Abstract
By analogy to mammals, odorant receptors (ORs) in insects, such as Drosophila melanogaster, have long been thought to belong to the G-protein coupled receptor superfamily. However, recent work has cast doubt on this assumption and has tentatively suggested an inverted topology compared to the canonical Nout-Cin 7TM GPCR topology, at least for some Drosophila ORs. Here, we report a detailed topology mapping of the Drosophila OR83b receptor using engineered glycosylation sites as topology markers. Our results are inconsistent with a classical GPCR topology and show that OR83b has an intracellular N-terminus, an extracellular C-terminus, and 7 transmembrane helices.
Keywords: odorant receptor, Drosophila melanogaster, membrane protein, topology
1. Introduction
In mammals, odorant receptors belong to the large superfamily of G-protein coupled receptors (GPRCs) and have a typical 7TM topology with an extracellular N-terminus and an intracellular C-terminus [1]. Because insects also have an expanded repertoire of GPCRs it has long been assumed that their odorant receptors have the same canonical 7TM topology, yet recent work on the Drosophila OR83b odorant receptor tentatively placed its N-terminus intracellularly rather than extracellularly [2,3]. This unexpected result is in accordance with theoretical topology predictions [1]. OR83b is a ubiquitously expressed and highly conserved member of the insect odorant receptor family and heteromerizes with other odorant receptors, forming active receptor complexes [2].
Here, we report a detailed study of the membrane topology of OR83b inserted into Drosophila rough microsomes (DRMs), using both an endogenous and engineered acceptor sites for N-linked glycosylation as topological markers. Our results support a 7TM Nin-Cout topology for OR83b.
2. Material and methods
Enzymes and chemicals
Unless otherwise stated, all enzymes, plasmid pGEM1, and the TNT® Quick transcription/translation system were from Promega (Madison, WI). [35S]-Methionine, [14C]-methylated marker proteins and deoxynucleotides were from GE Healthcare (Uppsala, Sweden). BigDye Terminator v1.1 Cycle Sequencing Kit was from AB Applied Biosystems (Foster City, CA) and oligonucleotides were from CyberGene AB (Stockholm, Sweden).
Plasmid construction
Fragments from full-length Or83b cDNA prepared Drosophila heads was modified in two ways during PCR amplification: (i) by the introduction of a 5′ XbaI site, and (ii) by changing the context of the region immediately upstream of the initiator ATG codon to a Kozak consensus ribosome binding sequence, GCCACCATGG [4]; both changes were encoded within the 5′PCR primer. The reverse primer encoded the 3′-end of the selected odorant receptor gene, two stop codons, and a SmaI site for cloning. The Or83b gene was amplified by PCR using the Expand High Fidelity PCR system from Roche Diagnostics GmbH (Mannheim, FRG) and cloned into pGEM1 downstream of the SP6 promoter as an XbaI-SmaI fragment. The amplified DNA products were purified using the QIAquick PCR Purification kit from QIAGEN (Hilden, FRG).
DNA manipulations
Glycosylation acceptor sites were designed as described previously [5], i.e. by replacing or insertion of one or more appropriately positioned codons for the acceptor tripeptide Asn-Ser-Thr (NST). To destroy the endogenous glycosylation acceptor site (Asn169-Ser-Ser (N169)), it was mutated to Gln-Ser-Ser (QSS). To create glycosylation acceptor sites the sequence was changed to N20NSTI21 (N21), V111NSTH114 (N112), E119NSTD121 (N120), V174NSTE175 (N175), A264NSTK267 (N265), and K439NSTF441 (N440). To introduce the C-terminal glycosylation acceptor site, the C-terminal end of OR83b was extended with the sequence K486PQSIYQKTMSFDKLIENSTQKT (C-term NST).
Site-specific mutagenesis was performed using the QuickChange™ Site-Directed Mutagenesis kit from Stratagene (La Jolla, USA). All mutants were confirmed by sequencing of plasmid DNA at BM labbet AB (Furulund, Sweden). All cloning steps were done according to standard procedures using restriction enzymes from Promega (Madison, USA).
Preparation of Drosophila rough microsomes
Drosophila S2 cells (ATCC: CRL-1963) from cultures growing in logarithmic phase were washed twice with PBS and once with buffer H (50 mM Hepes-KOH pH 7.4, 165 mM KOAc, 2 mM Mg(OAc)2) with centrifugation steps for 3 min at 200 g, 20°C. Cells were resuspended in 3 volumes buffer H containing 0.01% saponin (Sigma) and incubated 10 min at 20°C. Buffer H was added to decrease the saponin concentration to 0.002%, and the Drosophila rough microsomes (DRMs) were pelleted for 1 min at 2500 g, 20°C. DRMs were adjusted to 20 A280/ml with RM buffer (50 mM Hepes-KOH pH 7.6, 50 mM KOAc, 2 mM Mg(OAc)2, 250 mM sucrose, 1 mM DTT), CaCl2 and PMSF were added to a final concentration of 1 mM and 0.2 mg/ml, respectively. DRMs were incubated with 150 U/ml micrococcal nuclease (Nuclease S7 from Staphylococcus aureus, Roche) for 10 min at 25°C, and the reaction was stopped by the addition of EGTA to a final concentration of 2 mM. DRMs were layered on a sucrose cushion (50 mM Hepes-KOH pH 7.6, 50 mM KOAc, 2 mM Mg(OAc)2, 500 mM sucrose, 1 mM DTT) and separated by centrifugation for 30 min at 35.000 g, 4°C. The pellet was resuspended in RM buffer and DRMs adjusted to 100 A280/ml (i.e. 2 eq/μl).
Expression in vitro
Constructs in pGEM1 were transcribed and translated in the TnT Quick systems from Promega. 1μg DNA template, 1 μl [35S]-Met (15 μCi) and 1 equivalent of Drosophila (DRM) or 2 equivalents of dog pancreas rough microsomes (CRM) [6] were added at the start of the reaction, and samples were incubated for 90 min at 30°C [7]. Samples were analyzed by SDS-PAGE, and proteins were visualized in a Fuji FLA-3000 phosphorimager using the Image Reader V1.8J/Image Gauge V 3.45 software.
3. Results
Prediction models and topology assay
The Phobius [8], TMHMM [9], HMMTOP [10], Memsat 2.0 [11] and Toppred [12] algorithms all predict the same 7TM Nin-Cout topology for OR83b, Fig. 1A. In an attempt to experimentally map the topology of OR83b, we took advantage of a potential acceptor site for N-linked glycosylation (N169SS) present in the second predicted extracellular loop (EC2). N-linked glycosylation is a reliable topology marker, as the ER-resident oligosaccharide transferase enzyme can only transfer glycans to lumenally exposed parts of membrane proteins inserted into the ER [13]. OR83b was cloned behind the SP6 promoter in the pGEM1 vector and transcribed and translated in vitro in the absence or presence of DRMs. In the presence of DRMs, a more slowly migrating form of the protein was observed (Fig. 1B, compare lanes 1 and 2), which disappeared when the glycosylation site was mutated from N169SS (N169) to Q169SS (QSS) (lanes 3 and 4). We conclude that the EC2 loop is located in the lumen of the microsomes. This result also shows that the other two potential glycosylation sites (N33FT (N33) and N188AS (N188)) in OR83b are not utilized, possibly because they are located in a cytoplasmic part of the protein (N33FT) or too close to a transmembrane segment (N188AS) [14].
Figure 1.
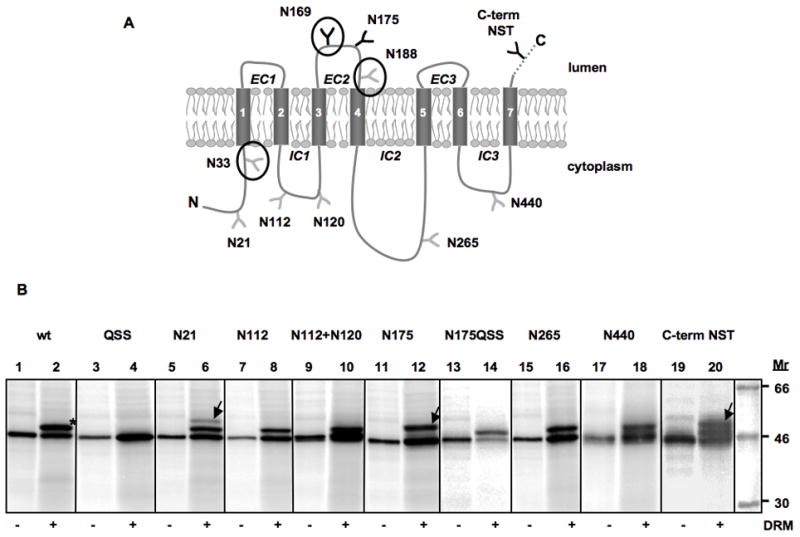
The topology of the Or83b protein. (A) Predicted topology of OR83b. Phobius [8] predicts an Nin-Cout topology with the following seven transmembrane helices: 50–68, 80–98, 137–158, 194–213, 350–371, 391–413, 462–483. Engineered and endogenous (N33FT, N169SS and N188AS encircled) putative glycosylation sites are indicated; sites that become modified upon insertion into DRMs are in black, non-modified sites are in gray. (B) Glycosylation mapping of OR83b. In vitro translation of OR83b wild type and variants with engineered glycosylation sites in the absence (-DRM) and presence (+DRM) of Drosophila rough microsomes. Molecules glycosylated only on the endogenous N169SS site are indicated by * and molecules glycosylated on an additional engineered site are indicated by arrows.
Topology mapping Or83b
Additional acceptor sites for N-linked glycosylation were engineered into the N-terminal tail of OR83b, as well as into loops IC1, IC2, IC3, and EC2. A 22-residue peptide (PQSIYQKTMSFDKLIENSTQKT) containing a glycosylation acceptor site (underlined) was also fused to the C terminus. The EC1 and EC3 loops were not targeted, as they are too short to be modified by the oligosaccharyl transferase [14,15]. The engineered glycosylation site (N175) in loop EC2 was efficiently modified in the presence of DRMs, resulting in a protein carrying two N-linked glycans (Fig. 1B, lanes 11 and 12). When the natural glycosylation site N169SS was mutated to N169QSS in this construct (N175QSS), only the N175 site was modified (lanes 13 and 14). The extended C-terminal tail (C-term NST) was also modified in the presence of DRMs (lanes 19 and 20); the somewhat lower modification efficiency of this site is probably caused by its location very close to the C terminus of the protein [16]. In contrast, none of the acceptor sites in loops IC1 (N112), IC2 (N265), or IC3 (N440) were modified. The engineered site (N21) in the N-terminal tail was mostly non-glycosylated, although a faint doubly glycosylated product (modified on both N21 and N169) was also seen for this construct (lanes 5 and 6). Since no faint doubly glycosylated bands were seen for the N112 and N112+N120 constructs (lanes 7–10), loop IC1 faces the cytosol in all molecules. Molecules glycosylated on N21 thus represent a minor, probably misfolded, fraction of the protein in which the first or second transmembrane segment does not span the membrane. Earlier work using GFP and YFP fusions and epitope staining is consistent with a cytosolic location of the N terminus and a lumenal location of the EC2 loop [2].
As a control, the constructs were also translated in the presence of mammalian (dog pancreas) RMs (Supplementary Fig. S1). The results were the same as those obtained with the Drosophila RMs, except that the glycosylated bands were hard to resolve for the construct with an extended C-terminal tail.
4. Discussion
The Drosophila OR83b protein is an ubiquitously expressed member of the insect odorant receptor family, and it forms functional heteromers with other OR proteins [2]. Mammalian odorant receptors are 7TM G-protein coupled receptors (GPCRs) with an extracellular N terminus, but there is no detectable sequence similarity between mammalian and insect ORs [2]. Recent work has tentatively located the N terminus of OR83b to the cytosol [2], arguing that this protein is not a classical GPCR. Given this rather surprising conclusion, we decided to perform a detailed study on the topology of OR83b using glycosylation mapping, an approach that has been widely applied to eukaryotic membrane proteins [17].
In short, our results confirm the suggested intracellular location of the N-terminal tail of OR83b and in addition show that the EC2 loop and the C-terminal tail are extracellular. We also find that glycosylation acceptor sites engineered into loops IC1, IC2, and IC3 are not modified by the lumenally disposed oligosaccharide transferase, in accordance with the proposed 7TM Nin-Cout topology [2].
When the available experimental data is used to constrain [18] the Phobius predictor, the predicted topology for OR83b is as shown in Fig. 2, i.e., essentially the same as predicted by the unconstrained Phobius, TMHMM, HMMTOP, Memsat 2.0, and Toppred algorithms (cf., Fig. 1). We conclude that OR83b has 7 transmembrane helices, an intracellular N terminus and extracellular C terminus, and is thus inverted compared to the canonical Nout-Cin 7TM topology of the GPCR family of mammalian odorant receptors.
Figure 2.
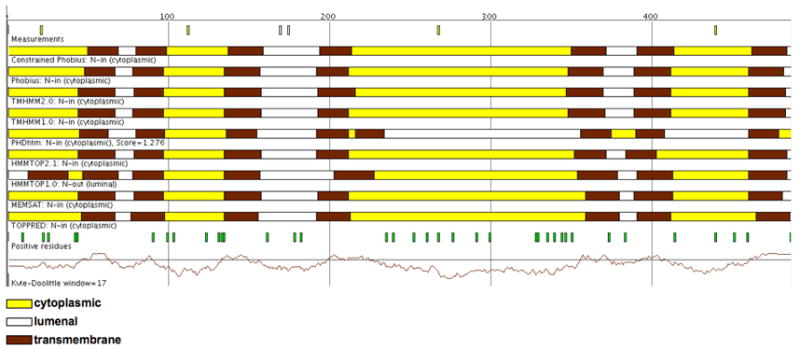
Predictions of OR83b transmembrane topology, adapted from the Sfinx metaserver (http://sfinx.cgb.ki.se; [19]). A Phobius prediction obtained by using the full set of glycosylation data as constraints is shown on top, below are the default predictions of the Sfinx server: unconstrained predictions from Phobius [8], TMHMM (1.0 and 2.0) [9], HMMTOP (2.1 and 1.0) [10], PHDhtm [20], Memsat 2.0 [11], and Toppred [12]. OR83b is predicted to have seven transmembrane helices with the N-terminus in the cytoplasm by eight of the nine methods. Predicted transmembrane segments (brown segments), cytoplasmic loops (yellow segments), and lumenal loops (white segments) are indicated above the respective prediction method.
Supplementary Material
Acknowledgments
We thank Prof. Bernard Dobberstein for advice concerning Drosophila microsomes and Prof. Arthur E. Johnson for providing dog pancreas microsomes. This work was supported by grants from the Swedish Cancer Foundation to IN and GvH, from the Swedish Research Council to GvH, from the Swedish Foundation for International Cooperation in Research and Higher Education (STINT) to IN, from Magnus Bergvalls Stiftelse, Henrik Granholms Stiftelse, and Carl Tryggers Stiftelse to IN, and from the NIH to JC.
The abbreviations used are
- OR
odorant receptor
- GPCR
G-protein coupled receptor
- TM
transmembrane
- DRM
Drosophila rough microsome
- CRM
column-washed dog pancreas rough microsomes
- IC
intracellular
- EC
extracellular
- ER
endoplasmatic reticulum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wistrand M, Kall L, Sonnhammer EL. A general model of G protein-coupled receptor sequences and its application to detect remote homologs. Protein Sci. 2006;15:509–21. doi: 10.1110/ps.051745906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–33. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 4.Kozak M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol Cell Biol. 1989;9:5073–80. doi: 10.1128/mcb.9.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsson I, Whitley P, von Heijne G. The COOH-terminal ends of internal signal and signal-anchor sequences are positioned differently in the ER translocase. J Cell Biol. 1994;126:1127–32. doi: 10.1083/jcb.126.5.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter P, Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- 7.Hessa T, et al. Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature. 2005;433:377–81. doi: 10.1038/nature03216. [DOI] [PubMed] [Google Scholar]
- 8.Käll L, Krogh A, Sonnhammer EL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 10.Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001;17:849–50. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- 11.Jones DT, Taylor WR, Thornton JM. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry. 1994;33:3038–49. doi: 10.1021/bi00176a037. [DOI] [PubMed] [Google Scholar]
- 12.von Heijne G. Membrane Protein Structure Prediction-Hydrophobicity Analysis and the Positive-Inside Rule. J Mol Biol. 1992;225:487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan HA, Welply JK, Lennarz WJ. Oligosaccharyl transferase: the central enzyme in the pathway of glycoprotein assembly. Biochim Biophys Acta. 1987;906:161–173. doi: 10.1016/0304-4157(87)90010-4. [DOI] [PubMed] [Google Scholar]
- 14.Popov M, Tam LY, Li J, Reithmeier RA. Mapping the ends of transmembrane segments in a polytopic membrane protein. Scanning N-glycosylation mutagenesis of extracytosolic loops in the anion exchanger, band 3. J Biol Chem. 1997;272:18325–32. doi: 10.1074/jbc.272.29.18325. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson I, von Heijne G. Determination of the Distance Between the Oligosaccharyltransferase Active Site and the Endoplasmic Reticulum Membrane. J Biol Chem. 1993;268:5798–5801. [PubMed] [Google Scholar]
- 16.Nilsson I, von Heijne G. Glycosylation efficiency of Asn-Xaa-Thr sequons depends both on the distance from the C terminus and on the presence of a downstream transmembrane segment. J Biol Chem. 2000;275:17338–43. doi: 10.1074/jbc.M002317200. [DOI] [PubMed] [Google Scholar]
- 17.von Heijne G. Membrane-protein topology. Nat Rev Mol Cell Biol. 2006;7:909–18. doi: 10.1038/nrm2063. [DOI] [PubMed] [Google Scholar]
- 18.Melen K, Krogh A, von Heijne G. Reliability measures for membrane protein topology prediction algorithms. J Mol Biol. 2003;327:735–44. doi: 10.1016/s0022-2836(03)00182-7. [DOI] [PubMed] [Google Scholar]
- 19.Sonnhammer EL, Wootton JC. Integrated graphical analysis of protein sequence features predicted from sequence composition. Proteins. 2001;45:262–73. doi: 10.1002/prot.1146. [DOI] [PubMed] [Google Scholar]
- 20.Rost B, Fariselli P, Casadio R. Topology prediction for helical transmembrane proteins at 86% accuracy. Protein Sci. 1996;5:1704–1718. doi: 10.1002/pro.5560050824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


