Abstract
Fusarium species cause a broad spectrum of infections in humans, including superficial, locally invasive, and disseminated infections. The clinical form of fusariosis depends largely on the immune status of the host and the portal of entry, with superficial and localized disease occurring mostly in immunocompetent patients and invasive and disseminated disease affecting immunocompromised patients. Risk factors for severe fusariosis include prolonged neutropenia and T-cell immunodeficiency, especially in hematopoietic stem cell transplant recipients with severe graft-versus-host disease. The most frequent presentation of disseminated fusariosis is a combination of characteristic cutaneous lesions and positive blood cultures, with or without lung or sinus involvement. The prognosis is poor and is determined largely by degree of immunosuppression and extent of infection, with virtually a 100% death rate among persistently neutropenic patients with disseminated disease. These infections may be clinically suspected on the basis of a constellation of clinical and laboratory findings, which should lead to prompt therapy. Treatment options include the lipid formulations of amphotericin B, voriconazole, and posaconazole. Prevention of fusarial infection among high-risk patients should be considered.
INTRODUCTION
Fusarium species are important plant pathogens causing various diseases such as crown rot, head blight, and scab on cereal grains (72), and they may occasionally cause infection in animals (32). In humans, Fusarium species cause a broad spectrum of infections, including superficial (such as keratitis and onychomycosis), locally invasive, or disseminated infections, with the last occurring almost exclusively in severely immunocompromised patients (74). Fusarium species may also cause allergic diseases (sinusitis) in immunocompetent individuals (111) and mycotoxicosis in humans and animals following ingestion of food contaminated by toxin-producing Fusarium spp. (72).
Fusarium species are widely distributed in soil, subterranean and aerial plant parts, plant debris, and other organic substrates (72) and are present in water worldwide as part of water structure biofilms (28). The widespread distribution of Fusarium species may be attributed to their ability to grow on a wide range of substrates and their efficient mechanisms for dispersal (14).
More than 50 species of Fusarium have been identified, including plant and animal pathogens, but a few cause human infections. In a literature review of 259 cases of fusariosis (excluding cases of keratitis and onyclomycosis) (74), updated with 35 additional cases published between 2001 and 2005 (4, 5, 7, 9, 18, 19, 24, 25, 36, 37, 41-43, 48, 54, 58, 65, 68, 69, 71, 82, 87, 91, 93, 95, 101, 102, 106, 107, 110, 113), the infecting species was found to have been reported in 124 cases. Twelve species were associated with infection; Fusarium solani was the most frequent (∼50% of cases), followed by Fusarium oxysporum (∼20%) and Fusarium verticillioidis and Fusarium moniliforme (∼10% each). Other infecting species included Fusarium dimerum, Fusarium proliferatum, Fusarium chlamidosporum, Fusarium sacchari, Fusarium nygamai, Fusarium napiforme, Fusarium antophilum, and Fusarium vasinfectum. Fusarium solani is also the most frequent pathogen in fusarial keratitis (23) and, with F. oxysporum, accounts for most cases of onychomycosis caused by Fusarium species (12, 39, 73).
PATHOGENESIS
Host Defenses
Although little information is available regarding host defenses against Fusarium species, invasive fusariosis shares many features with invasive aspergillosis and other invasive mold infections, including its occurrence in patients receiving high doses of corticosteroids and those with prolonged and profound neutropenia. The importance of immunity in the pathogenesis of fusariosis is supported by in vitro and in vivo experimental studies (38, 57, 94, 112), the unique susceptibility of severely immunocompromised patients to disseminated fusariosis (11), and the strong correlation between immune reconstitution and outcome (75).
The innate immunity plays a major role in the defense against mold infections (100). Macrophages and neutrophils damage fusarial hyphae, and their effect is primed by gamma interferon, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) (38), and interleukin-15 (112). The effect of interleukin-15 is mediated by the release of interleukin-8 and by direct stimulation of hyphal damage. More recently, the role of Toll-like receptors in the innate immune recognition of fungi has been recognized (94), and although little is known about fusariosis and Toll-like receptors, this system is likely important in invasive fusariosis as well.
Animal models of fusariosis have been developed to study the pathogenicity of Fusarium species. Immunocompetent and neutropenic mice were challenged with F. solani conidia. Mortality correlated with inoculum size. In nonneutropenic mice the infection was characterized by necrotizing abscesses with hyphae, hemorrhage, and neutrophil and macrophage infiltration. By contrast, neutropenic mice did not exhibit an inflammatory cellular reaction and had a significantly higher fungal burden (57).
The importance of T-cell defenses against Fusarium is illustrated by the occurrence of disseminated fusariosis in nonneutropenic hematopoietic stem cell transplant (HSCT) recipients (76). These patients have severe T-cell immunodeficiency caused by multiple therapies for their underlying disease and for graft-versus-host disease (GvHD). Further supporting the importance of T-cell immunity and phagocytes is the major impact of corticosteroid therapy on the outcome of fusariosis, as shown by the much higher death rate among recipients of such therapy than among patients who were not receiving corticosteroids (75).
Virulence Factors
Fusarium species possess several virulence factors, including the ability to produce mycotoxins, including trichothecenes, which suppress humoral and cellular immunity and may also cause tissue breakdown (72). In addition, Fusarium species have the ability to adhere to prosthetic material and to produce proteases and collagenases (55).
Fusarium solani is the most virulent species, as shown in a murine model of fusariosis in immunocompetent animals. In that study, 13 isolates belonging to four Fusarium species were injected intravenously into immunocompetent mice. Five F. solani strains caused death in all animals tested, as opposed to 100% survival of animals infected with F. oxysporum, F. verticillioides, or F. proliferatum (66).
Genetic virulence determinants of Fusarium oxysporum have been recently studied in immunosuppressed mice. Animals were inoculated with microconidia of a well-characterized tomato-pathogenic isolate (wild type), which resulted in disseminated infection and death. Inoculation of mutants with knockout mutations in genes encoding three known virulence factors for tomato plants (a mitogen-activated protein kinase, a pH response transcription factor, or a class V chitin synthase) led to discrepant results regarding pathogenicity for plants and animals. The mitogen-activated protein kinase gene, which is essential for virulence in fungal plant pathogens, was not necessary for virulence of F. oxysporum in this model. Conversely, the pH response transcription factor was required for animal virulence but not for plant virulence. Most mice infected with the chitin synthase knockout mutant isolates died within 24 h, as opposed to 5- to 12-day survival with the wild-type strain. Postmortem studies suggested that these animals died of respiratory insufficiency, probably as a result of severe lung damage, rather than the usual pattern of more generalized lesions seen in the other experiments. This unusual fast-killing effect was thought to be due to the presence of numerous large (30- by 25-μm) lemon-shaped or irregularly swollen mutant conidia, causing physical obstruction to lung interstitial capillaries. These morphological alterations in conidia of the chitin synthase knockout mutants are caused by defects in cell wall integrity (79).
EPIDEMIOLOGY AND CLINICAL SPECTRUM OF FUSARIOSIS
Fusarium species cause a broad spectrum of infections in humans, including superficial, locally invasive, and disseminated infections. The clinical form of fusariosis depends largely on the immune status of the host and the portal of entry of the infection.
Among immunocompetent hosts, keratitis and onychomycosis are the most common infections. Less frequently, the infection may occur as a result of skin breakdown, such as burns and wounds (74), or the presence of foreign bodies, such as keratitis in contact lens wearers (23), which at times causes outbreaks of fusarial keratitis (16). Peritonitis in patients receiving continuous ambulatory peritoneal dialysis has also been described (34, 52, 92). Other infections in immunocompetent patients include sinusitis (56), pneumonia (62, 97), thrombophlebitis (69), fungemia with or without organ involvement (74, 104), endophtalmitis (35, 86), septic arthritis (51), and osteomyelitis (10). Two recent outbreaks of fusarial keratitis were recently described in the United States (164 cases) and Singapore (66 cases). Case-control studies in the two populations of patients showed that keratitis was more likely to occur in patients who used a specific contact lens solution (ReNu with MoistureLock) (17, 98).
Immunocompromised patients at high risk for fusariosis are those with prolonged and profound neutropenia and/or severe T-cell immunodeficiency (11). Unlike infection in the normal host, fusariosis in the immunocompromised population is typically invasive and disseminated (74). In a study of 84 patients with hematologic diseases, the infection occurred more frequently in patients with acute leukemia (56%), and most patients (83%) were neutropenic at diagnosis (75). In the allogeneic HSCT population, the infection has a trimodal distribution, with a first peak in the early posttransplant period (during neutropenia), a second peak at a median of 70 days after transplant among patients with acute GvHD receiving corticosteroids, and a third peak >1 year after transplant during treatment for chronic extensive GvHD. Severe T-cell immunodeficiency and not neutropenia is the major risk factor for fusariosis in these patients (76). The overall incidence of fusariosis is ∼6 cases per 1,000 HSCTs; the incidence is lowest (∼1.5 to 2/1,000) among autologous recipients, intermediate (∼2.5 to 5/1,000) in matched related and matched unrelated allogeneic recipients, and highest (20/1,000) among recipients of mismatched related donor allogeneic HSCTs (76). Locally invasive and usually late infections may also develop among solid organ transplant recipients (96), but these appear to be less common than those among HSCT patients.
The principal portal of entry for Fusarium spp. is the airways, followed by the skin at site of tissue breakdown and possibly the mucosal membranes.
Airborne fusariosis is thought to be acquired by the inhalation of airborne fusarial conidia, as suggested by the occurrence of sinusitis and or pneumonia in absence of dissemination. The role of skin as a portal of entry is supported by the development of infection following skin breakdowns due to trauma (automobile accidents, bamboo), burns or onychomycosis in normal hosts (74), and the development of cellulitis (typically at sites of tissue breakdown such as toes and fingers), which may remain localized or lead to disseminated infection in immunocompromised patients (11, 75).
Given the ubiquity of Fusarium species in the environment, fusariosis may potentially be acquired in the community, as suggested by the presence of airborne fusarial conidia in outdoor air samples (2, 11, 89). In a prospective study, Fusarium species were recovered from a hospital water system (water, water storage tanks, shower and sink drains, shower heads, and sink faucet aerators) and from hospital air and other environments (2). Fusarium species were also present in the outdoor air. Showering and other water-related activities appeared to be an efficient mechanism for the dispersion of airborne fusarial conidia and transmission to the immunocompromised host, as shown by the close molecular relatedness between water and patient isolates. The genetic diversity of patient and environmental isolates of Fusarium oxysporum recovered from three locations in the United States was recently studied. The results indicated that a geographically widespread clonal lineage was responsible for >70% of all clinical isolates, and strains of this clonal lineage were genetically similar to those isolated from the water systems of three U.S. hospitals (78), further supporting the risk of nosocomial waterborne fusariosis.
CLINICAL MANIFESTATIONS OF FUSARIOSIS IN IMMUNOCOMPROMISED PATIENTS
The clinical manifestations of fusariosis depend on the portal of entry of the infection and the intensity and duration of immunosuppression. Disseminated infections typically occur among the severely immunocompromised patients, as opposed to more localized infection when immune function is somewhat preserved.
Endophtalmitis
While fusarial endophtalmitis in immunocompetent individuals usually occurs as a complication of advanced keratitis (26) or ocular surgery, such as cataract extraction (33), fusarial endophtalmitis in the immunocompromised host more commonly results from hematogenous seeding in the setting of disseminated infection (91, 106).
Sinusitis
In the immunocompetent host, Fusarium spp. may cause allergic sinusitis (111) or chronic noninvasive or invasive sinusitis (56, 103). By contrast, sinusitis is always invasive in immunocompromised hosts (60, 99, 108). Fifty-four of 294 reported cases of fusariosis (18%) had sinus involvement, more commonly among patients with acute leukemia and prolonged and profound neutropenia (52/54) and in the context of disseminated fusariosis, suggesting that sinuses may serve as site for dissemination (75).
The clinical manifestations of fusarial sinusitis are indistinguishable from those caused by Aspergillus spp.: nasal discharge and obstruction. Necrosis of the mucosa is a hallmark and is a consequence of the angioinvasive nature of these mycoses. Periorbital and paranasal cellulitis may be present (75).
Pneumonia
Lung involvement is common in invasive fusariosis (114 of 294 cases [39%]) and almost always occurs among immunocompromised (109/114) patients with disseminated infection. Isolated fusarial pneumonia was reported in 14 patients (11 immunocompromised), usually manifesting as nodular and cavitary lesions. As expected, lung involvement is associated with higher mortality, even after controlling for immune status.
In a series of 84 patients with fusariosis and an underlying hematologic disease, lung infiltrates (proven or presumed to be due to fusariosis) were present in 54% of patients and, like in aspergillosis, consisted of nonspecific alveolar or interstitial infiltrates, nodules, and cavities. The clinical presentation was nonspecific, with some presenting with a clinical picture similar to invasive aspergillosis, with dry cough, pleuritic chest pain, and shortness of breath (75).
Skin Involvement
Skin involvement in fusariosis can represent a primary site of infection, usually a cellulitis of the toes, or a manifestation of metastatic infection in patients with disseminated fusariosis. Skin involvement in fusariosis was present in 181 patients (70%) among 259 published cases of fusariosis (232 immunocompromised and 27 immunocompetent) (74).
Among immunocompetent hosts, lesions are usually localized (13 of 14 patients) and occur after skin breakdown (trauma or preexisting onychomycosis). Three patients presented with ulcerated lesions resembling chromoblastomycosis. The single case of disseminated metastatic skin lesion occurred in a child with no apparent underlying disease who developed fever, pulmonary infiltrates, multiple erythematous papules and nodules, and several blood cultures yielding Fusarium sp. Two cases of mycetoma caused by Fusarium spp. have been recently reported (107, 113).
Among immunocompromised patients, skin lesions may also be localized, usually as a result of skin breakdown caused by trauma, or may lead to disseminated infection. Among 16 patients with metastatic skin lesions, a recent history of cellulitis at the site of onychomycosis (11 patients), local trauma (3 patients), or an insect bite (2 patients) was reported (74). Patients with disseminated disease typically have multiple erythematous papular or nodular and painful lesions, frequently with central necrosis giving the lesions an echthyma gangrenosum-like appearance. Target lesions (a thin rim of erythema of 1 to 3 cm in diameter surrounding the above-mentioned papular or nodular lesions) may be present in approximately 10% of patients, while bullae develop rarely. Fusarial skin lesions can involve practically any site, with a predominance in the extremities, and evolve rapidly, usually over a few days. Lesions at different stages of evolution (papules, nodules, and necrotic lesions) may be present in a third of patients, and concomitant myalgias (suggesting muscle involvement) were described in 15%. Skin lesions were the single source of diagnosis in the majority of patients with such lesions (100/181 [55%]).
Fungemia
A striking characteristic of fusariosis, as opposed to aspergillosis, is the high frequency of positive blood cultures, mostly in the context of disseminated disease. Among 294 reported cases, blood cultures yielded the organism in 119 (41%). Occasionally fungemia is the only manifestation of fusariosis, usually in absence of neutropenia, among patients with central venous catheters. Antifungal treatment and catheter removal result in cure in most such cases (1, 15, 27, 53, 70, 88, 109).
Disseminated Infection
Disseminated disease is the most frequent and challenging clinical form of fusariosis in immunocompromised patients, accounting for approximately 70% of all cases of fusariosis in this population. Patients at risk for disseminated fusariosis include those with acute leukemia and prolonged and profound neutropenia and patients undergoing HSCT.
The most frequent pattern of disseminated disease is a combination of cutaneous lesions and positive blood cultures, with or without involvement at other sites (sinuses, lungs, and others). The typical clinical presentation is that of a patient with prolonged (>10 days) and profound (<100/mm3) neutropenia who is persistently febrile and develops disseminated and characteristic skin lesions, with a positive blood culture for a mold (sometimes with adventitious sporulation reported as yeasts). As expected, the death rate is higher among patients with disseminated disease (141/188 [75%] versus 38/106 [36%]; P < 0.001), and this association remained statistically significant even after controlling for the presence of neutropenia, HSCT, underlying disease, and organ involvement.
DIAGNOSIS
The diagnosis of fusariosis depends on the clinical form of the disease. The clinical picture is not of help in the diagnosis of keratitis, since the clinical manifestations are similar regardless of etiology (bacteria or fungi). Culture of corneal scrapings (most frequent) or tissue biopsy is usually required for a definitive diagnosis.
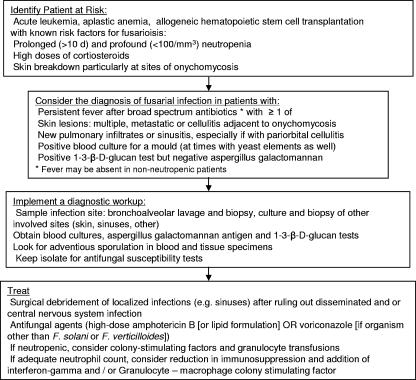
Two characteristics suggest the diagnosis of disseminated fusariosis in the severely immunocompromised host: skin lesions (either cellulitis or metastatic lesions) and positive blood cultures for mold (Fig. 1). Unlike in aspergillosis, blood cultures are frequently positive in fusariosis. This is possibly due to the fact that Fusarium species produce yeast-like structures (adventitious sporulation) that facilitate their dissemination and growth in the blood (59).
FIG. 1.
Algorithm for the diagnosis and management of fusariosis in immunocompromised patients.
While there are no specific recommendations for the collection and processing of blood cultures for the diagnosis of fusariosis, a study compared the performances of a specific fungal medium and a standard aerobic medium from Bactec. When the inoculum was low (102 and 103 CFU/ml), fungal growth was detected earlier in the fungal medium, with a mean difference of 10 h for Fusarium dimerum, 14 h for Fusarium solani, and 35 h for Fusarium verticillioides. These data suggest that fungal medium should be preferably used in patients with suspected invasive fusariosis (47).
The interpretation of the growth of Fusarium species from different biological materials depends on the clinical context. The clinician and the microbiologist must be cautious, because Fusarium species may contaminate laboratory specimens and pseudo-outbreaks of fusariosis may occur (40). In support of infection is the isolation of several colonies from the same specimen or of the same fungus from different specimens (as opposed to isolating a single colony from only one biological sample), a positive direct examination of the biological material, and, most importantly, the site of isolation and the host. For example, culture of sinus aspirate or respiratory secretions in severely immunocompromised hosts should always be considered as diagnostic of fusarial infection, as opposed to isolating Fusarium spp. from skin scrapings in an immunocompetent host.
Confirmatory diagnosis of fusariosis may require histopathology. In tissue, the hyphae are similar to those of Aspergillus species, with hyaline and septate filaments that typically dichotomize in acute and right angles. However, adventitious sporulation may be present in tissue, and the finding of hyphae and yeast-like structures together is highly suggestive of fusariosis in the high-risk population. In the absence of microbial growth, distinguishing fusariosis from other hyalohyphomycoses may be difficult and requires the use of in situ hybridization in paraffin-embedded tissue specimens (44).
Fusarium species grow easily and rapidly in most media without cycloheximide. Although the genus Fusarium can be identified by the production of hyaline, banana-shaped, multicellular macroconidia with a foot cell at the base, species identification is difficult and may require molecular methods. Recently, a commercially available PCR-based method was tested with 21 clinical isolates of Fusarium species and 5 ATCC isolates. Using sequencing identification as a gold standard, seven of nine different species were identified (45).
The 1,3-β-d-glucan test is usually positive in invasive fusarial infections but cannot distinguish Fusarium from other fungal infections (Candida, Aspergillus, Trichosporon, and others) which are also detected by the assay (77, 80). However, a positive 1,3-β-d-glucan test and a negative galactomannan test in a high-risk patient with mold infection is highly suggestive of fusariosis.
A PCR technique was developed for the detection of Fusarium species in blood and tissues. Two primers were developed and tested in a mouse model of disseminated fusariosis, as well as in human blood inoculated with fusarial mycelia. The primers were highly specific for 11 medically important Fusarium species, and the method was able to detect Fusarium species in all blood samples. In the mouse model, four immunosuppressed mice were inoculated intravenously with conidia of Fusarium solani. Cultures were positive for 15 of 27 tissue samples (including blood). The correlation between PCR and cultures of tissues was only 46%; 18% were culture positive and PCR negative, and 11 were culture negative and PCR positive. The authors attributed these discrepancies to poor efficiency of the extraction protocol, reducing the sensitivity of the PCR, and the presence of necrotic abscesses, rendering cultures negative (50).
PROGNOSIS AND TREATMENT
Prognosis
The prognosis of fusariosis in the immunocompromised host is directly related to the immune status of the patient, with high death rates in patients with persistent immunodeficiency. An analysis of 84 patients with hematologic diseases revealed survival rates at 30 and 90 days after diagnosis of 50% and 21%, respectively (75). Multivariate predictors of poor outcome were persistent neutropenia (hazard ratio, 5.43; 95% confidence interval, 2.64 to 11.11) and recent corticosteroid therapy (hazard ratio, 2.18; 95% confidence interval, 1.98 to 3.96). The actuarial survival of patients was 0% for patients with both unfavorable prognostic factors and 4% for those with persistent neutropenia only. By contrast, patients who had no risk factors or whose only risk factor was corticosteroid therapy had a 67% and 30% survival rates, respectively (P < 0.0001). Among HSCT recipients, 90-day survival after diagnosis was only 13%, and the single predictor of poor outcome was persistent neutropenia (hazard ratio, 12.05; 95% confidence interval, 1.46 to 100) (76). A separate analysis of 294 reported cases of fusariosis indicated that receipt of HSCT and presence of neutropenia, disseminated disease, and lung involvement predicted death (74).
Treatment
In general, patients with localized infection are likely to benefit from surgical debridement, while disseminated infection requires the use of systemic agents and immunotherapy, when possible. Table 1 summarizes the therapeutic strategies in invasive fusariosis.
TABLE 1.
Summary of recommendations for the management of invasive fusariosis
| Strategy | Recommendation(s) |
|---|---|
| Antifungal agents | F. solani and F. verticillioides, high-dose amphotericin B; other Fusarium species, high-dose amphotericin B or voriconazole; perform susceptibility testing |
| Immunotherapy | Growth factors (G-CSF or GM-CSF) or granulocyte transfusions for neutropenic patients; gamma interferon and/or GM-CSF for patients with adequate neutrophil counts |
| Surgery | Debride necrotic tissue |
| Catheter management | Remove central venous catheter if isolated fungemia |
Localized infection.
Keratitis is usually treated with topical antifungal agents, and natamycin is the drug of choice (23). More recently, successful treatment with topical and oral voriconazole has been reported (13). Localized skin lesions in immunocompromised patients deserve special attention. Since the skin may be the source for disseminated and frequently life-threatening fusarial infections, local debridement should be performed and topical antifungal agents (natamycin or amphotericin B) should be used, prior to commencing immunosuppressive therapies.
Invasive and disseminated infection. (i) Antifungal susceptibility.
The typical antifungal susceptibility profile of Fusarium spp. is that of relative resistance to most antifungal agents (Table 2). However, different species may have different patterns of susceptibility. Fusarium solani and Fusarium verticillioides are usually resistant to azoles and exhibit higher amphotericin B MICs than other Fusarium spp. By contrast, Fusarium oxysporum and Fusarium moniliforme may be susceptible to voriconazole and posaconazole (6, 20, 21, 29, 31, 67, 81, 85, 105). The relevance of these in vitro data is not clear, because there are not enough data documenting a correlation between MICs and the clinical outcome.
TABLE 2.
Antifungal susceptibility of Fusarium species
| Species | Reference | No. of isolates | MIC (μg/ml)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amphotericin B
|
Itraconazole
|
Voriconazole
|
Posaconazole
|
|||||||||||
| Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | |||
| Fusarium solani | 21 | 24 | NRa | 1.0 | 4.0 | NR | >8.0 | >8.0 | NR | >8.0 | >8.0 | NR | NR | NR |
| 20 | 18 | NR | 1.0 | 4.0 | NR | >8.0 | >8.0 | NR | >8.0 | >8.0 | NR | >8.0 | >8.0 | |
| 29 | 5 | 0.25-8 | NR | NR | ≥8.0 | NR | NR | NR | NR | NR | >8.0 | NR | NR | |
| 81 | 18 | 2->16 | NR | NR | NR | NR | NR | 1-8 | NR | NR | 4->16 | NR | NR | |
| 85 | 6 | 1-2 | 1.3b | NR | 1->16 | 8 | NR | 8-16 | 10.5b | NR | NR | NR | NR | |
| 31 | 3 | 0.12-4 | NR | NR | >8 | NR | NR | 4->8 | NR | NR | >8 | NR | NR | |
| 30 | 10 | 0.5-4 | NR | 4 | 8->8 | NR | >8 | NR | NR | NR | NR | NR | NR | |
| 67 | 5 | 0.5-2 | NR | NR | 2-32 | NR | NR | 1-16 | NR | NR | NR | NR | NR | |
| 6 | 18 | 1->4 | NR | NR | >16 | NR | NR | 1-16 | NR | NR | NR | NR | NR | |
| 105 | 10 | 1-2 | 1 | 2 | >16 | >16 | >16 | NR | NR | NR | NR | NR | NR | |
| Fusarium oxysporum | 21 | 19 | NR | 0.5 | 2 | NR | >8 | >8 | NR | 4 | >8 | NR | NR | NR |
| 20 | 15 | NR | 0.5 | 1 | NR | >8 | >8 | NR | 4 | >8 | NR | NR | NR | |
| 29 | 3 | 0.5-2 | NR | NR | ≥8 | NR | NR | NR | NR | NR | 1->8 | NR | NR | |
| 81 | 4 | 16 | NR | NR | NR | NR | NR | 2-4 | NR | NR | 1-2 | NR | NR | |
| 85 | 6 | 2 | 2 | NR | 1->16 | 8b | NR | NR | NR | NR | NR | NR | NR | |
| 67 | 5 | 0.25-2 | NR | NR | 0.5->32 | NR | NR | 0.5-16 | NR | NR | NR | NR | NR | |
| 6 | 4 | 2-4 | NR | NR | 0.5 | NR | NR | 1-4 | NR | NR | NR | NR | NR | |
| Fusarium verticillioides | 21 | 10 | NR | 2 | 8 | NR | >8 | >8 | NR | >8 | >8 | NR | NR | NR |
| 20 | 11 | NR | 2 | >16 | NR | >8 | >8 | NR | >8 | >8 | NR | NR | NR | |
| Fusarium moniliforme | 29 | 3 | 1-2 | NR | NR | 2->8 | NR | NR | NR | NR | NR | NR | NR | NR |
NR, not reported.
Geometric mean MIC.
(ii) Clinical experience.
Because of a lack of clinical trials and the critical role of immune reconstitution in the outcome of fusariosis, the optimal treatment strategy for patients with severe fusarial infection remains unclear. Hence, comparisons between different treatment reports are problematic. In a retrospective analysis of 84 patients with hematologic diseases and invasive fusariosis, treatment consisted of deoxycholate amphotericin B (69 patients) or a lipid formulation of amphotericin B (13 patients), with 2 patients not receiving treatment. Twenty-seven patients (32%) responded to treatment, but only 18 patients (21%) were still alive 90 days after diagnosis. The response rate to a lipid formulation of amphotericin B appeared to be superior to that to deoxycholate amphotericin B (46% versus 32%, respectively), but the difference was not statistically significant (P = 0.36) (75).
The outcome was very poor among the 45 patients who underwent HSCT, regardless of the receipt of deoxycholate amphotericin B (30 patients), a lipid formulation of amphotericin B (14 patients), or caspofungin (1 patient) (76).
In a retrospective study, amphotericin B lipid complex was given to 26 evaluable patients with hematological malignancy and fusariosis, usually as salvage therapy as a result of intolerance or lack of response to primary therapy (83). Thirteen patients (46%) were neutropenic. At a median daily dose of 4.5 mg/kg (total cumulative dose, 5 g), the response rate (cure or improvement) in the 26 evaluable patients was 46%. In another study, voriconazole was given to 11 patients with fusariosis, all intolerant or refractory to primary therapy (84). The response rate (complete or partial response) was 45%, with a 90-day actuarial survival of 71%. Posaconazole has also been used as salvage therapy among 21 patients with proven or probable fusariosis (90). The underlying disease was a hematologic malignancy in 76%, and 38% of patients were neutropenic. All but one patient were initially treated with a lipid-based formulation of amphotericin B. The overall success rate (complete plus partial response) was 48%. As expected, patients with disseminated disease had a lower response rate (3/10 [30%]) than patients with localized disease (7/11, 64%). The positive results of these salvage therapy studies are in sharp contrast with the 21% 90-day survival among 84 patients with hematologic diseases (75) and the 13% 90-day survival among 61 HSCT recipients (76), suggesting that a selection bias was commonly present in trials of salvage antifungal therapy, i.e., that patients treated with salvage regimens may have a better prognosis simply by the fact that they survived long enough to receive a second treatment.
(iii) Combination therapy.
Data on combination therapy for fusariosis are limited to a few case reports: caspofungin plus amphotericin B (64), amphotericin B plus voriconazole (25, 42), amphotericin B and terbinafine (95), and voriconazole plus terbinafine (49). Given the scarcity of data and the potential publication bias, no solid recommendations can be provided.
(iv) Adjunctive therapies.
In addition to antifungal treatment, the optimal management of patients with fusariosis includes surgical debulking of infected tissues (61) and removal of venous catheters in the occasional patient with confirmed catheter-related fusariosis (109). The role of G-CSF or GM-CSF, G-CSF-stimulated granulocyte transfusions, and gamma interferon in the adjuvant treatment of fusariosis is not established. However, given the poor prognosis of fusariosis, especially in persistently neutropenic patients, G-CSF and granulocyte transfusions are frequently used. In support, there are isolated case reports of the successful treatment of invasive fusariosis with a combination of medical treatment and some of these measures (93).
(v) Monitoring of therapy.
The criteria for response in invasive fusariosis include disappearance of fever and clinical symptoms attributed to the infection and resolution of fungemia and radiologic abnormalities. In patients with fusarial sinusitis, nasal endoscopy should be repeated in order to ascertain that no new necrotic lesions developed. The interpretation of radiologic images may be problematic, since residual (not necessarily active) lesions in the lungs and sinuses may remain. Imaging methods that detect inflammation may be used, such as positron emission tomography (63) or indium-labeled white blood cell scintigraphy (8).
PREVENTION
Because of the poor prognosis associated with fusariosis and the limited susceptibility of Fusarium spp. to antifungal agents, prevention of infection remains the cornerstone of management. In severely immunocompromised patients, every effort should be made to prevent patient exposure (e.g., by putting high-risk patients in rooms with HEPA filters and positive pressure, avoiding contact with reservoirs of Fusarium spp. such as tap water [2], and/or cleaning showers prior to use by high-risk patients [3]).
Decreasing of immunosuppression should be attempted in patients with prior history of Fusarium infection and can be achieved by a reduction in or discontinuation of immunosuppressive agents, shortening the duration of neutropenia (selection of nonmyeloablative as opposed to myeloablative preparative regimens for allogeneic HSCT and the use of preemptive G-CSF-elicited or G-CSF- and dexamethasone-elicited white blood cell transfusions) (22, 46). If the organism is available, antifungal susceptibility testing should be performed and antifungal prophylaxis with an agent active against the recovered fusarial strain should be considered. In addition, thorough evaluation and treatment of skin lesions (particularly onychomycoses, which serve as a portal of entry for fusariosis) should be done prior to commencing antineoplastic therapy (74).
CONCLUSIONS
Infections by Fusarium species can be superficial or limited to single organs in otherwise healthy patients. Such infections are rare and tend to respond well to therapy. By contrast, disseminated fusariosis affects the immunocompromised host, especially HSCT recipients and patients with severe and prolonged neutropenia. Infection in this setting is frequently fatal, and successful outcome is determined largely by the degree and persistence of immunosuppression and the extent of infection, with virtually a 100% death rate for persistently neutropenic patients with disseminated disease. These infections may be clinically suspected on the basis of a constellation of clinical and laboratory findings, which should lead to prompt therapy.
Acknowledgments
Marcio Nucci received financial support from CNPq grant 300235/93-3.
REFERENCES
- 1.Ammari, L. K., J. M. Puck, and K. L. McGowan. 1993. Catheter-related Fusarium solani fungemia and pulmonary infection in a patient with leukemia in remission. Clin. Infect. Dis. 16:148-150. [DOI] [PubMed] [Google Scholar]
- 2.Anaissie, E. J., R. T. Kuchar, J. H. Rex, A. Francesconi, M. Kasai, F. M. Muller, M. Lozano-Chiu, R. C. Summerbell, M. C. Dignani, S. J. Chanock, and T. J. Walsh. 2001. Fusariosis associated with pathogenic Fusarium species colonization of a hospital water system: a new paradigm for the epidemiology of opportunistic mold infections. Clin. Infect. Dis. 33:1871-1878. [DOI] [PubMed] [Google Scholar]
- 3.Anaissie, E. J., S. L. Stratton, M. C. Dignani, C. K. Lee, T. H. Mahfouz, J. H. Rex, R. C. Summerbell, and T. J. Walsh. 2002. Cleaning patient shower facilities: a novel approach to reducing patient exposure to aerosolized Aspergillus species and other opportunistic molds. Clin. Infect. Dis. 35:E86-E88. [DOI] [PubMed] [Google Scholar]
- 4.Anandi, V., P. Vishwanathan, S. Sasikala, M. Rangarajan, C. S. Subramaniyan, and N. Chidambaram. 2005. Fusarium solani breast abscess. Indian J. Med. Microbiol. 23:198-199. [DOI] [PubMed] [Google Scholar]
- 5.Apostolidis, J., M. Bouzani, E. Platsouka, H. Belasiotou, M. Stamouli, N. Harhalakis, E. I. Boutati, O. Paniara, and E. Nikiforakis. 2003. Resolution of fungemia due to Fusarium species in a patient with acute leukemia treated with caspofungin. Clin. Infect. Dis. 36:1349-1350. [DOI] [PubMed] [Google Scholar]
- 6.Arikan, S., M. Lozano-Chiu, V. Paetznick, S. Nangia, and J. H. Rex. 1999. Microdilution susceptibility testing of amphotericin B, itraconazole, and voriconazole against clinical isolates of Aspergillus and Fusarium species. J. Clin. Microbiol. 37:3946-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bader, M., A. K. Jafri, T. Krueger, and V. Kumar. 2003. Fusarium osteomyelitis of the foot in a patient with diabetes mellitus. Scand. J. Infect. Dis. 35:895-896. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin, J. E., and E. P. Wraight. 1990. Indium labelled leucocyte scintigraphy in occult infection: a comparison with ultrasound and computed tomography. Clin. Radiol. 42:199-202. [DOI] [PubMed] [Google Scholar]
- 9.Bigley, V. H., R. F. Duarte, R. D. Gosling, C. C. Kibbler, S. Seaton, and M. Potter. 2004. Fusarium dimerum infection in a stem cell transplant recipient treated successfully with voriconazole. Bone Marrow Transplant. 34:815-817. [DOI] [PubMed] [Google Scholar]
- 10.Bourguignon, R. L., A. F. Walsh, J. C. Flynn, C. Baro, and E. Spinos. 1976. Fusarium species osteomyelitis. Case report. J. Bone Joint Surg. Am. 58:722-723. [PubMed] [Google Scholar]
- 11.Boutati, E. I., and E. J. Anaissie. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999-1008. [PubMed] [Google Scholar]
- 12.Brilhante, R. S., R. A. Cordeiro, D. J. Medrano, M. F. Rocha, A. J. Monteiro, C. S. Cavalcante, T. E. Meireles, and J. J. Sidrim. 2005. Onychomycosis in Ceara (Northeast Brazil): epidemiological and laboratory aspects. Mem. Inst. Oswaldo Cruz 100:131-135. [DOI] [PubMed] [Google Scholar]
- 13.Bunya, V. Y., K. M. Hammersmith, C. J. Rapuano, B. D. Ayres, and E. J. Cohen. 2007. Topical and oral voriconazole in the treatment of fungal keratitis. Am. J. Ophthalmol. 143:151-153. [DOI] [PubMed] [Google Scholar]
- 14.Burgess, L. W. 1981. General ecology of the fusaria, p. 225-235. In P. E. Nelson, T. A. Toussoun, and R. J. Cook (ed.), Fusarium: diseases, biology, and taxonomy. Pennsylvania State University Press, Philadelphia, PA.
- 15.Castagnola, E., A. Garaventa, M. Conte, A. Barretta, E. Faggi, and C. Viscoli. 1993. Survival after fungemia due to Fusarium moniliforme in a child with neuroblastoma. Eur. J. Clin. Microbiol. Infect. Dis. 12:308-309. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. 2006. Update: Fusarium keratitis—United States, 2005-2006. Morb. Mortal. Wkly. Rep. 55:563-564. [PubMed] [Google Scholar]
- 17.Chang, D. C., G. B. Grant, K. O'Donnell, K. A. Wannemuehler, J. Noble-Wang, C. Y. Rao, L. M. Jacobson, C. S. Crowell, R. S. Sneed, F. M. Lewis, J. K. Schaffzin, M. A. Kainer, C. A. Genese, E. C. Alfonso, D. B. Jones, A. Srinivasan, S. K. Fridkin, and B. J. Park. 2006. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA 296:953-963. [DOI] [PubMed] [Google Scholar]
- 18.Cocuroccia, B., J. Gaido, E. Gubinelli, G. Annessi, and G. Girolomoni. 2003. Localized cutaneous hyalohyphomycosis caused by a Fusarium species infection in a renal transplant patient. J. Clin. Microbiol. 41:905-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Consigny, S., N. Dhedin, A. Datry, S. Choquet, V. Leblond, and O. Chosidow. 2003. Successsful voriconazole treatment of disseminated Fusarium infection in an immunocompromised patient. Clin. Infect. Dis. 37:311-313. [DOI] [PubMed] [Google Scholar]
- 20.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, M. J. Buitrago, A. Monzon, and J. L. Rodriguez-Tudela. 2006. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 50:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, G. Garcia-Effron, A. Monzon, and J. L. Rodriguez-Tudela. 2005. In vitro activity of ravuconazole against 923 clinical isolates of nondermatophyte filamentous fungi. Antimicrob. Agents Chemother. 49:5136-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dignani, M. C., E. J. Anaissie, J. P. Hester, S. O'Brien, S. E. Vartivarian, J. H. Rex, H. Kantarjian, D. B. Jendiroba, B. Lichtiger, B. S. Andersson, and E. J. Freireich. 1997. Treatment of neutropenia-related fungal infections with granulocyte colony-stimulating factor-elicited white blood cell transfusions: a pilot study. Leukemia 11:1621-1630. [DOI] [PubMed] [Google Scholar]
- 23.Doczi, I., T. Gyetvai, L. Kredics, and E. Nagy. 2004. Involvement of Fusarium spp. in fungal keratitis. Clin. Microbiol. Infect. 10:773-776. [DOI] [PubMed] [Google Scholar]
- 24.Dornbusch, H. J., W. Buzina, R. C. Summerbell, C. Lass-Florl, H. Lackner, W. Schwinger, P. Sovinz, and C. Urban. 2005. Fusarium verticillioides abscess of the nasal septum in an immunosuppressed child: case report and identification of the morphologically atypical fungal strain. J. Clin. Microbiol. 43:1998-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durand-Joly, I., S. Alfandari, Z. Benchikh, M. Rodrigue, A. Espinel-Ingroff, B. Catteau, C. Cordevant, D. Camus, E. Dei-Cas, F. Bauters, L. Delhaes, and B. S. De. 2003. Successful outcome of disseminated Fusarium infection with skin localization treated with voriconazole and amphotericin B-lipid complex in a patient with acute leukemia. J. Clin. Microbiol. 41:4898-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dursun, D., V. Fernandez, D. Miller, and E. C. Alfonso. 2003. Advanced Fusarium keratitis progressing to endophthalmitis. Cornea 22:300-303. [DOI] [PubMed] [Google Scholar]
- 27.Eljaschewitsch, J., J. Sandfort, K. Tintelnot, I. Horbach, and B. Ruf. 1996. Port-a-cath-related Fusarium oxysporum infection in an HIV-infected patient: treatment with liposomal amphotericin B. Mycoses 39:115-119. [DOI] [PubMed] [Google Scholar]
- 28.Elvers, K. T., K. Leeming, C. P. Moore, and H. M. Lappin-Scott. 1998. Bacterial-fungal biofilms in flowing water photo-processing tanks. J. Appl. Microbiol. 84:607-618. [DOI] [PubMed] [Google Scholar]
- 29.Espinel-Ingroff, A. 2003. In vitro antifungal activities of anidulafungin and micafungin, licensed agents and the investigational triazole posaconazole as determined by NCCLS methods for 12,052 fungal isolates: review of the literature. Rev. Iberoam. Micol. 20:121-136. [PubMed] [Google Scholar]
- 30.Espinel-Ingroff, A. 2001. Comparison of the E-test with the NCCLS M38-P method for antifungal susceptibility testing of common and emerging pathogenic filamentous fungi. J. Clin. Microbiol. 39:1360-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espinel-Ingroff, A., V. Chaturvedi, A. Fothergill, and M. G. Rinaldi. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J. Clin. Microbiol. 40:3776-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans, J., D. Levesque, L. A. de, and H. E. Jensen. 2004. Intracranial fusariosis: a novel cause of fungal meningoencephalitis in a dog. Vet. Pathol. 41:510-514. [DOI] [PubMed] [Google Scholar]
- 33.Ferrer, C., J. Alio, A. Rodriguez, M. Andreu, and F. Colom. 2005. Endophthalmitis caused by Fusarium proliferatum. J. Clin. Microbiol. 43:5372-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn, J. T., D. Meislich, B. A. Kaiser, M. S. Polinsky, and H. J. Baluarte. 1996. Fusarium peritonitis in a child on peritoneal dialysis: case report and review of the literature. Perit. Dial. Int. 16:52-57. [PubMed] [Google Scholar]
- 35.Gabriele, P., and R. K. Hutchins. 1996. Fusarium endophthalmitis in an intravenous drug abuser. Am. J. Ophthalmol. 122:119-121. [DOI] [PubMed] [Google Scholar]
- 36.Garbino, J., I. Uckay, P. Rohner, D. Lew, and D. C. Van. 2005. Fusarium peritonitis concomitant to kidney transplantation successfully managed with voriconazole: case report and review of the literature. Transpl. Int. 18:613-618. [DOI] [PubMed] [Google Scholar]
- 37.Gardner, J. M., M. M. Nelson, and M. P. Heffernan. 2005. Chronic cutaneous fusariosis. Arch. Dermatol. 141:794-795. [DOI] [PubMed] [Google Scholar]
- 38.Gaviria, J. M., J. A. van Burik, D. C. Dale, R. K. Root, and W. C. Liles. 1999. Comparison of interferon-gamma, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor for priming leukocyte-mediated hyphal damage of opportunistic fungal pathogens. J. Infect. Dis. 179:1038-1041. [DOI] [PubMed] [Google Scholar]
- 39.Godoy, P., E. Nunes, V. Silva, J. Tomimori-Yamashita, L. Zaror, and O. Fischman. 2004. Onychomycosis caused by Fusarium solani and Fusarium oxysporum in Sao Paulo, Brazil. Mycopathologia 157:287-290. [DOI] [PubMed] [Google Scholar]
- 40.Grigis, A., C. Farina, F. Symoens, N. Nolard, and A. Goglio. 2000. Nosocomial pseudo-outbreak of Fusarium verticillioides associated with sterile plastic containers. Infect. Control Hosp. Epidemiol. 21:50-52. [DOI] [PubMed] [Google Scholar]
- 41.Guimera-Martin-Neda, F., M. Garcia-Bustinduy, A. Noda-Cabrera, R. Sanchez-Gonzalez, and R. G. Montelongo. 2004. Cutaneous infection by Fusarium: successful treatment with oral voriconazole. Br. J. Dermatol. 150:777-778. [DOI] [PubMed] [Google Scholar]
- 42.Guzman-Cottrill, J. A., X. Zheng, and E. G. Chadwick. 2004. Fusarium solani endocarditis successfully treated with liposomal amphotericin B and voriconazole. Pediatr. Infect. Dis. J. 23:1059-1061. [DOI] [PubMed] [Google Scholar]
- 43.Hamaki, T., M. Kami, A. Kishi, E. Kusumi, Y. Kishi, H. Iwata, S. Miyakoshi, J. Ueyama, S. Morinaga, S. Taniguchi, K. Ohara, and Y. Muto. 2004. Vesicles as initial skin manifestation of disseminated fusariosis after non-myeloablative stem cell transplantation. Leuk. Lymphoma 45:631-633. [DOI] [PubMed] [Google Scholar]
- 44.Hayden, R. T., P. A. Isotalo, T. Parrett, D. M. Wolk, X. Qian, G. D. Roberts, and R. V. Lloyd. 2003. In situ hybridization for the differentiation of Aspergillus, Fusarium, and Pseudallescheria species in tissue section. Diagn. Mol. Pathol. 12:21-26. [DOI] [PubMed] [Google Scholar]
- 45.Healy, M., K. Reece, D. Walton, J. Huong, S. Frye, I. I. Raad, and D. P. Kontoyiannis. 2005. Use of the Diversi Lab System for species and strain differentiation of Fusarium species isolates. J. Clin. Microbiol. 43:5278-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hennequin, C., M. Benkerrou, J. L. Gaillard, S. Blanche, and S. Fraitag. 1994. Role of granulocyte colony-stimulating factor in the management of infection with Fusarium oxysporum in a neutropenic child. Clin. Infect. Dis. 18:490-491. [DOI] [PubMed] [Google Scholar]
- 47.Hennequin, C., C. Ranaivoarimalala, T. Chouaki, M. Tazerout, T. Ancelle, J. J. Cabaud, and C. P. Raccurt. 2002. Comparison of aerobic standard medium with specific fungal medium for detecting Fusarium spp in blood cultures. Eur. J. Clin. Microbiol. Infect. Dis. 21:748-750. [DOI] [PubMed] [Google Scholar]
- 48.Herbrecht, R., R. Kessler, C. Kravanja, M. H. Meyer, J. Waller, and V. Letscher-Bru. 2004. Successful treatment of Fusarium proliferatum pneumonia with posaconazole in a lung transplant recipient. J. Heart Lung Transplant. 23:1451-1454. [DOI] [PubMed] [Google Scholar]
- 49.Howden, B. P., M. A. Slavin, A. P. Schwarer, and A. M. Mijch. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 22:111-113. [DOI] [PubMed] [Google Scholar]
- 50.Hue, F. X., M. Huerre, M. A. Rouffault, and C. de Bievre. 1999. Specific detection of Fusarium species in blood and tissues by a PCR technique. J. Clin. Microbiol. 37:2434-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jakle, C., J. C. Leek, D. A. Olson, and D. L. Robbins. 1983. Septic arthritis due to Fusarium solani. J. Rheumatol. 10:151-153. [PubMed] [Google Scholar]
- 52.Kerr, C. M., J. R. Perfect, P. C. Craven, J. H. Jorgensen, D. J. Drutz, J. D. Shelburne, H. A. Gallis, and R. A. Gutman. 1983. Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Ann. Intern. Med. 99:334-336. [DOI] [PubMed] [Google Scholar]
- 53.Kiehn, T. E., P. E. Nelson, E. M. Bernard, F. F. Edwards, B. Koziner, and D. Armstrong. 1985. Catheter-associated fungemia caused by Fusarium chlamydosporum in a patient with lymphocytic lymphoma. J. Clin. Microbiol. 21:501-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kivivuori, S. M., L. Hovi, K. Vettenranta, and U. M. Saarinen-Pihkala. 2004. Invasive fusariosis in two transplanted children. Eur. J. Pediatr. 163:692-693. [DOI] [PubMed] [Google Scholar]
- 55.Kratka, J., and E. Kovacikova. 1979. The effect of temperature and age of strains of Fusarium oxysporum on its enzymatic activity. Zentbl. Bakteriol. Naturwiss. 134:154-158. [DOI] [PubMed] [Google Scholar]
- 56.Kurien, M., V. Anandi, R. Raman, and K. N. Brahmadathan. 1992. Maxillary sinus fusariosis in immunocompetent hosts. J. Laryngol. Otol. 106:733-736. [DOI] [PubMed] [Google Scholar]
- 57.Legrand, C., E. Anaissie, R. Hashem, P. Nelson, G. P. Bodey, and J. Ro. 1991. Experimental fusarial hyalohyphomycosis in a murine model. J. Infect. Dis. 164:944-948. [DOI] [PubMed] [Google Scholar]
- 58.Letscher-Bru, V., F. Campos, J. Waller, R. Randriamahazaka, E. Candolfi, and R. Herbrecht. 2002. Successful outcome of treatment of a disseminated infection due to Fusarium dimerum in a leukemia patient. J. Clin. Microbiol. 40:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu, K., D. N. Howell, J. R. Perfect, and W. A. Schell. 1998. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am. J. Clin. Pathol. 109:45-54. [DOI] [PubMed] [Google Scholar]
- 60.Lopes, J. O., E. S. de Mello, and C. Klock. 1995. Mixed intranasal infection caused by Fusarium solani and a zygomycete in a leukaemic patient. Mycoses 38:281-284. [DOI] [PubMed] [Google Scholar]
- 61.Lupinetti, F. M., R. H. Giller, and M. E. Trigg. 1990. Operative treatment of Fusarium fungal infection of the lung. Ann. Thorac. Surg. 49:991-992. [DOI] [PubMed] [Google Scholar]
- 62.Madhavan, M., C. Ratnakar, A. J. Veliath, R. Kanungo, S. R. Smile, and S. Bhat. 1992. Primary disseminated fusarial infection. Postgrad. Med. J. 68:143-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mahfouz, T., M. H. Miceli, F. Saghafifar, S. Stroud, L. Jones-Jackson, R. Walker, M. L. Grazziutti, G. Purnell, A. Fassas, G. Tricot, B. Barlogie, and E. Anaissie. 2005. 18F-fluorodeoxyglucose positron emission tomography contributes to the diagnosis and management of infections in patients with multiple myeloma: a study of 165 infectious episodes. J. Clin. Oncol. 23:7857-7863. [DOI] [PubMed] [Google Scholar]
- 64.Makowsky, M. J., D. I. Warkentin, and M. L. Savoie. 2005. Caspofungin and amphotericin B for disseminated Fusarium verticillioides in leukemia. Ann. Pharmacother. 39:1365-1366. [DOI] [PubMed] [Google Scholar]
- 65.Mansoory, D., N. A. Roozbahany, H. Mazinany, and A. Samimagam. 2003. Chronic Fusarium infection in an adult patient with undiagnosed chronic granulomatous disease. Clin. Infect. Dis. 37:e107-e108. [DOI] [PubMed] [Google Scholar]
- 66.Mayayo, E., I. Pujol, and J. Guarro. 1999. Experimental pathogenicity of four opportunist Fusarium species in a murine model. J. Med. Microbiol. 48:363-366. [DOI] [PubMed] [Google Scholar]
- 67.Meletiadis, J., J. F. Meis, J. W. Mouton, J. P. Donnelly, and P. E. Verweij. 2000. Comparison of NCCLS and 3-(4,5-dimethyl-2-thiazyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) methods of in vitro susceptibility testing of filamentous fungi and development of a new simplified method. J. Clin. Microbiol. 38:2949-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moschovi, M., G. Trimis, J. Anastasopoulos, M. Kanariou, A. Raftopoulou, and F. Tzortzatou-Stathopoulou. 2004. Subacute vertebral osteomyelitis in a child with diabetes mellitus associated with Fusarium. Pediatr. Int. 46:740-742. [DOI] [PubMed] [Google Scholar]
- 69.Murray, C. K., M. L. Beckius, and K. McAllister. 2003. Fusarium proliferatum superficial suppurative thrombophlebitis. Mil. Med. 168:426-427. [PubMed] [Google Scholar]
- 70.Musa, M. O., A. Al Eisa, M. Halim, E. Sahovic, M. Gyger, N. Chaudhri, F. Al Mohareb, P. Seth, M. Aslam, and M. Aljurf. 2000. The spectrum of Fusarium infection in immunocompromised patients with haematological malignancies and in non-immunocompromised patients: a single institution experience over 10 years. Br. J. Haematol. 108:544-548. [DOI] [PubMed] [Google Scholar]
- 71.Nakar, C., G. Livny, I. Levy, Z. Samra, N. Linder, S. Ashkenazi, P. Livne, and L. Sirota. 2001. Mycetoma of the renal pelvis caused by Fusarium species. Pediatr. Infect. Dis. J. 20:1182-1183. [DOI] [PubMed] [Google Scholar]
- 72.Nelson, P. E., M. C. Dignani, and E. J. Anaissie. 1994. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 7:479-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ninet, B., I. Jan, O. Bontems, B. Lechenne, O. Jousson, D. Lew, J. Schrenzel, R. G. Panizzon, and M. Monod. 2005. Molecular identification of Fusarium species in onychomycoses. Dermatology 210:21-25. [DOI] [PubMed] [Google Scholar]
- 74.Nucci, M., and E. Anaissie. 2002. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: implications for diagnosis and management. Clin. Infect. Dis. 35:909-920. [DOI] [PubMed] [Google Scholar]
- 75.Nucci, M., E. J. Anaissie, F. Queiroz-Telles, C. A. Martins, P. Trabasso, C. Solza, C. Mangini, B. P. Simoes, A. L. Colombo, J. Vaz, C. E. Levy, S. Costa, V. A. Moreira, J. S. Oliveira, N. Paraguay, G. Duboc, J. C. Voltarelli, A. Maiolino, R. Pasquini, and C. A. Souza. 2003. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer 98:315-319. [DOI] [PubMed] [Google Scholar]
- 76.Nucci, M., K. A. Marr, F. Queiroz-Telles, C. A. Martins, P. Trabasso, S. Costa, J. C. Voltarelli, A. L. Colombo, A. Imhof, R. Pasquini, A. Maiolino, C. A. Souza, and E. Anaissie. 2004. Fusarium infection in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 38:1237-1242. [DOI] [PubMed] [Google Scholar]
- 77.Odabasi, Z., G. Mattiuzzi, E. Estey, H. Kantarjian, F. Saeki, R. J. Ridge, P. A. Ketchum, M. A. Finkelman, J. H. Rex, and L. Ostrosky-Zeichner. 2004. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199-205. [DOI] [PubMed] [Google Scholar]
- 78.O'Donnell, K., D. A. Sutton, M. G. Rinaldi, K. C. Magnon, P. A. Cox, S. G. Revankar, S. Sanche, D. M. Geiser, J. H. Juba, J. A. van Burik, A. Padhye, E. J. Anaissie, A. Francesconi, T. J. Walsh, and J. S. Robinson. 2004. Genetic diversity of human pathogenic members of the Fusarium oxysporum complex inferred from multilocus DNA sequence data and amplified fragment length polymorphism analyses: evidence for the recent dispersion of a geographically widespread clonal lineage and nosocomial origin. J. Clin. Microbiol. 42:5109-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ortoneda, M., J. Guarro, M. P. Madrid, Z. Caracuel, M. I. Roncero, E. Mayayo, and P. A. Di. 2004. Fusarium oxysporum as a multihost model for the genetic dissection of fungal virulence in plants and mammals. Infect. Immun. 72:1760-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ostrosky-Zeichner, L., B. D. Alexander, D. H. Kett, J. Vazquez, P. G. Pappas, F. Saeki, P. A. Ketchum, J. Wingard, R. Schiff, H. Tamura, M. A. Finkelman, and J. H. Rex. 2005. Multicenter clinical evaluation of the (1→3)beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654-659. [DOI] [PubMed] [Google Scholar]
- 81.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2002. In vitro activities of investigational triazoles against Fusarium species: effects of inoculum size and incubation time on broth microdilution susceptibility test results. Antimicrob. Agents Chemother. 46:3298-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pereiro, M., Jr., M. T. Abalde, A. Zulaica, J. L. Caeiro, A. Florez, C. Peteiro, and J. Toribio. 2001. Chronic infection due to Fusarium oxysporum mimicking lupus vulgaris: case report and review of cutaneous involvement in fusariosis. Acta Derm. Venereol. 81:51-53. [DOI] [PubMed] [Google Scholar]
- 83.Perfect, J. R. 2005. Treatment of non-Aspergillus moulds in immunocompromised patients, with amphotericin B lipid complex. Clin. Infect. Dis. 40(Suppl. 6):S401-S408. [DOI] [PubMed] [Google Scholar]
- 84.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 85.Pfaller, M. A., and D. J. Diekema. 2004. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J. Clin. Microbiol. 42:4419-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pflugfelder, S. C., H. W. Flynn, Jr., T. A. Zwickey, R. K. Forster, A. Tsiligianni, W. W. Culbertson, and S. Mandelbaum. 1988. Exogenous fungal endophthalmitis. Ophthalmology 95:19-30. [DOI] [PubMed] [Google Scholar]
- 87.Pushker, N., M. Chra, M. S. Bajaj, S. Ghose, N. Naik, S. Kashyap, and G. Satpathy. 2002. Necrotizing periorbital Fusarium infection—an emerging pathogen in immunocompetent individuals. J. Infect. 44:236-239. [DOI] [PubMed] [Google Scholar]
- 88.Raad, I., and R. Hachem. 1995. Treatment of central venous catheter-related fungemia due to Fusarium oxysporum. Clin. Infect. Dis. 20:709-711. [DOI] [PubMed] [Google Scholar]
- 89.Raad, I., J. Tarrand, H. Hanna, M. Albitar, E. Janssen, M. Boktour, G. Bodey, M. Mardani, R. Hachem, D. Kontoyiannis, E. Whimbey, and K. Rolston. 2002. Epidemiology, molecular mycology, and environmental sources of Fusarium infection in patients with cancer. Infect. Control Hosp. Epidemiol. 23:532-537. [DOI] [PubMed] [Google Scholar]
- 90.Raad, I. I., R. Y. Hachem, R. Herbrecht, J. R. Graybill, R. Hare, G. Corcoran, and D. P. Kontoyiannis. 2006. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin. Infect. Dis. 42:1398-1403. [DOI] [PubMed] [Google Scholar]
- 91.Rezai, K. A., D. Eliott, O. Plous, J. A. Vazquez, and G. W. Abrams. 2005. Disseminated Fusarium infection presenting as bilateral endogenous endophthalmitis in a patient with acute myeloid leukemia. Arch. Ophthalmol. 123:702-703. [DOI] [PubMed] [Google Scholar]
- 92.Rippon, J. W., R. A. Larson, D. M. Rosenthal, and J. Clayman. 1988. Disseminated cutaneous and peritoneal hyalohyphomycosis caused by Fusarium species: three cases and review of the literature. Mycopathologia 101:105-111. [DOI] [PubMed] [Google Scholar]
- 93.Rodriguez, C. A., J. Lujan-Zilbermann, P. Woodard, M. Andreansky, and E. E. Adderson. 2003. Successful treatment of disseminated fusariosis. Bone Marrow Transplant. 31:411-412. [DOI] [PubMed] [Google Scholar]
- 94.Romani, L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 4:1-23. [DOI] [PubMed] [Google Scholar]
- 95.Rothe, A., M. Seibold, T. Hoppe, H. Seifert, A. Engert, C. Caspar, M. Karthaus, G. Fatkenheuer, U. Bethe, K. Tintelnot, and O. A. Cornely. 2004. Combination therapy of disseminated Fusarium oxysporum infection with terbinafine and amphotericin B. Ann. Hematol. 83:394-397. [DOI] [PubMed] [Google Scholar]
- 96.Sampathkumar, P., and C. V. Paya. 2001. Fusarium infection after solid-organ transplantation. Clin. Infect. Dis. 32:1237-1240. [DOI] [PubMed] [Google Scholar]
- 97.Sander, A., U. Beyer, and R. Amberg. 1998. Systemic Fusarium oxysporum infection in an immunocompetent patient with an adult respiratory distress syndrome (ARDS) and extracorporal membrane oxygenation (ECMO). Mycoses 41:109-111. [DOI] [PubMed] [Google Scholar]
- 98.Saw, S. M., P. L. Ooi, D. T. Tan, W. B. Khor, C. W. Fong, J. Lim, H. Y. Cajucom-Uy, D. Heng, S. K. Chew, T. Aung, A. L. Tan, C. L. Chan, S. Ting, P. A. Tambyah, and T. Y. Wong. 2007. Risk factors for contact lens-related fusarium keratitis: a case-control study in Singapore. Arch. Ophthalmol. 125:611-617. [DOI] [PubMed] [Google Scholar]
- 99.Segal, B. H., T. J. Walsh, J. M. Liu, J. D. Wilson, and K. J. Kwon-Chung. 1998. Invasive infection with Fusarium chlamydosporum in a patient with aplastic anemia. J. Clin. Microbiol. 36:1772-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shoham, S., and S. M. Levitz. 2005. The immune response to fungal infections. Br. J. Haematol. 129:569-582. [DOI] [PubMed] [Google Scholar]
- 101.Sierra-Hoffman, M., S. Paltiyevich-Gibson, J. L. Carpenter, and D. L. Hurley. 2005. Fusarium osteomyelitis: case report and review of the literature. Scand. J. Infect. Dis. 37:237-240. [DOI] [PubMed] [Google Scholar]
- 102.Sridhar, H., J. R. Subramanyam, L. Appaji, M. Shafiulla, and B. R. Vijaykumar. 2001. Fusarium solani fungemia in a patient with acute lymphoblastic leukemia. Indian J. Cancer 38:19-21. [PubMed] [Google Scholar]
- 103.Stammberger, H. 1985. Endoscopic surgery for mycotic and chronic recurring sinusitis. Ann. Otol. Rhinol. Laryngol. Suppl. 119:1-11. [DOI] [PubMed] [Google Scholar]
- 104.Sturm, A. W., W. Grave, and W. S. Kwee. 1989. Disseminated Fusarium oxysporum infection in patient with heatstroke. Lancet i:968. [DOI] [PubMed] [Google Scholar]
- 105.Szekely, A., E. M. Johnson, and D. W. Warnock. 1999. Comparison of E-test and broth microdilution methods for antifungal drug susceptibility testing of molds. J. Clin. Microbiol. 37:1480-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tiribelli, M., F. Zaja, C. Fili, T. Michelutti, S. Prosdocimo, A. Candoni, and R. Fanin. 2002. Endogenous endophthalmitis following disseminated fungemia due to Fusarium solani in a patient with acute myeloid leukemia. Eur. J. Haematol. 68:314-317. [DOI] [PubMed] [Google Scholar]
- 107.Tomimori-Yamashita, J., M. M. Ogawa, S. H. Hirata, O. Fischman, N. S. Michalany, H. K. Yamashita, and M. Alchorne. 2002. Mycetoma caused by Fusarium solani with osteolytic lesions on the hand: case report. Mycopathologia 153:11-14. [DOI] [PubMed] [Google Scholar]
- 108.Valenstein, P., and W. A. Schell. 1986. Primary intranasal Fusarium infection. Potential for confusion with rhinocerebral zygomycosis. Arch. Pathol. Lab. Med. 110:751-754. [PubMed] [Google Scholar]
- 109.Velasco, E., C. A. Martins, and M. Nucci. 1995. Successful treatment of catheter-related fusarial infection in immunocompromised children. Eur. J. Clin. Microbiol. Infect. Dis. 14:697-699. [DOI] [PubMed] [Google Scholar]
- 110.Vincent, A. L., J. E. Cabrero, J. N. Greene, and R. L. Sandin. 2003. Successful voriconazole therapy of disseminated Fusarium solani in the brain of a neutropenic cancer patient. Cancer Control 10:414-419. [DOI] [PubMed] [Google Scholar]
- 111.Wickern, G. M. 1993. Fusarium allergic fungal sinusitis. J. Allergy Clin. Immunol. 92:624-625. [DOI] [PubMed] [Google Scholar]
- 112.Winn, R. M., C. Gil-Lamaignere, E. Roilides, M. Simitsopoulou, C. A. Lyman, A. Maloukou, and T. J. Walsh. 2005. Effects of interleukin-15 on antifungal responses of human polymorphonuclear leukocytes against Fusarium spp. and Scedosporium spp. Cytokine 31:1-8. [DOI] [PubMed] [Google Scholar]
- 113.Yera, H., M. E. Bougnoux, C. Jeanrot, M. T. Baixench, P. G. De, and J. Dupouy-Camet. 2003. Mycetoma of the foot caused by Fusarium solani: identification of the etiologic agent by DNA sequencing. J. Clin. Microbiol. 41:1805-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]