar]
- 86.Li, L., M. Ledizet, K. Kar, R. Koski, and B. Kazmierczak. 2005. An indirect enzyme-linked immunosorbent assay for rapid and quantitative assessment of type III virulence phenotypes of Pseudomonas aeruginosa isolates. Ann. Clin. Microbiol. Antimicrob. 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lugert, R., M. Kuhns, T. Polch, and U. Gross. 2004. Expression and localization of type III secretion-related proteins of Chlamydia pneumoniae. Med. Microbiol. Immunol. 193:163-171. [DOI] [PubMed] [Google Scholar]
- 88.Luperchio, S., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333-340. [DOI] [PubMed] [Google Scholar]
- 89.Lyons, S., L. Wang, J. E. Casanova, S. V. Sitaraman, D. Merlin, and A. T. Gewirtz. 2004. Salmonella typhimurium transcytoses flagellin via an SPI2-mediated vesicular transport pathway. J. Cell Sci. 117:5771-5780. [DOI] [PubMed] [Google Scholar]
- 90.Ma, C., M. Wickham, J. Guttman, W. Deng, J. Walker, K. Madsen, K. Jacobson, W. Vogl, B. Finlay, and B. Vallance. 2006. Citrobacter rodentium infection causes both mitochondrial dysfunction and intestinal epithelial barrier disruption in vivo: role of mitochondrial associated protein (Map). Cell. Microbiol. 8:1669-1686. [DOI] [PubMed] [Google Scholar]
- 91.Makino, K., K. Oshima, K. Kurokawa, K. Yokoyama, T. Uda, K. Tagomori, Y. Iijima, M. Najima, M. Nakano, A. Yamashita, Y. Kubota, S. Kimura, T. Yasunaga, T. Honda, H. Shinagawa, M. Hattori, and T. Iida. 2003. Genome sequence of Vibrio parahaemolyticus: a pathogenic mechanism distinct from that of V. cholerae. Lancet 361:743-749. [DOI] [PubMed] [Google Scholar]
- 92.Marcus, S., M. Wenk, O. Steele-Mortimer, and B. Finlay. 2001. A synaptojanin-homologous region of Salmonella typhimurium SigD is essential for inositol phosphatase activity and Akt activation. FEBS Lett. 494:201-207. [DOI] [PubMed] [Google Scholar]
- 93.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 309:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matson, J., K. Durick, D. Bradley, and M. Nilles. 2005. Immunization of mice with YscF provides protection from Yersinia pestis infections. BMC Microbiol. 5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McGhie, E., R. Hayward, and V. Koronakis. 2004. Control of actin turnover by a salmonella invasion protein. Mol. Cell 13:497-510. [DOI] [PubMed] [Google Scholar]
- 96.Menard, R., M. Prevost, P. Gounon, P. Sansonetti, and C. Dehio. 1996. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc. Natl. Acad. Sci. USA 93:1254-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meumann, E., R. Novak, D. Gal, M. Kaestli, M. Mayo, J. Hanson, E. Spencer, M. Glass, J. Gee, P. Wilkins, and B. Currie. 2006. Clinical evaluation of a type III secretion system real-time PCR assay for diagnosing melioidosis. J. Clin. Microbiol. 44:3028-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miao, E. A., C. M. Alpuche-Aranda, M. Dors, A. E. Clark, M. W. Bader, S. I. Miller, and A. Aderem. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat. Immunol. 7:569-575. [DOI] [PubMed] [Google Scholar]
- 99.Miura, M., J. Terajima, H. Izumiya, J. Mitobe, T. Komano, and H. Watanabe. 2006. OspE2 of Shigella sonnei is required for the maintenance of cell architecture of bacterium-infected cells. Infect. Immun. 74:2587-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monack, D. M., J. Mecsas, N. Ghori, and S. Falkow. 1997. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl. Acad. Sci. USA 94:10385-10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mueller, C., P. Broz, S. Muller, P. Ringler, F. Erne-Brand, I. Sorg, M. Kuhn, A. Engel, and G. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674-676. [DOI] [PubMed] [Google Scholar]
- 102.Mukherjee, S., G. Keitany, Y. Li, Y. Wang, H. Ball, E. Goldsmith, and K. Orth. 2006. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science 312:1211-1214. [DOI] [PubMed] [Google Scholar]
- 103.Muschiol, S., L. Bailey, A. Gylfe, C. Sundin, K. Hultenby, S. Bergstrom, M. Elofsson, H. Wolf-Watz, S. Normark, and B. Henriques-Normark. 2006. A small-molecule inhibitor of type III secretion inhibits different stages of the infectious cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 103:14566-14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neely, A., I. Holder, J. Wiener-Kronish, and T. Sawa. 2005. Passive anti-PcrV treatment protects burned mice against Pseudomonas aeruginosa challenge. Burns 31:153-158. [DOI] [PubMed] [Google Scholar]
- 105.Nishikawa, H., E. Sato, G. Briones, L. Chen, M. Matsuo, Y. Nagata, G. Ritter, E. Jager, H. Nomura, S. Kondo, I. Tawara, T. Kato, H. Shiku, L. Old, J. Galan, and S. Gnjatic. 2006. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J. Clin. Investig. 116:1946-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nordfelth, R., A. M. Kauppi, H. A. Norberg, H. Wolf-Watz, and M. Elofsson. 2005. Small-molecule inhibitors specifically targeting type III secretion. Infect. Immun. 73:3104-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oaks, E., and K. Turbyfill. 2006. Development and evaluation of a Shigella flexneri 2a and S. sonnei bivalent invasin complex (Invaplex) vaccine. Vaccine 24:2290-2301. [DOI] [PubMed] [Google Scholar]
- 108.Ogawa, M., T. Yoshimori, T. Suzuki, H. Sagara, N. Mizushima, and C. Sasakawa. 2005. Escape of intracellular Shigella from autophagy. Science 307:727-731. [DOI] [PubMed] [Google Scholar]
- 109.Ohya, K., Y. Handa, M. Ogawa, M. Suzuki, and C. Sasakawa. 2005. IpgB1 is a novel Shigella effector protein involved in bacterial invasion of host cells. Its activity to promote membrane ruffling via Rac1 and Cdc42 activation. J. Biol. Chem. 280:24022-24034. [DOI] [PubMed] [Google Scholar]
- 110.Ono, T., K. Park, M. Ueta, T. Iida, and T. Honda. 2006. Identification of proteins secreted via Vibrio parahaemolyticus type III secretion system 1. Infect. Immun. 74:1032-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Orr, N., D. Katz, J. Atsmon, P. Radu, M. Yavzori, T. Halperin, T. Sela, R. Kayouf, Z. Klein, R. Ambar, D. Cohen, M. Wolf, M. Venkatesan, and T. Hale. 2005. Community-based safety, immunogenicity, and transmissibility study of the Shigella sonnei WRSS1 vaccine in Israeli volunteers. Infect. Immun. 73:8027-8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ou, T., J. Liu, and H. Leu. 2003. Independent prognostic factors for fatality in patients with invasive Vibrio cholerae non-O1 infections. J. Microbiol. Immunol. Infect. 36:117-122. [PubMed] [Google Scholar]
- 113.Panthel, K., K. Meinel, L. Sevil, V. Domenech, G. Geginat, K. Linkemann, D. Busch, and H. Russmann. 2006. Prophylactic anti-tumor immunity against a murine fibrosarcoma triggered by the Salmonella type III secretion system. Microbes Infect. 8:2539-2546. [DOI] [PubMed] [Google Scholar]
- 114.Park, K., T. Ono, M. Rokuda, M. Jang, K. Okada, T. Iida, and T. Honda. 2004. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 72:6659-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Persson, C., R. Nordfelth, K. Andersson, A. Forsberg, H. Wolf-Watz, and M. Fallman. 1999. Localization of the Yersinia PTPase to focal complexes is an important virulence mechanism. Mol. Microbiol. 33:828-838. [DOI] [PubMed] [Google Scholar]
- 116.Phalipon, A., and P. J. Sansonetti. 2007. Shigella's ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol. Cell Biol. 85:119-129. [DOI] [PubMed] [Google Scholar]
- 117.Phillips, N., R. Hayward, and V. Koronakis. 2004. Phosphorylation of the enteropathogenic E. coli receptor by the Src-family kinase c-Fyn triggers actin pedestal formation. Nat. Cell Biol. 6:618-625. [DOI] [PubMed] [Google Scholar]
- 118.Potter, A., S. Klashinsky, Y. Li, E. Frey, H. Townsend, D. Rogan, G. Erickson, S. Hinkley, T. Klopfenstein, R. Moxley, D. Smith, and B. Finlay. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362-369. [DOI] [PubMed] [Google Scholar]
- 119.Power, M. R., Y. Peng, E. Maydanski, J. S. Marshall, and T. J. Lin. 2004. The development of early host response to Pseudomonas aeruginosa lung infection is critically dependent on myeloid differentiation factor 88 in mice. J. Biol. Chem. 279:49315-49322. [DOI] [PubMed] [Google Scholar]
- 120.Raffatellu, M., R. Wilson, D. Chessa, H. Andrews-Polymenis, Q. Tran, S. Lawhon, S. Khare, L. Adams, and A. Baumler. 2005. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect. Immun. 73:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ridley, A. J. 2006. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 16:522-529. [DOI] [PubMed] [Google Scholar]
- 122.Rocha, C., J. Coburn, E. Rucks, and J. Olson. 2003. Characterization of Pseudomonas aeruginosa exoenzyme S as a bifunctional enzyme in J774A.1 macrophages. Infect. Immun. 71:5296-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosenberger, C., and B. Finlay. 2003. Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat. Rev. Mol. Cell. Biol. 4:385-396. [DOI] [PubMed] [Google Scholar]
- 124.Rosenberger, C., and B. Finlay. 2004. Bacterial responses to neutrophil phagocytosis. Curr. Opin. Hematol. 11:1-6. [DOI] [PubMed] [Google Scholar]
- 125.Rosqvist, R., K. Magnusson, and H. Wolf-Watz. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roy-Burman, A., R. H. Savel, S. Racine, B. L. Swanson, N. S. Revadigar, J. Fujimoto, T. Sawa, D. W. Frank, and J. P. Wiener-Kronish. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J. Infect. Dis. 183:1767-1774. [DOI] [PubMed] [Google Scholar]
- 127.Ruckdeschel, K., O. Mannel, K. Richter, C. A. Jacobi, K. Trulzsch, B. Rouot, and J. Heesemann. 2001. Yersinia outer protein P of Yersinia enterocolitica simultaneously blocks the nuclear factor-kappa B pathway and exploits lipopolysaccharide signaling to trigger apoptosis in macrophages. J. Immunol. 166:1823-1831. [DOI] [PubMed] [Google Scholar]
- 128.Russmann, H., and K. Panthel. 2004. “One size fits it all”: translocation of foreign antigens by Yersinia type III secretion system (TTSS) leads to concomitant CD4 and CD8 T-cell priming. Int. J. Med. Microbiol. 294:313-317. [DOI] [PubMed] [Google Scholar]
- 129.Russmann, H. 2003. Bacterial type III translocation: a unique mechanism for cytosolic display of heterologous antigens by attenuated Salmonella. Int. J. Med. Microbiol. 293:107-112. [DOI] [PubMed] [Google Scholar]
- 130.Sambri, V., M. Donati, E. Storni, K. Di Leo, M. Agnusdei, R. Petracca, O. Finco, G. Grandi, G. Ratti, and R. Cevenini. 2004. Experimental infection by Chlamydia pneumoniae in the hamster. Vaccine 22:1131-1137. [DOI] [PubMed] [Google Scholar]
- 131.Sani, M., A. Botteaux, C. Parsot, P. Sansonetti, E. Boekema, and A. Allaoui. 2007. IpaD is localized at the tip of the Shigella flexneri type III secretion apparatus. Biochim. Biophys. Acta 1770:307-311. [DOI] [PubMed] [Google Scholar]
- 132.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 133.Sato, H., D. W. Frank, C. J. Hillard, J. B. Feix, R. R. Pankhaniya, K. Moriyama, V. Finck-Barbancon, A. Buchaklian, M. Lei, R. M. Long, J. Wiener-Kronish, and T. Sawa. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schlumberger, M., and W. Hardt. 2006. Salmonella type III secretion effectors: pulling the host cell's strings. Curr. Opin. Microbiol. 9:46-54. [DOI] [PubMed] [Google Scholar]
- 135.Schotte, P., G. Denecker, B. A. Van Den, P. Vandenabeele, G. R. Cornelis, and R. Beyaert. 2004. Targeting Rac1 by the Yersinia effector protein YopE inhibits caspase-1-mediated maturation and release of interleukin-1beta. J. Biol. Chem. 279:25134-25142. [DOI] [PubMed] [Google Scholar]
- 136.Shao, F., P. Merritt, Z. Bao, R. Innes, and J. Dixon. 2002. A Yersinia effector and a Pseudomonas avirulence protein define a family of cysteine proteases functioning in bacterial pathogenesis. Cell 109:575-588. [DOI] [PubMed] [Google Scholar]
- 137.Shea, J. E., M. Hensel, C. Gleeson, and D. W. Holden. 1996. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 93:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shime, N., T. Sawa, J. Fujimoto, K. Faure, L. Allmond, T. Karaca, B. Swanson, E. Spack, and J. Wiener-Kronish. 2001. Therapeutic administration of anti-PcrV F(ab′)(2) in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 167:5880-5886. [DOI] [PubMed] [Google Scholar]
- 139.Sing, A., D. Reithmeier-Rost, K. Granfors, J. Hill, A. Roggenkamp, and J. Heesemann. 2005. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. USA 102:16049-16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Suzuki, T., Y. Yoshikawa, H. Ashida, H. Iwai, T. Toyotome, H. Matsui, and C. Sasakawa. 2006. High vaccine efficacy against shigellosis of recombinant noninvasive Shigella mutant that expresses Yersinia invasin. J. Immunol. 177:4709-4717. [DOI] [PubMed] [Google Scholar]
- 141.Swanson, M. 2006. Autophagy: eating for good health. J. Immunol. 177:4945-4951. [DOI] [PubMed] [Google Scholar]
- 142.Swietnicki, W., B. Powell, and J. Goodin. 2005. Yersinia pestis Yop secretion protein F: purification, characterization, and protective efficacy against bubonic plague. Protein Expr. Purif. 42:166-172. [DOI] [PubMed] [Google Scholar]
- 143.Tafazoli, F., A. Holmstrom, A. Forsberg, and K. Magnusson. 2000. Apically exposed, tight junction-associated beta1-integrins allow binding and YopE-mediated perturbation of epithelial barriers by wild-type Yersinia bacteria. Infect. Immun. 68:5335-5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tammiruusu, A., T. Penttila, R. Lahesmaa, M. Sarvas, M. Puolakkainen, and J. Vuola. 2007. Intranasal administration of chlamydial outer protein N (CopN) induces protection against pulmonary Chlamydia pneumoniae infection in a mouse model. Vaccine 25:283-290. [DOI] [PubMed] [Google Scholar]
- 145.Terebiznik, M., O. Vieira, S. Marcus, A. Slade, C. Yip, W. Trimble, T. Meyer, B. Finlay, and S. Grinstein. 2002. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat. Cell Biol. 4:766-773. [DOI] [PubMed] [Google Scholar]
- 146.Thorpe, C., L. Edwards, R. Snelgrove, O. Finco, A. Rae, G. Grandi, R. Guilio, and T. Hussell. 2007. Discovery of a vaccine antigen that protects mice from Chlamydia pneumoniae infection. Vaccine 25:2252-2260. [DOI] [PubMed] [Google Scholar]
- 147.Tomson, F., V. Viswanathan, K. Kanack, R. Kanteti, K. Straub, M. Menet, J. Kaper, and G. Hecht. 2005. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol. Microbiol. 56:447-464. [DOI] [PubMed] [Google Scholar]
- 148.Tran Van Nhieu, G., E. Caron, A. Hall, and P. Sansonetti. 1999. IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J. 18:3249-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Troisfontaines, P., and G. R. Cornelis. 2005. Type III secretion: more systems than you think. Physiology (Bethesda) 20:326-339. [DOI] [PubMed] [Google Scholar]
- 150.Trulzsch, K., T. Sporleder, E. I. Igwe, H. Russmann, and J. Heesemann. 2004. Contribution of the major secreted Yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infect. Immun. 72:5227-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsukita, S., M. Furuse, and M. Itoh. 1999. Structural and signalling molecules come together at tight junctions. Curr. Opin. Cell Biol. 11:628-633. [DOI] [PubMed] [Google Scholar]
- 152.Tsukita, S., M. Furuse, and M. Itoh. 2001. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell. Biol. 2:285-293. [DOI] [PubMed] [Google Scholar]
- 153.Tu, X., I. Nisan, C. Yona, E. Hanski, and I. Rosenshine. 2003. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol. Microbiol. 47:595-606. [DOI] [PubMed] [Google Scholar]
- 154.Turbyfill, K., A. Hartman, and E. Oaks. 2000. Isolation and characterization of a Shigella flexneri invasin complex subunit vaccine. Infect. Immun. 68:6624-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van Donkersgoed, J., D. Hancock, D. Rogan, and A. Potter. 2005. Escherichia coli O157:H7 vaccine field trial in 9 feedlots in Alberta and Saskatchewan. Can. Vet. J. 46:724-728. [PMC free article] [PubMed] [Google Scholar]
- 156.Vazquez-Torres, A., Y. Xu, J. Jones-Carson, D. Holden, S. Lucia, M. Dinauer, P. Mastroeni, and F. Fang. 2000. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science 287:1655-1658. [DOI] [PubMed] [Google Scholar]
- 157.Viboud, G., and J. Bliska. 2005. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 59:69-89. [DOI] [PubMed] [Google Scholar]
- 158.Viswanathan, V., A. Koutsouris, S. Lukic, M. Pilkinton, I. Simonovic, M. Simonovic, and G. Hecht. 2004. Comparative analysis of EspF from enteropathogenic and enterohemorrhagic Escherichia coli in alteration of epithelial barrier function. Infect. Immun. 72:3218-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wickham, M., C. Lupp, A. Vazquez, M. Mascarenhas, B. Coburn, B. Coombes, M. Karmali, J. Puente, W. Deng, and B. B. Finlay. 2007. Citrobacter rodentium virulence in mice associates with bacterial load and the type III effector NleE. Microbes Infect. 9:400-407. [DOI] [PubMed] [Google Scholar]
- 160.Worley, M., G. Nieman, K. Geddes, and F. Heffron. 2006. Salmonella typhimurium disseminates within its host by manipulating the motility of infected cells. Proc. Natl. Acad. Sci. USA 103:17915-17920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang, X., X. Qu, K. Wang, J. Zheng, L. Si, X. Dong, and Y. Wang. 2005. Construction of prophylactic human papillomavirus type 16 L1 capsid protein vaccine delivered by live attenuated Shigella flexneri strain sh42. Acta Biochim. Biophys. Sin. 37:743-750. [DOI] [PubMed] [Google Scholar]
- 162.Yoshida, S., Y. Handa, T. Suzuki, M. Ogawa, M. Suzuki, A. Tamai, A. Abe, E. Katayama, and C. Sasakawa. 2006. Microtubule-severing activity of Shigella is pivotal for intercellular spreading. Science 314:985-989. [DOI] [PubMed] [Google Scholar]
- 163.Yoshida, S., E. Katayama, A. Kuwae, H. Mimuro, T. Suzuki, and C. Sasakawa. 2002. Shigella deliver an effector protein to trigger host microtubule destabilization, which promotes Rac1 activity and efficient bacterial internalization. EMBO J. 21:2923-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yu, X., J. Ruiz-Albert, K. Unsworth, S. Garvis, M. Liu, and D. Holden. 2002. SpiC is required for secretion of Salmonella pathogenicity island 2 type III secretion system proteins. Cell. Microbiol. 4:531-549. [DOI] [PubMed] [Google Scholar]
- 165.Yu, Y., H. Zeng, S. Lyons, A. Carlson, D. Merlin, A. S. Neish, and A. T. Gewirtz. 2003. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 285:G282-G290. [DOI] [PubMed] [Google Scholar]
- 166.Zeng, H., H. Wu, V. Sloane, R. Jones, Y. Yu, P. Lin, A. T. Gewirtz, and A. S. Neish. 2006. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G96-G108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang, S., R. Santos, R. Tsolis, S. Stender, W. Hardt, A. Baumler, and L. Adams. 2002. The Salmonella enterica serotype Typhimurium effector proteins SipA, SopA, SopB, SopD, and SopE2 act in concert to induce diarrhea in calves. Infect. Immun. 70:3843-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou, D., and J. Galan. 2001. Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect. 3:1293-1298. [DOI] [PubMed] [Google Scholar]
- 169.Zhou, D., M. Mooseker, and J. Galán. 1999. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science 283:2092-2095. [DOI] [PubMed] [Google Scholar]
- 170.Zychlinsky, A., C. Fitting, J. M. Cavaillon, and P. J. Sansonetti. 1994. Interleukin 1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J. Clin. Investig. 94:1328-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zychlinsky, A., M. C. Prevost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-169. [DOI] [PubMed] [Google Scholar]