Abstract
Dictyostelium discoideum expresses multiple Gα subunits but only a single Gβ and Gγ subunit suggesting the specific response to an external signal depends largely on Gα subunit function or G protein-independent signaling from the receptor. To test the contribution of Gα subunit functional specificity, the chimeric Gα subunits, Gα2/4 and Gα5/4, were created and analyzed along with wild-type subunits for the ability to substitute for the Gα4 subunit in mediating responses from folate receptors. The Gα2/4 subunit, but not the Gα2 or Gα5/4 subunits, partly rescued chemotaxis and cGMP accumulation in folate-stimulated gα4− cells. Expression of the Gα5/4 or Gα5 subunits resulted in an inhibition of gα4− and wild-type cell movement and a reduced aggregate size in developing wild-type and gα5− cells suggesting these subunits mediate similar responses. Only the Gα4 subunit was capable of correcting developmental morphology in gα4− multicellular aggregates suggesting the chimeric Gα2/4 or Gα5/4 subunits were insufficient to provide the Gα4 function necessary for proper development. These results indicate Dictyostelium Gα subunit specificity is not limited to receptor coupling and that Gα subunit sequences outside of the carboxyl terminus are important for cell movement and developmental processes.
Keywords: signal transduction, G protein, receptor, Dictyostelium, development, chemotaxis, folate
Introduction
G protein-coupled receptors are capable of detecting a wide variety of extracellular signals that regulate many cellular activities, including metabolism, growth, differentiation and migration (Neves et al., 2002). Many responses are mediated through receptor activation of heterotrimeric G proteins allowing the Gα subunit and Gβγ dimer to separate and interact with other signaling components. The defining roles for specific G protein subunits is complicated in many eukaryotes by the different potential combinations of G protein subunits that can form a heterotrimeric unit and the diversity of interactions between specific G protein subunits and other signaling components such as receptors and downstream effectors (Albert and Robillard, 2002). Furthermore, some receptor-stimulated responses can occur in the absence of G protein subunits, indicating that some signaling occurs independently of G proteins (Brzostowski and Kimmel, 2001; Luttrell, 2005). While seemingly complex, the assessment of G protein subunit specificity can benefit from the analysis of signal transduction in cells with a relatively low number of potential G protein subunit combinations.
In many systems, Gα subunits have been demonstrated to be the critical determinants for receptor specificity whereas the Gβγ dimers often serve as the primary mediators of downstream signal transduction events that regulate cell division, chemotaxis, and gene regulation (Conklin et al., 1993; Hur and Kim, 2002). For instance, specific Gα subunits are necessary for mammalian cell chemotaxis but the role of these subunits appears to be primarily for the release of Gβγ dimers from chemoattractant receptors (Neptune and Bourne, 1997; Neptune et al., 1999). Likewise, the over-expression of the Gβ subunit in yeast activates cell cycle arrest and gene expression in the absence of mating pheromone and Gα subunit function (Cole et al., 1990). These observations highlight the importance of Gβγ dimer release but question the significance of Gα subunits in downstream signaling. However, some Gα subunits are known to interact with downstream signaling components (e.g., adenylyl cyclases, phosphodiesterases, phospholipases, etc.) other than Gβγ dimers implying additional roles for Gα subunits in at least some cellular responses (Offermanns and Simon, 1998).
The microbe Dictyostelium discoideum offers an excellent system for the genetic analysis of Gα subunit specificity and function because the genome contains 12 different genes for Gα subunits but only single genes for the Gβ and Gγ subunits (Brzostowski and Kimmel, 2001; Wu et al., 1995; Zhang et al., 2001). Therefore, all the heterotrimeric G proteins in Dictyostelium are predicted to contain the same Gβγ dimer and this prediction is supported by phenotypic analyses of gβ− mutants (Wu et al., 1995). Loss of some individual Gα subunits (e.g., Gα2, Gα4, or Gα5) results in a wide variety of developmental phenotypes indicating that the respective signal transduction pathways can regulate different cellular functions for the developmental life cycle of this organism (Brandon et al., 1997; Brzostowski et al., 2002; Hadwiger and Firtel, 1992; Hadwiger et al., 1996; Kumagai et al., 1991). The Gα4 subunit couples with folate receptors, mediates the chemotactic movement to folate but not to cAMP, and promotes spore development (Hadwiger et al., 1994). Cells lacking the Gα4 subunit can aggregate when starved but then terminate development prior to fruiting body formation with deficiencies in spore development (Hadwiger and Firtel, 1992). In contrast, the loss of the Gα5 subunit results in enhanced chemotactic responses to folate and large aggregates with delayed anterior tip formation (Hadwiger et al., 1996; Natarajan et al., 2000). Over-expression of the Gα5 subunit mildly inhibits responses to folate responses, decreases aggregate size, and accelerates tip formation whereas over-expression of the Gα4 subunit increases aggregate size and inhibits anterior tip formation. While the signal that stimulates the Gα5-mediated pathway remains to be identified it appears to promote developmental processes opposite of those promoted by the Gα4-mediated pathway. The Gα2 subunit couples to cAMP receptors, mediates chemotaxis to cAMP but not to folate, and allows for chemotactic aggregation of starved cells (Kumagai et al., 1991). The Gα2 subunit is also likely to function after aggregation as suggested by the developmental expression of this subunit and the requirement for cAMP receptors in later stages of development (Firtel, 1996). The Gα2 and Gα4 subunits are required for the accumulation of both cGMP and cAMP in response to external signals but the accumulation of at least cAMP is thought be mediated through the release of Gβγ dimers (Hadwiger et al., 1994; Kumagai et al., 1991; Wu et al., 1995). The mutant phenotypes resulting from the absence or over-expression of Gα subunits indicate differences between signaling pathways but do not determine a specific role for Gα subunits in downstream signaling because the functions of the receptor and Gβγ dimer can be dependent on specific Gα subunit-receptor coupling. Direct interactions of between G protein subunits with downstream signaling components have not been established in Dictyostelium.
Many previous studies have shown that the carboxyl terminus of Gα subunits is the primary determinant of receptor coupling (Brown et al., 2000; Conklin et al., 1993; Conklin et al., 1996; Coward et al., 1999; Neptune et al., 1999). Chimeric Gα subunits with changes in the last several amino acid residues at the carboxyl terminus (typically within the last 12 residues) are sufficient to alter receptor specificity allowing Gα subunits to gain functional interactions with different mammalian hormone receptors or yeast mating type receptors. In one case, a chimeric Gαq subunit with a carboxyl terminus resembling that of Gαi subunits was shown to mediate chemotaxis to signals that stimulate Gαi-specific receptors (Neptune and Bourne, 1997). In this study, Dictyostelium Gα subunit function and specificity were examined using the chimeric Gα subunits, Gα2/4 and Gα5/4, that contain a carboxyl terminus specific to the Gα4 subunit so as to allow the chimeric subunits to interact with Gα4-specific receptors. These chimeric Gα subunits, along with wild-type Gα subunits, were tested for the ability to compensate for the absence of the Gα4 subunit with respect to developmental morphogenesis, chemotaxis, and cGMP accumulation. The Gα subunits were also analyzed for the ability to rescue the loss of Gα specific function in gα2− and gα5− cells. The results of this study support critical roles for Gα subunits in chemotaxis and development in addition to the release of the Gβγ dimers from specific receptors.
Materials and methods
Strains and Media
Analysis of Gα subunit function was conducted in the Dictyostelium strain KAx-3 and Gα subunit gene disruption mutants derived from this strain. The creation of gα4−, gα2−, and gα5− strains was previously described (Hadwiger and Firtel, 1992; Hadwiger et al., 1996; Kumagai et al., 1991). Dictyostelium were grown in axenic medium HL5 or on bacterial lawns (Watts and Ashworth, 1970). DNA vectors were electroporated into Dictyostelium as previously described except the healing solution treatment omitted (Dynes and Firtel, 1989). Transformed cells were selected and maintained in medium containing 4–10 μg/ml of the drug G418 but cells were grown in the absence of the drug at least 18 hours prior to analysis. Folate solutions were prepared by neutralizing folic acid with NaHCO3.
Recombinant DNA Constructs and Nucleic Acid Detection
Chimeric Gα subunit genes were created by PCR amplification of cloned Gα subunit genomic or cDNA sequences using oligonucleotides with HindIII (upstream) or XbaI (downstream) restriction sites. The upstream oligonucleotide used in the amplification of Gα2 and Gα2/4 subunits was (gccggcaagCTTAAAAAATGGGTATTTGTGCATCATCAATGGAAGGAG) and the downstream oligonucleotides were (cggcgctctagaTTAAGAATATAAACCAGCTTTCATAACACATTG) and (cggcgctctagattagaagtgttctaatgcttgagatAAAATAATATCTTTTACAGCTTCAAAAACG), respectively (lower case nucleotides differ from those in the template DNA). The upstream oligonucleotide used in the amplification of the Gα5 and Gα5/4 subunits was (gccggcaagcTTAAAAAGATGGGTTGTATATTAACAATTGAAGCAAAGAAATC) and the downstream oligonucleotides were (cggcgctctagaTTAATAATTTATGATTGTATTAAAGATATTTTTTAATAAAG) and (cggcgctctagattagaagtgttctaatgcttgagaTAATAAAGTTTCTCTAACAGCATGAAATAC), respectively. The upstream and downstream oligonucleotides used in the amplification of the Gα4 subunit were (gccggcaagcttAAAAAAATGAGATTCAAGTGTTTTGGATCAGAAGAAACTG) and (cggcgctctagaTTAGAAGTGTTCTAAtGCTTGAGATAAAATTGTTTGTCTAAC), respectively. All of the downstream oligonucleotides coding for the Gα4 subunit carboxyl terminus had a silent mutation to remove a HindIII site to facilitate the cloning of the PCR products. Each amplified Gα subunit gene was sequenced to screen out any PCR-related mutations and then inserted into the Dictyostelium expression vector pDXA-GFP2 (Ddp2-based plasmid), replacing the HindIII/XbaI fragment that contained the GFP2 reading frame downstream of the act15 promoter (Levi et al., 2000). These vectors integrate into the genome unless the cell also contains the pREP vector. All pact15::Gα gene constructs were also transferred as SalI/XbaI fragments into the Ddp1-based pTX-GFP vector replacing the pact15::GFP2 gene (Levi et al., 2000). Each Gα subunit expression vector was electroporated into Dictyostelium and multiple transformants were examined for folate chemotaxis, growth on bacterial lawns, and multicellular development. The Ddp2-based Gα subunit expression vectors were also co-electroporated with the pREP vector allowing the Ddp2-based vectors to be maintained as extrachromosomal plasmids. Total RNA was isolated from Dictyostelium and then RNA blots and hybridizations were performed as described (Hadwiger et al., 1996).
Development and chemotaxis assays
Dictyostelium were grown to mid-log phase (approximately 2 × 106 cells/ml), washed twice in phosphate buffer (12 mM NaH2PO4 adjusted to pH 6.1 with KOH), and suspended in phosphate buffer before spotting on nonnutrient plates (phosphate buffer, 1.5% agar) for development or chemotaxis as described (Hadwiger et al., 1996). Chemotaxis assays were performed by spotting droplets of cell suspensions (107 to 108 cell/ml) on non-nutrient plates followed by the spotting of 1 μl droplets of folate solutions (10−2–10−4 M) approximately 2–3 mm away from the cell droplet. Cell movement was monitored with a dissecting microscope and a digital camera. Cell density and folate concentrations were varied experiments but all strains were treated identically for each experiment.
Cyclic GMP assays
Dictyostelium were grown to mid-log phase (approximately 2 × 106 cells/ml), washed twice in phosphate buffer and suspended in phosphate at 5 × 107 cells/ml. Cell suspensions were bubbled with air for 10 minutes prior to treatment with 100 μM folate. At times indicated cellular responses were terminated by the addition of perchloric acid and then the samples were neutralized with ammonium sulfate as described (Hadwiger et al., 1994). The concentration of cGMP in each sample was determined using a radioimmunoassay kit (Amersham).
Results
Creation and expression of chimeric and wild-type Gα subunits
The distinct chemotactic and developmental phenotypes associated with the loss of either Gα2, Gα4, or Gα5 subunits in Dictyostelium suggest these subunits and/or their respective coupled-receptors provide signaling specificity in chemotactic and developmental pathways but the extent of Gα subunit contribution to the specificity of these pathways beyond receptor coupling has not previously been determined. Sequence comparison between the Dictyostelium Gα2, Gα4 and Gα5 subunits indicates a slightly greater overall sequence similarity between the Gα4 and Gα5 subunits and this is similarity is also indicated by a phylogenetic analysis of Dictyostelium Gα subunits (Fig. 1A and B) (Chisholm et al., 2006; Eichinger et al., 2005). However, the Gα4 and Gα2 subunits share some identity between residues that are distinct from those found at the same positions in the Gα5 subunit, including a cluster of residues near the amino terminus. All three subunits share considerable identity with each other in regions highly conserved among all Gα subunits (Simon et al., 1991). Each of these Dictyostelium Gα subunits differs from each other in the last several residues of the carboxyl terminus consistent with the coupling of these subunits to different receptors. All of the Dictyostelium Gα subunits are expected to interact with the same Gβγ dimer because only single genes for these subunits are found in BLAST searches of the sequenced Dictyostelium genome (Eichinger et al., 2005).
Fig. 1.
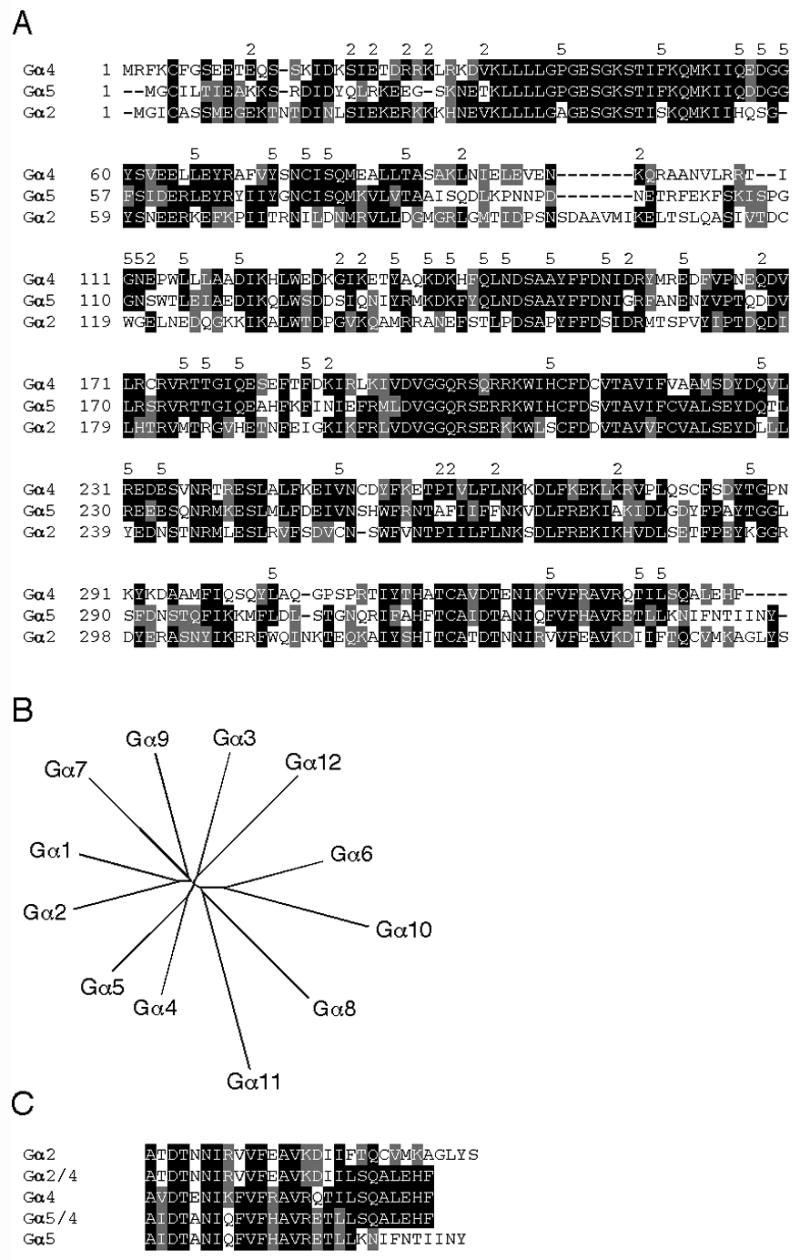
Comparison of Dictyostelium Gα subunits. (A) Alignment of Dictyostelium Gα4, Gα5 and Gα2 subunit amino acid sequences using ClustalW program and formatted using BOXSHADE program to highlight identical (black background) and similar residues (gray background). The number (2) above the aligned sequences indicates residues identical between the Gα4 and Gα2 but not Gα5 subunit. The number (5) above the aligned sequences indicates identity between the Gα4 and Gα5 but not Gα2 subunit. (B) Unrooted phylogenic tree of Dictyostelium Gα subunits generated at http://clustalw.genome.ad.jp/ by the neighbor-joining method from a ClustalW alignment. Sequence information was obtained using dictyBase at http://www.dictybase.org/. (C) Alignment of wild-type and chimeric Gα subunit carboxyl terminal amino acid sequences formatted using the BOXSHADE program as described above.
To determine if regions other than the carboxyl terminus of Dictyostelium Gα subunits provide functional specificity, the carboxyl terminus of the Gα2 and Gα5 subunits were replaced with the corresponding sequence specific to the Gα4 subunit (Fig. 1C). In these chimeric Gα genes, only the sequence coding for the last 12 residues of the Gα2 subunit and the last 8 residues of the Gα5 subunit were replaced with the corresponding Gα4 sequence because the adjacent sequences coded for conserved residues (Brown et al., 2000). To eliminate distinctions in Gα subunit expression, each chimeric subunit gene, as well as each wild-type subunit gene, was inserted into a Dictyostelium Ddp1-based expression vector downstream of the act15 promoter. Others have shown gene expression from this vector to be relatively homogeneous among individual cells within a clonal population of cells (Levi et al., 2000). RNA blot analysis indicated the expression the level of the Gα subunit transcripts was abundant for all constructs in this Ddp1-based expression vector (Fig. 2). Expression of these Gα subunit genes from integrating Ddp2-based vectors gave similar phenotypes but with more variability in the strength of the phenotype from clone to clone suggesting that differences in gene copy number might be the basis for such variability (data not shown except where noted).
Fig. 2.
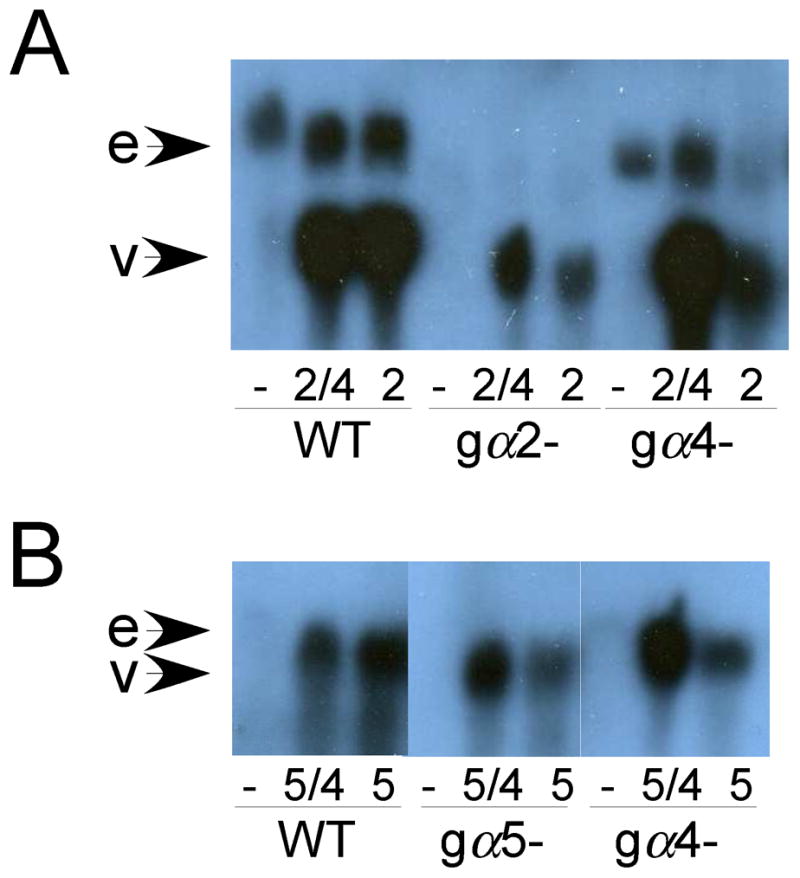
Expression of Gα subunit transcripts in wild-type and Gα subunit mutants. Total RNA was isolated from cells in late log phase of growth and subjected to RNA blot analysis using Gα2- or Gα5-specific probes as describe in Materials and methods. Each lane was loaded with 4 μg of total RNA. All lanes from each panel were run on same gel and then blotted and hybridized together but the scanned image of the autoradiograph was spliced to bring relevant lanes together. (A) Endogenous Gα2 (e) and vector-expressed Gα2/4 or Gα2 transcripts (v) in wild-type, gα2−, and gα4− cells with no vector (−), the Gα2/4 vector (2/4), or the Gα2 vector (2). (B) Endogenous Gα5 (e) and vector-expressed Gα5/4 or Gα5 transcripts (v) in wild-type and gα4− cells with the Gα5/4 vector (5/4), Gα5 vector (5), or no vector (−). Variability in the level of vector-expressed transcripts is likely due to gel loading inconsistencies but in each case the level of vector-expressed transcripts is higher than the level of endogenous gene transcripts. Endogenous Gα2 and Gα5 transcripts were more visible upon longer exposures.
Analysis of developmental morphology
Expression of Gα subunit genes from a heterologous promoter (e.g., act15) rather than Gα-specific promoters could potentially alter the spatial and temporal distributions of Gα subunits during development and possibly affect the ability of the Gα expression vectors to rescue phenotypes in Gα subunit mutants. Therefore, Gα subunit expression vectors were introduced into strains with Gα subunit gene knockout strains (gα2−, gα4−, or gα5− mutants) and then examined for the ability to complement Gα subunit-specific developmental phenotypes. Only the Gα4 subunit expression vector complemented gα4−cells restoring wild-type morphological development (Fig. 3). The Gα2/4, Gα2, Gα5/4, or Gα5 subunit expression vectors failed to complement or noticeably change the terminal gα4− morphology of arrested aggregates with extended tip structures. The Gα2 but not the Gα2/4 subunit expression vector was capable of restoring aggregate formation and fruiting body development in gα2− cells (Fig. 4). The formation of cell aggregates was slightly delayed but subsequent morphological development was similar to wild-type aggregates. Consistent with this result, gα2− cells carrying the Gα2 but not the Gα2/4 subunit expression vector were capable of chemotactic movement to cAMP (data not shown). The Gα5 and Gα5/4 subunit expression vectors were both capable of rescuing the large aggregate phenotype of gα5− cells and producing a precocious tip development phenotype associated with the over-expression of Gα5 function (Fig. 5). The Gα5 and Gα5/4 subunit expression vectors also produced similar small aggregate and precocious tip development phenotypes in gα4− and wild-type cells (data not shown). The ability of Gα5/4 subunit to provide phenotypes similar to the Gα5 subunit suggested that Gα5/4 subunit might be activated by receptors that recognize the Gα4-specific carboxyl terminus.
Fig. 3.
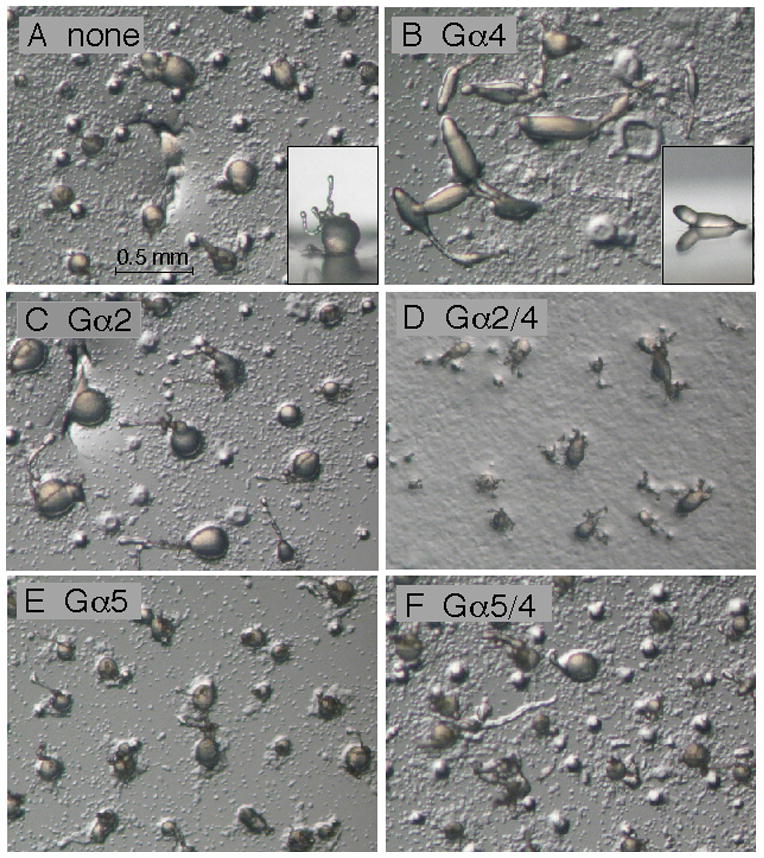
Development of gα4− cells with or without Gα subunit expression vectors. Cells were grown in axenic medium and washed in phosphate buffer and then cell suspensions (2 × 108 cells/ml) were plated for development as described in the Materials and methods section. Panel (A) aggregates of gα4− cells with no Gα subunit expression vector. Insert displays a side view of a typical aggregate with the round mound and extended tip morphology. Panel (B) aggregates of gα4− cells with Gα4 subunit expression vector in the early stages of culmination. Insert displays a side view of a typical aggregate displaying wild-type slug morphology. Panel (C) gα4− cells with Gα2 subunit expression vector. Panel (D) gα4−cells with Gα2/4 subunit expression vector. Panel E: gα4− cells with Gα5 subunit expression vector. Panel F: gα4− cells with Gα5/4 subunit expression vector. Developing cell aggregates were photographed 23 hrs after initial plating using a dissecting microscope (20X magnification).
Fig. 4.
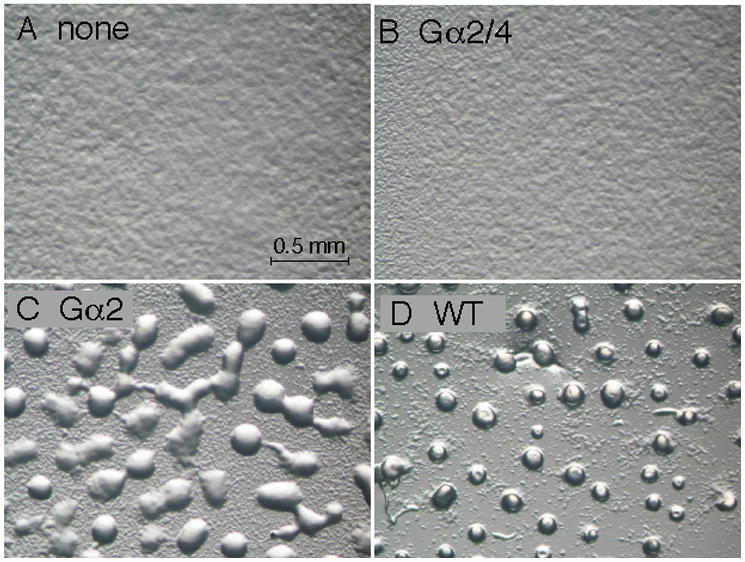
Development of gα2− cells with or without Gα subunit expression vectors. Cells were grown in axenic medium and washed in phosphate buffer and then cell suspensions (2 × 108 cells/ml) were plated for development as described in the Materials and methods section. Panel (A) gα2− cells with no Gα subunit expression vector. Panel (B) gα2− cells with Gα2/4 subunit expression vector. Panel (C) aggregates of gα2− cells with Gα2 subunit expression vector. Panel (D) aggregates of wild-type cells with no Gα subunit expression vector. Developing cells were photographed 12 hrs after initial plating using a dissecting microscope (20X magnification).
Fig. 5.
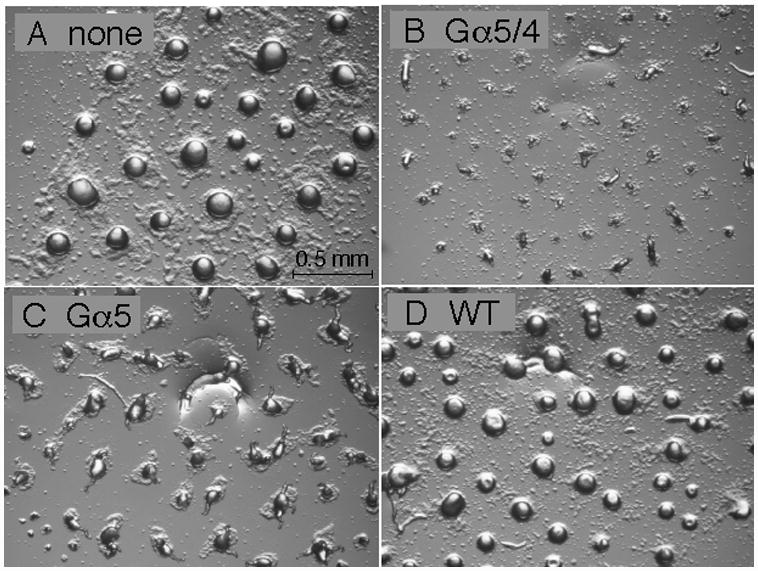
Development of gα5− cells with or without Gα subunit expression vectors. Cells were grown in axenic medium and washed in phosphate buffer and then cell suspensions (2 × 108 cells/ml) were plated for development as described in the Materials and methods section. Panel (A) aggregates of gα5− cells with no Gα subunit expression vector. Panel (B) aggregates of gα5− cells with Gα5/4 subunit expression vector. Panel (C) aggregates of gα5− cells with Gα5 subunit expression vector. Panel (D) aggregates of wild-type cells with no Gα subunit expression vector. Developing cell aggregates were photographed 12 hrs after initial plating using a dissecting microscope (20X magnification).
Chemotaxis to folate
To examine Gα subunit specificity in cellular responses, gα4− and wild-type cells with or without Gα subunit expression vectors were assayed for chemotaxis to folate. Chemotaxis assays were conducted on a nonnutrient agar surface where droplets of cells and folate solution are placed in close proximity of each other resulting in the chemotactic migration of cells toward the source of folate (Fig. 6A). The chemotaxis index (provided as a percentage) in this assay was defined as the number of cells migrating toward the source of folate divided the total number of cell migrating away from the original cell droplet location. Using this definition, chemotactic cells are expected to have a chemotaxis index significantly greater than 50% whereas cells without chemoattractant-directed movement are expect to have a chemotaxis index of near 50%. Only gα4− cells carrying the Gα4 or Gα2/4 subunit expression vectors displayed a chemotaxis index substantially larger than 50% suggesting that the other Gα subunit vectors were not capable of rescuing chemotactic movement (Fig. 6B). The distance traveled by cells expressing the Gα2/4 subunit was noticeably less than that exhibited by cells expressing the Gα4 subunit. This reduced migration distance of cells expressing the Gα2/4 subunit could possibly be due to a decrease in cell speed across the substratum and/or an increase in cell turning. To examine these possibilities, cell movement during chemotaxis assays was monitored by time-lapsed photography and paths of individual cells were mapped over a 30 min period (Fig 6C). Cells were plated at very low densities and only cells nearest to the source of folate were monitored. Only a few cells were examined in each strain but the migration distance of cells expressing the Gα4 subunit appeared to be much greater at each interval (5 minutes) than that observed for all other strains, regardless of the direction of movement. Cells expressing the Gα2/4 subunit migrated distances similar to cells without a Gα subunit expression vector and cells expressing the Gα5/4 subunit traveled the shortest distances each interval. In general, the paths observed for each strain appeared relatively similar with respect to changes in cell direction suggesting that cell speed is likely to account for much of the differences in cell migration distance. The maximum distance traveled by each strain toward the folate source was also examined by measuring the distance from the original cell droplet perimeter to the leading edge of cells after a specific length of time. The distance traveled by gα4− cells with the Gα4 subunit was substantially greater than that traveled by the other strains consistent with the Gα4 subunit providing a greater migratory speed than the other Gα subunits (Fig. 7A). The distance traveled by cells expressing the Gα5/4 or Gα5 subunit was less than that observed for gα4− cells without a Gα subunit expression vector suggesting these subunits actually inhibit cell migration. This inhibition of cell migration was also observed for wild-type cells with either the Gα5/4 or Gα5 subunit expression vector (Fig. 7B). The Gα2/4 or Gα2 subunit expression vectors only had marginal effects on the distance traveled by wild-type cells.
Fig. 6.
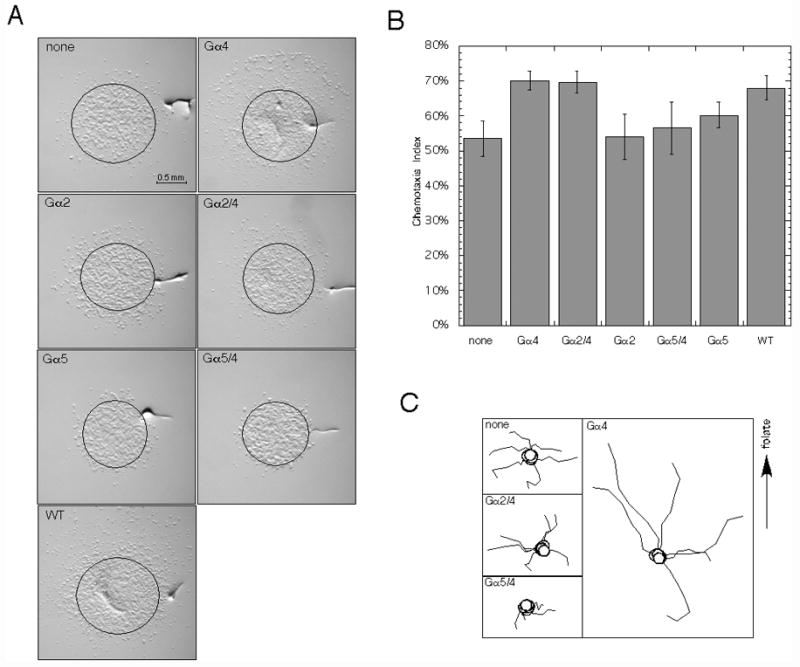
Chemotaxis of gα4− cells with or without Gα subunit expression vectors. Cells were grown in axenic medium, washed in phosphate buffer, and plated for chemotaxis as described in the Materials and methods section. Droplets of cell suspensions (2 × 107 cells/ml) were spotted onto nonnutrient plates followed by the spotting of one microliter droplets of 1 mM folate solution approximately 2–3 mm away from the cell droplet. (A) Micrographs of chemotaxis assays. In all panels the source of folate is above (upper side) cell droplet. Deformations in agar were created by a needle and used as a reference points to align images captured at different times during the assay. Cell distribution was photographed 3 hrs later using a dissecting microscope (20X magnification) and indirect light. Original cell droplet perimeter is indicated by drawn circle. Developing cell aggregates were photographed 23 hrs after initial plating using a dissecting microscope (20X magnification). Data is representative of one of several assays with similar results. (B) Chemotaxis index of gα4− cells with or without Gα subunit expression vectors. Cell suspensions (1 × 107 cells/ml) were plated for chemotaxis assays and cell distribution was photographed after 3 hrs. Chemotaxis index was determined as the number cells outside the original drop perimeter on the side facing the source of folate divided by the total number of cells outside the original droplet perimeter. Each index was determined as the mean of six separate droplets. Data is representative of one of three different experiments with similar results and the vertical error bars represent the standard deviation. (C) Migration maps of gα4− cells with or without Gα subunit expression vectors. Cell suspension (5 × 106 cells/ml) were plated for chemotaxis assays as described above and then 2.5 hours after the initial plating time-lapse photography was used to follow the movement of cells over a 30 minute period. Images corresponding to 5 min intervals were used to trace the movement of individual cells. Only cells with minimal or no cell-cell contact were chosen for creating maps. Multiple paths representing different cells of the each strain are shown in each panel and the initial point of each path is designated with an open circle. All initial points were positioned at a central location in each panel with the orientation of the folate source at the top of each panel. Data is representative of one of two experiments with similar results. In all panels and graph, gα4− cells without Gα subunit expression vector (none), gα4− cells with the Gα2/4 subunit (Gα2/4), Gα2 subunit (Gα2), Gα5/4 subunit (Gα5/4), or Gα5 subunit (Gα5) expression vectors, and wild-type cells with no Gα subunit expression vector
Fig. 7.
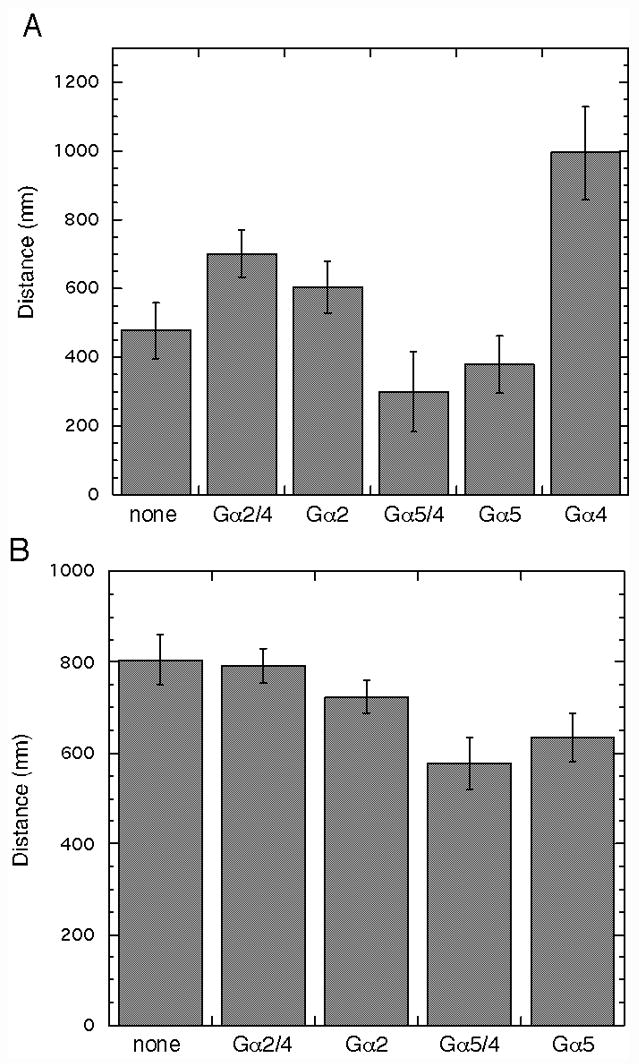
Chemotaxis distance of gα4− or wild-type cells with or without Gα subunit expression vectors. Chemotaxis assays were conducted as described in Figure 6 and the maximum distance from the original cell droplet perimeter to the leading edge of migrating cells in the direction of the folate source was recorded. (A) Chemotaxis distance traveled by gα4− cells without a Gα subunit expression vector (none) and cells with the Gα2/4 subunit (Gα2/4), Gα2 subunit (Gα2), Gα5/4 subunit (Gα5/4), Gα5 subunit (Gα5), or Gα4 subunit (Gα4) expression vectors after 3.5 hrs. (B) Chemotaxis distance traveled by wild-type cells without a Gα subunit expression vector (none) and cells with the Gα2/4 subunit (Gα2/4), Gα2 subunit (Gα2), Gα5/4 subunit (Gα5/4), or Gα5 subunit (Gα5) expression vectors after 3 hrs. Each value is the mean of six independent cell droplets. Error represents the standard deviation. Data is representative of one of at least two assays with similar results.
Some Dictyostelium mutants, such as gα4− or gβ− cells, do not chemotax to folate and they also form small plaques with slow expansion rates when grown on bacterial lawns (Hadwiger et al., 1994; Wu et al., 1995). These mutants also have less accumulation of cells on the plaque perimeter, probably reflecting a reduced capacity to migrate toward the bacterial food source surrounding the plaque. Several clones of wild-type cells expressing the Gα5/4 subunit from the integrating Ddp2-based vector displayed a reduction in the accumulated cells on the plaque perimeter and slightly retarded plaque expansion rate as compared to wild-type cells without a Gα subunit expression vector (Fig. 8A). These phenotypes were not observed for wild-type cells carrying any of the other Gα subunit expression vectors, including the Gα2/4 subunit expression vector. The impact of the Gα5/4 subunit on cell accumulation at the plaque perimeter is consistent with the ability of this Gα subunit to inhibit chemotactic responses to folate. However, cells expressing the Gα5/4 subunit were capable of aggregating and completing fruiting body development (Fig. 8B) with viable spores whereas aggregates of gα4− cells terminate development with aberrant morphology and a severe reduction in viable spores. The slow plaque growth rate, reduced cell accumulation on the perimeter, and aberrant developmental morphology of gα4− cells on bacterial lawns was not altered by the expression of the Gα5/4 or Gα2/4 subunit suggesting neither subunit can provide sufficient Gα4 subunit function to correct these phenotypes (Fig. 8A).
Fig. 8.
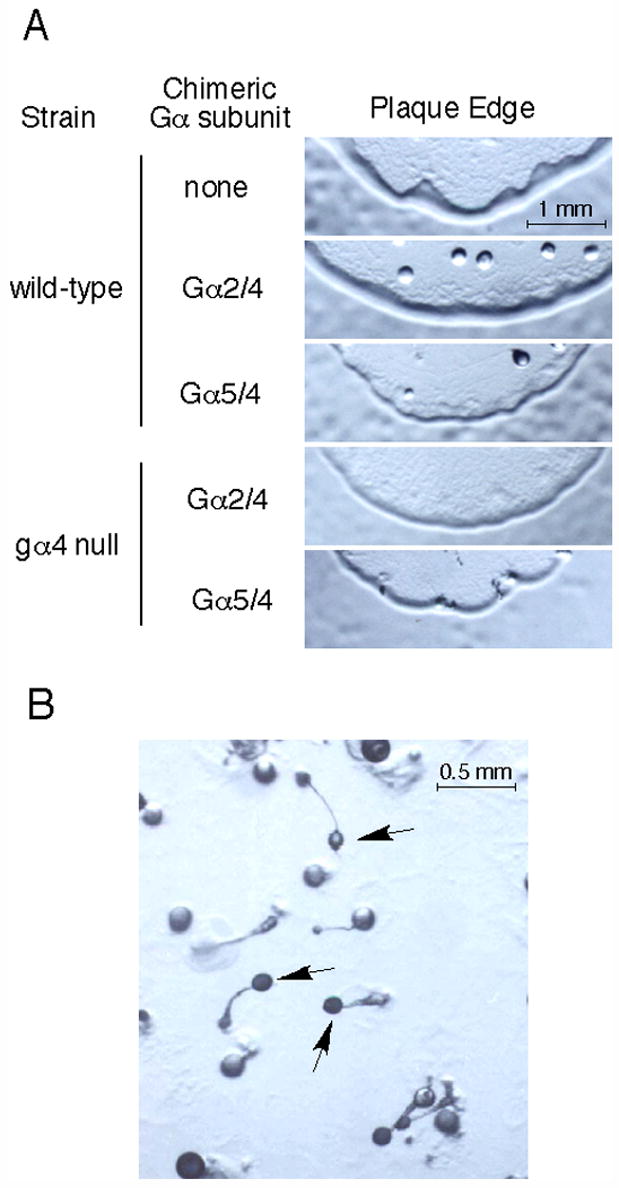
Growth and development phenotypes on bacterial lawns. (A) Plaque edge phenotype of wild-type and gα4− cells with chimeric Gα subunit vectors on bacterial lawns. Wild-type cells without Gα subunit (none) expression vector or wild-type and gα4− cells with the Gα2/4 subunit (Gα2/4) or Gα5/4 subunit (Gα5/4) expression vector were placed on a bacterial lawns and allowed to form plaques. Plaque edges were photographed after 4 days using the same magnification (20X) on a dissecting microscope. (B) Wild-type cells with the Gα5/4 subunit expression vector developing within a plaque on a bacterial lawn. Developing aggregates were photographed using a dissecting microscope (40X). Arrows indicate mature fruiting body formation.
cGMP accumulation
Folate stimulates a rapid accumulation of cGMP in Dictyostelium and this response is dependent on Gα4 subunit function (Fig. 9). Expression of the Gα2/4 but not the Gα2 subunit partly restores this response in gα4− cells but the rise in cGMP concentration was much less than that observed for wild-type cells. While lower in amplitude, the accumulation of cGMP in gα4− cells expressing the Gα2/4 subunit occurred with kinetics similar to those observed for wild-type cells. An increase in cGMP concentration was not observed for gα4− cells with the Gα5/4 or Gα5 subunit expression vector, suggesting the Gα5/4 subunit is not sufficient to rescue the stimulation of cGMP accumulation. However, cells with either of these Gα subunits displayed a higher basal level of cGMP than did cells without any of the Gα subunit expression vectors.
Fig. 9.
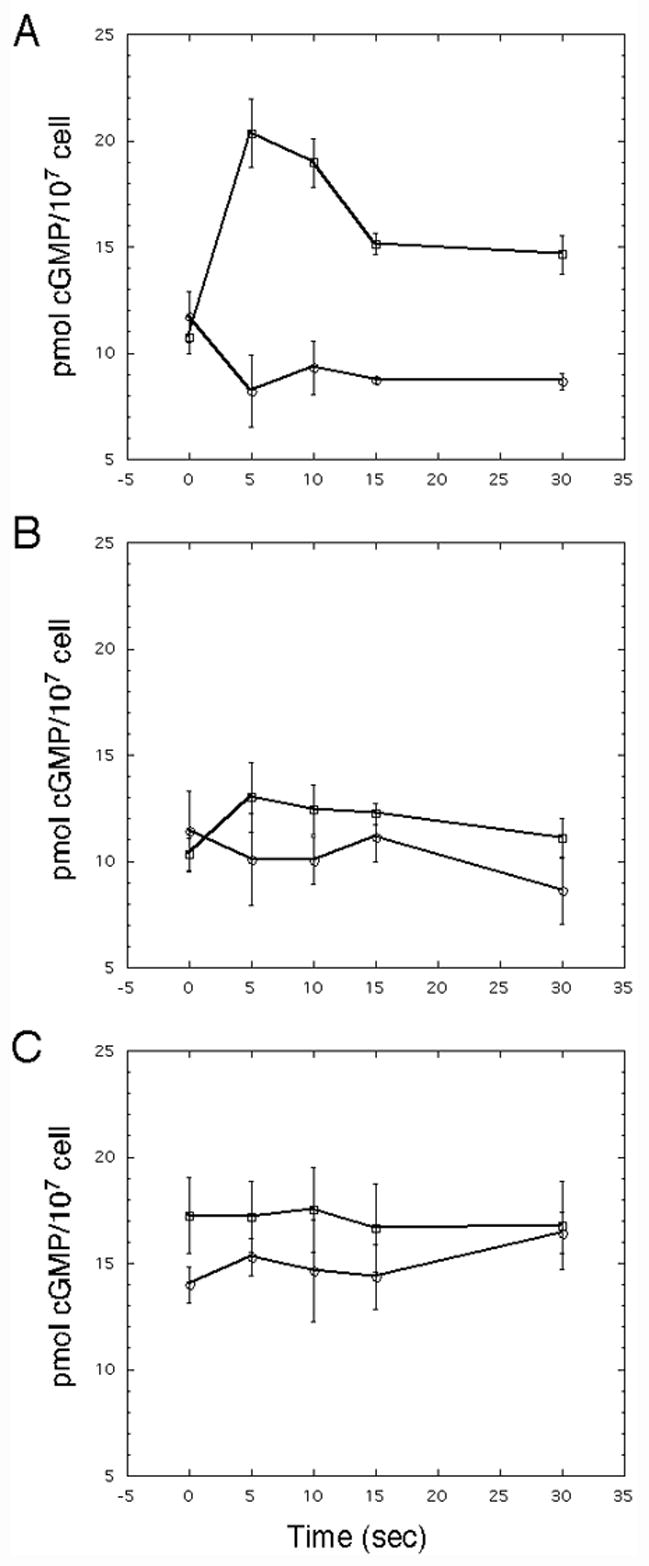
Folate-stimulated cGMP accumulation in gα4− cells with or without Gα expression vectors. Cells were grown, harvested, and stimulated with folate as described in the Materials and methods section. (A) cGMP accumulation in gα4− cells with the Gα4 subunit (open squares) or without a Gα subunit (open circles) expression vector. (B) cGMP accumulation in gα4− cells with the Gα2/4 subunit (open squares) or Gα2 subunit (open circles) expression vector. (C) cGMP accumulation in gα4− cells with the Gα5/4 subunit (open squares) or Gα5 subunit (open circles) expression vector. Concentration of cGMP was normalized with respect to cell extract protein concentration and each cell extract was assayed in duplicate. The data represent the average of three individual experiments and the vertical error bars represent deviation from the mean value.
Discussion
The phenotypic analysis of developing Gα mutant and wild-type cells containing the chimeric Gα2/4 or Gα5/4 subunits suggests that the functional specificity of these subunits impacts downstream interactions and not just the release of the Gβγ dimer from a specific receptor, consistent with the studies of similar chimeric Gα subunits in other organisms (Conklin et al., 1993; Conklin et al., 1996). The Gα5 over-expression phenotypes (i.e., small aggregates with precocious tip formation and folate chemotaxis inhibition) observed in gα5− and wild-type cells expressing the Gα5/4 subunit support this idea. The ability of the Gα5/4 subunit to produce these developmental phenotypes could potentially result from the activation of Gα5-specific function of this subunit by Gα4-specific receptors. This possibility cannot be confirmed without evidence of direct subunit activation by Gα4-specific receptors. However, the strong similarities in temporal and spatial parameters of signal transduction pathways mediated by the Gα5 and Gα4 subunits allow for the possibility that the endogenous signals activating these pathways might be available to the same subset of cells during the various stages of development (Hadwiger and Firtel, 1992; Hadwiger et al., 1991; Hadwiger et al., 1996). Alternatively, the developmental phenotypes might result from the Gα5/4 subunit acting as a dominant-negative component in Gα4-mediated signaling pathway. However, the Gα5/4 only inhibits chemotaxis and reduces aggregate size but does not alter subsequent morphological development suggesting the Gα5/4 subunit only affects some Gα4-specific functions. The inability of the Gα2/4 subunit to rescue aggregation of gα2− cells is likely due to the lack of coupling to cAMP receptors because these cell were incapable of chemotaxis to cAMP. Neither the Gα2/4 or Gα5/4 subunit could rescue developmental Gα4 function in gα4− cells but this observation agrees with chimeric Gα subunit function not being defined solely by the coupled receptor. The failure of Gα2/4 and Gα5/4 subunits to rescue development in gα4− cells cannot be explained by insufficient expression from a heterologous promoter because the same expression vectors provided sufficient wild-type Gα subunits (i.e., Gα4, Gα2, and Gα5) to restore wild-type development to the respective Gα subunit mutants.
The similar chemotactic indexes of gα4− cells expressing the Gα2/4 or Gα4 subunit suggest the Gα2/4 subunit can partially restore directional movement in folate gradients but the extent of the migration is significantly compromised. The very limited folate-stimulated cGMP response in cells expressing the Gα2/4 subunit is also consistent with this compromised chemotactic response. Given that the Gα4 and Gα2 subunits mediate chemotaxis for different cell fates (foraging versus aggregation, respectively) the failure of the Gα2/4 subunit to fully rescue folate chemotaxis might be attributed to inefficient coupling of the Gα2/4 subunit with the folate receptors or to Gα2/4 interactions with downstream signaling components that potentially desensitize responses to folate. The desensitization of folate responses by Gα2 function is consistent with the reduction in folate chemotaxis as the Gα2 subunit mediates chemotactic responses to cAMP at the onset of aggregation (De Wit and De Wit, 1986). Mechanisms of heterologous desensitization in Dictyostelium are poorly understood but Gα subunits could potentially play important roles in this process.
The ability of the Gα5/4 subunit to inhibit wild-type cell migration to folate is probably not due to an interference in the coupling of the Gα4 subunit to folate receptors because the inhibition of migration also occurs in gα4− cells. Consistent with this idea, activating mutations that impair GTPase activity and receptor association in Gα subunits produce Gα5 subunits (Gα5* or Gα5/4*) that are very effective in inhibiting chemotaxis to folate and also cAMP (Watson and Hadwiger, unpublished results, (Srinivasan et al., 1999)). Analogous activating mutations in the Gα4 or Gα2 subunit also inhibit folate and cAMP chemotaxis suggesting the constitutive activation of Gα subunits adversely affects chemotactic signaling. These observations suggest that the inhibition of migration is dependent on downstream Gα5/4 subunit function rather than specific receptor associations. The unexpectedly high basal level of cGMP in gα4− cells expressing the Gα5/4 or Gα5 subunit could possibly inhibit chemotaxis by altering myosin assembly through the cGMP binding protein GpbC (Bosgraaf et al., 2002). However, this possible mechanism of inhibition has not been verified.
The phenotypes associated with cells expressing the Gα2/4 or Gα5/4 subunit implies that Dictyostelium Gα subunit specificity plays an important role in regulating developmental morphogenesis, chemotaxis, and cGMP accumulation in response to folate. If Gα subunits were only important for the release and capture of Gβγ dimers from specific receptors then the Gα4, Gα2/4, and Gα5/4 subunits would be expected to give similar phenotypes but this is clearly not the case suggesting Gα subunits interact with other signaling components. The distinct functions(s) of each Dictyostelium Gα subunits could result from different interactions with RGS (regulators of G protein signaling) proteins or other downstream signaling components, allowing for pathway specific responses and heterologous desensitization. The involvement of Gα subunits in competing cellular processes, such as foraging versus aggregation or prespore versus prestalk cell development, make these proteins good candidates for mediators of heterologous desensitization.
The contrasting phenotypes produced by the Gα4, Gα2/4, and Gα5/4 subunits suggests that Gα subunit structure outside of the carboxyl terminus significantly contributes to the specificity of Gα function in Dictyostelium. The chimeric Gα5/4 subunit was more inhibitory to folate responses than the Gα2/4 subunit but this observation is somewhat surprising given that the Gα4 subunit primary sequence shares more identity overall with the Gα5/4 (52%) than the Gα2/4 (45%) subunit. However, some small regions of the Gα4 subunit have more identity with the Gα2 subunit than to the Gα5 subunit including a region (residues 12–32) near the amino. This particular region of the Gα4 subunit has 38% and 14% identity with the corresponding regions of the Gα2 and Gα5 subunit, respectively, and within this region only the Gα5 subunit contains a putative MAP kinase-docking site (KKSRDIDYQL) composed of a group of two or more basic residues separated by 2–8 residues from a hydrophobic-X-hydrophobic residue sequence (Bardwell et al., 2001). A similar MAP kinase-docking site (KRANDVIEQSLQL) has been previously identified in the analogous region of the yeast Gpa1 Gα subunit (Metodiev et al., 2002). The MAP kinase-docking site of the yeast Gpa1 protein is required for interactions with the Fus3 MAP kinase and for the desensitization to mating factor stimulation (Metodiev et al., 2002). The Dictyostelium MAP kinase Erk2 is activated in Gα4- and Gα2-mediated signaling pathways and therefore the Erk2 protein might represent a target for the putative Gα5 subunit MAP kinase docking site (Maeda and Firtel, 1997; Segall et al., 1995). Requirement of this docking site for Gα5-mediated developmental phenotypes has been recently determined (Raisley and Hadwiger, unpublished data).
The expression of chimeric Gα subunits in mammalian cell lines, yeast and now in Dictyostelium has allowed for the analysis of Gα subunit specificity by coupling receptors in these cells to activate new or altered responses (Brown et al., 2000; Conklin et al., 1993; Conklin et al., 1996). This manipulation of signaling has resulted in the development of heterologous signaling systems useful for the analysis of orphan receptors and the screening of compounds that interact with specific G protein-coupled receptors (Coward et al., 1999; Howard et al., 2001; Stables et al., 1997). The development of similar technologies should also be possible in Dictyostelium because Gα subunits play important roles in defining downstream signaling responses.
Acknowledgments
The author thanks B. Raisley for technical assistance. The research was supported by an NIH grant (R15 GM073698-01) and a grant from the Center for Sensors and Sensor Technology at Oklahoma State University awarded to J.A.H.
Abbreviations
- PCR
polymerase chain reaction
- MAP kinase
mitogen-activated protein kinase
- SDS
sodium dodecyl sulfate
- GTPγS
guanosine 5′[γ-thio]triphosphate
- cGMP
cyclic guanosine monophosphate
- cAMP
cyclic adenosine monophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert PR, Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–18. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- Bardwell AJ, et al. A conserved docking site in MEKs mediates high-affinity binding to MAP kinases and cooperates with a scaffold protein to enhance signal transmission. J Biol Chem. 2001;276:10374–86. doi: 10.1074/jbc.M010271200. Epub 2000 Dec 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosgraaf L, et al. A novel cGMP signalling pathway mediating myosin phosphorylation and chemotaxis in Dictyostelium. The EMBO journal. 2002;21:4560–70. doi: 10.1093/emboj/cdf438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon MA, et al. Molecular characterization of a Dictyostelium G-protein alpha-subunit required for development. Gene. 1997;200:99–105. doi: 10.1016/s0378-1119(97)00387-9. [DOI] [PubMed] [Google Scholar]
- Brown AJ, et al. Functional coupling of mammalian receptors to the yeast mating pathway using novel yeast/mammalian G protein alpha-subunit chimeras. Yeast. 2000;16:11–22. doi: 10.1002/(SICI)1097-0061(20000115)16:1<11::AID-YEA502>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Brzostowski JA, et al. Galpha-mediated inhibition of developmental signal response. Current biology. 2002;12:1199–208. doi: 10.1016/s0960-9822(02)00953-3. [DOI] [PubMed] [Google Scholar]
- Brzostowski JA, Kimmel AR. Signaling at zero G: G-protein-independent functions for 7-TM receptors. Trends Biochem Sci. 2001;26:291–7. doi: 10.1016/s0968-0004(01)01804-7. [DOI] [PubMed] [Google Scholar]
- Chisholm RL, et al. dictyBase, the model organism database for Dictyostelium discoideum. Nucleic acids research. 2006;34:D423–7. doi: 10.1093/nar/gkj090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GM, et al. Stoichiometry of G protein subunits affects the Saccharomyces cerevisiae mating pheromone signal transduction pathway. Mol Cell Biol. 1990;10:510–7. doi: 10.1128/mcb.10.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin BR, et al. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–6. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- Conklin BR, et al. Carboxyl-terminal mutations of Gq alpha and Gs alpha that alter the fidelity of receptor activation. Mol Pharmacol. 1996;50:885–90. [PubMed] [Google Scholar]
- Coward P, et al. Chimeric G proteins allow a high-throughput signaling assay of Gi-coupled receptors. Anal Biochem. 1999;270:242–8. doi: 10.1006/abio.1999.4061. [DOI] [PubMed] [Google Scholar]
- De Wit RW, De Wit TFR. Developmental regulation of the folic acid chemosensory system in Dictyostelium discoideum. Developmental Biology. 1986;118:385–391. [Google Scholar]
- Dynes JL, Firtel RA. Molecular complementation of a genetic marker in Dictyostelium using a genomic DNA library. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:7966–70. doi: 10.1073/pnas.86.20.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichinger L, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel RA. Interacting signaling pathways controlling multicellular development in Dictyostelium. Curr Opin Genet Dev. 1996;6:545–54. doi: 10.1016/s0959-437x(96)80082-7. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA, Firtel RA. Analysis of Gα4, a G-protein subunit required for multicellular development in Dictyostelium. Genes Dev. 1992;6:38–49. doi: 10.1101/gad.6.1.38. [DOI] [PubMed] [Google Scholar]
- Hadwiger JA, et al. The G alpha subunit G alpha 4 couples to pterin receptors and identifies a signaling pathway that is essential for multicellular development in Dictyostelium. Proc Natl Acad Sci U S A. 1994;91:10566–70. doi: 10.1073/pnas.91.22.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger JA, et al. Identification of Dictyostelium Gα genes expressed during multicellular development. Proc Natl Acad Sci U S A. 1991;88:8213–7. doi: 10.1073/pnas.88.18.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger JH, et al. Mutations in the Dictyostelium heterotrimeric G protein α subunit Gα5 alter the kinetics of tip morphogenesis. Development. 1996;122:1215–1224. doi: 10.1242/dev.122.4.1215. [DOI] [PubMed] [Google Scholar]
- Howard AD, et al. Orphan G-protein-coupled receptors and natural ligand discovery. Trends Pharmacol Sci. 2001;22:132–40. doi: 10.1016/s0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- Hur EM, Kim KT. G protein-coupled receptor signalling and cross-talk: achieving rapidity and specificity. Cell Signal. 2002;14:397–405. doi: 10.1016/s0898-6568(01)00258-3. [DOI] [PubMed] [Google Scholar]
- Kumagai A, et al. Molecular genetic analysis of two G alpha protein subunits in Dictyostelium. J Biol Chem. 1991;266:1220–8. [PubMed] [Google Scholar]
- Levi S, et al. Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid. 2000;44:231–8. doi: 10.1006/plas.2000.1487. [DOI] [PubMed] [Google Scholar]
- Luttrell LM. Composition and function of g protein-coupled receptor signalsomes controlling mitogen-activated protein kinase activity. Journal of molecular neuroscience. 2005;26:253–64. doi: 10.1385/JMN:26:2-3:253. [DOI] [PubMed] [Google Scholar]
- Maeda M, Firtel RA. Activation of the mitogen-activated protein kinase ERK2 by the chemoattractant folic acid in Dictyostelium. J Biol Chem. 1997;272:23690–5. doi: 10.1074/jbc.272.38.23690. [DOI] [PubMed] [Google Scholar]
- Metodiev MV, et al. Regulation of MAPK function by direct interaction with the mating-specific Galpha in yeast. Science. 2002;296:1483–6. doi: 10.1126/science.1070540. [DOI] [PubMed] [Google Scholar]
- Natarajan K, et al. Related Gα subunits play opposing roles during Dictyostelium development. Differentiation. 2000;66:136–46. doi: 10.1046/j.1432-0436.2000.660208.x. [DOI] [PubMed] [Google Scholar]
- Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci U S A. 1997;94:14489–94. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, et al. Galphai is not required for chemotaxis mediated by Gi-coupled receptors. J Biol Chem. 1999;274:2824–8. doi: 10.1074/jbc.274.5.2824. [published erratum appears in J Biol Chem 1999 Mar 12;274(11):7598] [DOI] [PubMed] [Google Scholar]
- Neves SR, et al. G protein pathways. Science. 2002;296:1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Simon MI. Genetic analysis of mammalian G-protein signalling. Oncogene. 1998;17:1375–81. doi: 10.1038/sj.onc.1202173. [DOI] [PubMed] [Google Scholar]
- Segall JE, et al. A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1995;128:405–13. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MI, et al. Diversity of G proteins in signal transduction. Science. 1991;252:802–8. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, et al. Activated Galpha Subunits Can Inhibit Multiple Signal Transduction Pathways during Dictyostelium Development. Dev Biol. 1999;215:443–452. doi: 10.1006/dbio.1999.9474. [Record as supplied by publisher] [DOI] [PubMed] [Google Scholar]
- Stables J, et al. A bioluminescent assay for agonist activity at potentially any G-protein-coupled receptor. Analytical biochemistry. 1997;252:115–26. doi: 10.1006/abio.1997.2308. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Ashworth JM. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. The Biochemical journal. 1970;119:171–4. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, et al. The G protein beta subunit is essential for multiple responses to chemoattractants in Dictyostelium. J Cell Biol. 1995;129:1667–75. doi: 10.1083/jcb.129.6.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, et al. Ggamma in dictyostelium: its role in localization of gbetagamma to the membrane is required for chemotaxis in shallow gradients. Molecular biology of the cell. 2001;12:3204–13. doi: 10.1091/mbc.12.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]


