Abstract
Bronchopulmonary dysplasia and emphysema are significant global health problems at the extreme stages of life. Both are characterized by arrested alveolar development or loss of alveoli, respectively. Both lack effective treatment strategies. Knowledge about the genetic control of branching morphogenesis in mammals derives from investigations of the respiratory system in Drosophila, but mechanisms that regulate alveolar development remain poorly understood. Even less is known about regulation of the growth and development of the pulmonary vasculature. Understanding how alveoli and the underlying capillary network develop, and how these mechanisms are disrupted in disease states, are critical for developing effective therapies for lung diseases characterized by impaired alveolar structure. Recent observations have challenged old notions that the development of the blood vessels in the lung passively follows that of the airways. Rather, increasing evidence suggests that lung blood vessels actively promote alveolar growth during development and contribute to the maintenance of alveolar structures throughout postnatal life. Our working hypothesis is that disruption of angiogenesis impairs alveolarization, and that preservation of vascular growth and endothelial survival promotes growth and sustains the architecture of the distal airspace. Furthermore, the explosion of interest in stem cell biology suggests potential roles for endothelial progenitor cells in the pathogenesis or treatment of lung vascular disease. In this Pulmonary Perspective, we review recent data on the importance of the lung circulation, specifically examining the relationship between dysmorphic vascular growth and impaired alveolarization, and speculate on how these new insights may lead to novel therapeutic strategies for bronchopulmonary dysplasia.
Keywords: lung injury, oxygen, angiogenesis, newborn, nitric oxide
Preterm delivery is a major health care problem, affecting more than 12% of all births and accounting for more than 85% of all perinatal complications and death (National Institute of Medicine of the National Academies, July 2006; available from: http://www.iom.edu/CMS/3740/25471/35813.aspx [accessed April, 2007]). Survival of extremely premature newborns (especially those born at less than 28 wk of gestation) has increased because of improvements in perinatal care (1). These infants, however, are at high risk for long-term medical and neurocognitive impairment, including chronic lung disease or bronchopulmonary dysplasia (BPD) (2). Each year, 10,000–15,000 newborns develop BPD (National Institute of Health, available from: http://www.nhlbi.nih.gov/health/dci/Diseases/Bpd/Bpd_WhatIs.html [accessed April, 2007]), a chronic lung disease that follows ventilator and oxygen therapy for acute respiratory failure after premature birth (3). Although surfactant therapy, antenatal steroids, and changes in neonatal intensive care have modified its phenotype, BPD remains a significant complication of premature birth. BPD is characterized by persistent respiratory signs, prolonged need for mechanical ventilation or oxygen therapy, recurrent hospitalizations for respiratory infections and distress, exercise intolerance, and other problems that reach beyond childhood (4).
Adult-onset emphysema, defined as airspace enlargement distal to terminal bronchioles, is a major component of chronic obstructive pulmonary disease (COPD), the fourth leading cause of death in the United States (5). Both BPD and COPD are characterized by marked enlargement of distal airspaces; however, whereas COPD is related to the destruction of established alveoli, BPD represents the disruption of normal lung development. Understanding how alveoli and the underlying capillary network develop and are maintained is critical for developing novel strategies for the treatment of these debilitating newborn and adult lung diseases. Recent evidence suggests that blood vessels in the lung actively promote normal alveolar development (6, 7) and contribute to maintenance of alveolar structures throughout life (8) (Figure 1). Consequently, modulation of angiogenic growth factors and vascular precursor cells may have therapeutic potential for lung diseases characterized by alveolar damage (Figure 2). Although this Pulmonary Perspective will focus on BPD, some knowledge acquired from the developing lung may also be relevant to adult diseases characterized by alveolar injury, as lung remodeling and regeneration may recapitulate ontogeny (9).
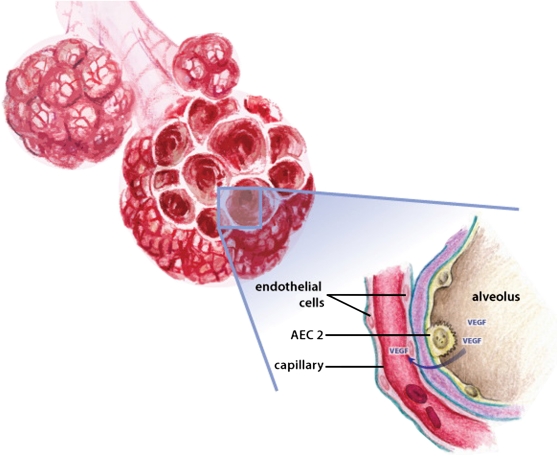
Figure 1.
Schematic of proposed interactions between vascular growth factors and their receptors during alveolarization. Vascular growth factors are secreted by the respiratory epithelium and signal to their receptors located on the vascular endothelium to promote angiogenesis and drive alveolarization. AEC2 = alveolar epithelial type 2 cell.
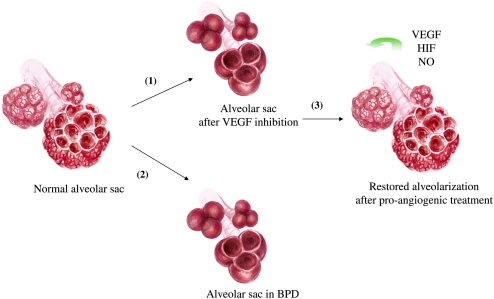
Figure 2.
Illustration of VEGF's fulfillment of Koch's alveolarization postulate. (1) Inhibition of VEGF signaling impairs alveolarization; (2) conversely, impaired alveolarization in human and experimental BPD is associated with decreased VEGF signaling; (3) lung overexpression or activation of VEGF in experimental BPD restores normal alveolar development.
LUNG ANGIOGENESIS—THE ORPHAN OF LUNG DEVELOPMENT
The Classical Stages of Lung Development
Lung development is classically subdivided into five overlapping stages in humans and rodents, on the basis of gross histologic features. The first four stages, termed the embryonic, pseudoglandular, canalicular, and saccular stages, occur during gestation. At the end of the saccular stage, at about 36 wk, lungs have formed alveolar ducts and air sacs. Alveolarization, the final stage of lung development, begins in the near-term lung before birth, but primarily occurs postnatally, during the first 2–3 yr of life, and may continue at a slower rate beyond childhood (10, 11). The formation of alveoli occurs by the outgrowth of secondary septae that subdivide terminal saccules into anatomic alveoli. Premature infants at greatest risk for BPD in the postsurfactant era are born at 24–28 wk, during the late canalicular or saccular stage of lung development, just as the airways become juxtaposed to pulmonary vessels. Although surfactant replacement therapy overcomes biochemical and functional aspects of the premature lung, it does not treat its underlying structural immaturity. Thus, a better understanding on how sacculi, alveoli, and the underlying capillary network develop, and how these mechanisms are disrupted in BPD is critical to developing efficient therapies for lung diseases characterized by impaired alveolar structure.
Lung Angiogenesis
Recently, there has been growing interest in mechanisms that regulate vascular growth and the role of vascular growth in lung development. Formation of the pulmonary circulation has been primarily described as dependent upon two basic processes: vasculogenesis and angiogenesis. Vasculogenesis is the de novo formation of blood vessels from angioblasts or endothelial precursor cells that migrate and differentiate in response to local cues (growth factors, extracellular matrix) to form vascular tubes. Angiogenesis is the formation of new blood vessels from preexisting ones. It has generally been accepted that the distal vasculature arises by vasculogenesis, and the proximal vasculature by angiogenesis, but this remains controversial (12). Vasculogenesis results in the de novo formation of blood vessels from blood islands present within the mesenchyme of the embryonic lung (Embryonic Day [E] 9 in the mouse) (13). Angiogenesis starts around E12, when arteries and veins begin to sprout from the central pulmonary vascular trunks. Around E14, peripheral sinusoids and central vessel sprouts connect and establish a vascular network. This union of peripheral and central vascular structures is accompanied by extensive branching of the arteries, which follow the branching pattern of the airways. Again, the relative contributions of vasculogenesis and angiogenesis to lung vascular growth during each stage of lung development are controversial, and additional studies with appropriate experimental models are required to better define these underlying mechanisms. Studies in human fetal lung suggest that airways act as a template for pulmonary artery development, and that endothelial tubes form around the terminal buds of distal airspaces, suggesting an inductive influence of the epithelium (14). More recently, Parera and colleagues (12) suggested distal angiogenesis as a new mechanism for lung vascular morphogenesis, based on morphologic analysis from the onset of lung development (E9.5) until the pseudoglandular stage (E13.5) in Tie2-LacZ–transgenic mice. In their model, capillary networks surrounding the terminal buds exist from the first morphologic sign of lung development and then expand by formation of new capillaries from preexisting vessels as the lung bud grows (12). Clearly, new tools, routinely used in developmental biology, need to be applied to deepen our understanding about the formation of the pulmonary circulation.
The final important step of microvascular maturation overlaps the alveolar stage of lung development (15). Capillaries, which are organized as double capillary layers in the immature gas-exchange region, later remodel to form a single capillary layer. This process is completed in the rat by about the third postnatal week. The alveolar wall thins and its cellular composition changes. In the rat, the thickness of the alveolar wall and the air–gas barrier (the distance between alveolar gas and capillary blood) decrease by 20–25%. Between birth and adulthood, the alveolar and capillary surface areas expand nearly 20-fold, and the capillary volume by 35-fold. Further expansion of the capillary network subsequently occurs via two angiogenic mechanisms: sprouting angiogenesis from preexisting vessels and intussusceptive growth (16). Little is known about intussusceptive microvascular growth in the lung. This novel mode of blood vessel formation and remodeling occurs by internal division of the preexisting capillary plexus (insertion of transcapillary tissue pillars) without sprouting, which may underlie alveolar growth and remodeling throughout adult life, and thus be amenable to therapeutic modulation for lung regeneration. Much more needs to be learned about the anatomic events underlying lung vascular development and time-specific mechanisms that regulate growth and function at each stage. Likewise, there is a need for endothelial cell–specific surface markers to identify and characterize angioblasts, endothelial progenitor cells (EPCs), and mature endothelial cells, as well as genetic tools allowing us to better define endothelial cell fate and lineage relationships in the embryonic and postnatal lung.
Arrested Lung Development in BPD
With premature birth, the normal sequence of lung development is disrupted, resulting in the histologic pattern of alveolar simplification (larger but fewer alveoli with decreased septation) and impaired or “dysmorphic” vascular growth. Various animal models of BPD and autopsy studies of humans who died from BPD have consistently shown a reduction in the number of small arteries and an abnormal distribution of vessels within the distal lung (17–22). A recent postmortem study of newborns who died after short and prolonged durations of mechanical ventilation quantified lung microvascular growth (23). This study confirmed the reduction in vascular branching arteries, but, interestingly, lung platelet endothelial cell adhesion molecule-1 (PECAM-1) protein content (a marker of endothelial cells) was decreased in infants dying after brief ventilation, but was increased after prolonged ventilation (23). These findings suggest a transient decrease in endothelial proliferation, followed by a brisk proliferative response, despite a reduction in vessel number. This observation suggests that dysmorphic lung vascular growth in BPD may not necessarily result simply from a reduction in the number of endothelial cells, suggesting the need to better discern distinct mechanisms regulating endothelial cell survival, proliferation, migration, vessel formation, and maturation, especially in response to injury. These findings further emphasize the need for more extensive studies in animal models of BPD to better define the mechanisms that underlie early events and the time-dependent sequence of events that precede the development of impaired distal lung structure. There may be interesting parallels with similar time-specific events that alter the vascular response to hyperoxic injury to the developing retina and lead to retinopathy of prematurity (24). In addition to dysmorphic growth, the pulmonary vasculature in BPD undergoes hypertensive structural remodeling, which includes medial hypertrophy and distal muscularization of small peripheral arteries (25). Abnormal vasoreactivity, reduced arterial number, and structural abnormalities of the vessel wall can contribute to pulmonary hypertension in BPD, leading to significant morbidity and mortality (26). Interestingly, recent animal studies demonstrated that hypertension itself can inhibit vascular growth and impair alveolarization in the developing lung, suggesting that hemodynamic stress may be an additional mechanism for abnormal lung structure in BPD (27).
LINK BETWEEN ANGIOGENESIS AND LUNG DISEASE—KOCH'S POSTULATES AND THE “VASCULAR HYPOTHESIS”
Multiple signaling pathways have been identified as contributing to alveolarization, such as elastin, platelet-derived growth factor, fibroblast growth factors, transforming growth factors, and others (reviewed in Reference 28). Still, the molecular mechanisms and signal-transduction pathways that regulate normal alveolar development, and how these pathways are disrupted in premature infants with BPD, remain incompletely understood. Based on experimental and clinical studies, an additional mechanism, involving vascular growth and signaling, has been proposed as a contributing factor in alveolarization (29). It has been hypothesized that disruption of angiogenesis during critical periods of lung growth can impair alveolarization and contribute to lung hypoplasia, especially in the pathogenesis of BPD (29). Evidence supporting this hypothesis, as illustrated in Figure 2, includes: (1) disruption of angiogenesis impairs alveolarization in various models (7, 20, 30, 31); (2) BPD is characterized by reduced and dysmorphic vascular growth and decreased alveolarization, and downregulation of angiogenic growth factors (20, 23, 32–38); and (3) enhancing angiogenesis and preserving endothelial survival promote vascular and alveolar growth and improve lung structure in experimental BPD (20, 39–41).
In 1959, a similar concept was proposed for adults with COPD by Liebow (42), who observed that alveolar septa in centrilobular emphysema were thin and nearly avascular, suggesting that a reduction in the blood supply of the small precapillary blood vessels might induce the disappearance of alveolar septa. Despite this observation, pulmonary vessels were considered as passive by-standers during destruction of alveolar septae in COPD. Similarly, the lung vasculature, during normal lung development, had received little attention, and was thought to simply follow the branching pattern of the airways. Recent evidence in experimental and human neonatal BPD and emphysema supports the concept that coordination of distal air space and vascular growth is essential for normal alveolarization. That is, the role of the pulmonary vasculature goes beyond delivery of nutrients and oxygen, and includes a crucial cross talk between epithelial and endothelial cells (as well as the fibroblast) present in the distal airspaces. Supporting evidence comes from other organs, such as the pancreas and the liver. For example, in the pancreas, endothelial cells induce pancreas development, including islet formation adjacent to the vessels (43). Islets from vascular endothelial growth factor (VEGF)–A–deficient animals have few capillaries, which are not fenestrated, resulting in impaired fine tuning of blood glucose regulation, suggesting a key role for paracrine VEGF-A signaling in pancreatic structure and function (43).
Among several angiogenic growth factors, VEGF is absolutely critical for vascular development. VEGF is a potent endothelial cell–specific mitogen and survival factor that promotes vessel growth and remodeling. The absolute requirement of VEGF for development of the embryonic vasculature in mice has been demonstrated by inactivation studies of VEGF alleles (44, 45) and knockouts of VEGF receptor (VEGFR)–1 (46) and VEGFR-2 (47). In each of these studies, inactivation of target genes resulted in lethal phenotypes, characterized by deficient organization of endothelial cells. Koch's postulates, formulated in 1884 and designed to establish a causal relationship between a causative microbe and a disease, provide a useful tool to demonstrate the crucial role of VEGF-driven lung angiogenesis during alveolar development and homeostasis. Koch applied the postulates to establish the etiology of anthrax and tuberculosis, but they have been generalized to other diseases. Koch's postulates are:
The organism must be found in all animals suffering from the disease, but not in healthy animals.
The organism must be isolated from a diseased animal and grown in pure culture.
The cultured organism should cause disease when introduced into a healthy animal.
The organism must be reisolated from the experimentally infected animal.
Pierce and Shipley (48) adapted Koch's postulate to test whether a particular candidate factor is required for and promotes alveolarization:
The candidate agent should be present in all models of alveolarization.
The agent or its receptors should be detected specifically at sites of secondary crest formation.
Blocking expression or activity of the agent should block alveolarization.
Alveolarization should be enhanced when the agent is ectopically administered.
Although multiple growth factors are involved in alveolar development, VEGF fulfills Koch's stringent criteria (Table 1).
TABLE 1.
VASCULAR ENDOTHELIAL GROWTH FACTOR IS CRUCIAL FOR ALVEOLAR DEVELOPMENT AND FULFILLS KOCH'S POSTULATE
| Modified Koch's Postulate | Evidence | References |
|---|---|---|
| 1. VEGF is present in all models of alveolarization | VEGF is identified in the lungs of various species, including mice, rats, rabbits, sheep, nonhuman primates, and humans. | 20, 23, 36, 37, 50, 51 |
| 2. VEGF is detected specifically at sites of secondary crest formation | VEGF mRNA and protein are localized to distal airway epithelial cells and the basement membrane subjacent to the airway epithelial cells, suggesting that translocation of VEGF protein occurs after its synthesis in the epithelium. VEGFR-1 and VEGFR-2 mRNA expression increases during normal mouse lung development and is localized to the pulmonary endothelial cells closely apposed to the developing epithelium. | 49, 50 |
| 3. VEGF inhibition blocks alveolarization | Pharmacologic (SU5416, VEGF trap, mFlt(1-3)-Ig) and genetic (targeted VEGF164 and VEGF188 exon deletion) inhibition of VEGF results in fewer and larger distal airspaces and capillary rarefaction. | 7, 20, 30, 31 |
| 4. Alveolarization is enhanced when VEGF is ectopically administered | VEGF treatment (via intratracheal adenovirus–mediated gene therapy or intramuscular injection of recombinant VEGF) promotes lung angiogenesis and alveolarization in experimental, oxygen-induced BPD in newborn rats. | 20, 40, 41 |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; VEGF = vascular endothelial growth factor; VEGFR = VEGF receptor.
Angiogenic Growth Factors Promote Alveolar Development—Koch's Modified Postulates 1 and 2
Disruption of vascular growth with diverse antiangiogenesis agents, including VEGF antagonists, impairs alveolarization in infant rats (7, 20). Beyond the embryonic period, a crucial role for VEGF signaling during alveolar development has also been recognized. Inducible Cre-loxP–mediated gene targeting or administration of a soluble VEGFR chimeric protein (mFlt[1-3]-IgG) to inactivate VEGF in early postnatal life results in increased mortality, stunted body growth, and impaired organ development (31). VEGF inhibition resulted in less significant alterations as the animal matured, and VEGF dependence is less beyond the fourth postnatal week (31).
The spatial relationship between receptor and ligand suggests that VEGF plays a role in the development of the alveolar capillary bed (49). Furthermore, various VEGF isoforms (VEGF120, VEGF164, and VEGF188) are present in alveolar type II cells in the developing mouse lung, and their expression peaks during the canalicular stage, when most of the vessel growth occurs in the lung, then decreases until Postnatal Day 10, when it increases to levels that are maintained through adulthood (50). Findings that pharmacologic and genetic inhibition of VEGF expression/function impairs alveolar architecture in newborn and adult animals (features encountered in clinical BPD and emphysema) suggest that VEGF is required for the formation, but also for the maintenance, of the pulmonary vasculature and alveolar structures throughout adulthood. As noted in neonatal rats (6), treatment of adult rats with SU5416, a VEGFR blocker, causes enlargement of the air spaces, indicative of emphysema (8). These findings suggest that VEGF is required for the maintenance not only of the pulmonary vasculature, but also of the alveolar structures throughout adulthood. However, newborns are more susceptible to disruption of VEGF signaling than adults. Whereas even brief treatment with VEGF inhibitors impairs alveolar growth and induces pulmonary hypertension in the fetus and newborns (7), adult rats require repetitive and prolonged treatment (8) or a second injury (such as hypoxia) (51) to induce more subtle impairment of lung architecture. Work by MacDonald and collaborators may explain differences between mechanisms of “developmental” versus “late-onset” emphysema, which may have important therapeutic implications. The adult microvasculature is capable of rapid regrowth after VEGF inhibition–induced regression due to persistence of vascular basement membranes after endothelial cells degenerated, providing a ghost-like record of pretreatment vessel number and location, and a potential scaffold for vessel regrowth (52). Whether such scaffolding is present in the developing lung is unknown.
Recent studies suggest that VEGF-induced lung angiogenesis is in part mediated by nitric oxide (NO). Neonatal treatment with the VEGF inhibitor, SU5416, downregulates endothelial NO synthase protein and NO production, and treatment with inhaled NO improves vascular and alveolar growth in this model of BPD (53). Lungs of late fetal and neonatal endothelial NO synthase–deficient mice can have a paucity of distal arteries and reduced alveolarization (54), and are more susceptible for failed vascular and alveolar growth after exposure to mild hypoxia and hyperoxia (55). Administration of an anti–PECAM-1 antibody that inhibits endothelial cell migration, but not proliferation or survival in vitro, also impairs septation in neonatal rats, without reducing endothelial cell content (56). Overall, these data suggest that the loss of PECAM-1 function compromises postnatal lung development and provides evidence that inhibition of endothelial cell function, in contrast to loss of viable endothelial cells, inhibits alveolarization.
Decreased VEGF Signaling in Experimental and Clinical BPD—Koch's Modified Postulate 3
Lung VEGF expression is reduced in several animal models of BPD, including hyperoxic exposure of newborn rabbits and rats (20, 36, 57), antenatal endotoxin exposure (58), and prolonged mechanical ventilation of preterm baboons (37, 39). Preterm infants with more severe respiratory distress syndrome and who subsequently develop BPD have lower VEGF tracheal aspirate levels than those who recover (34, 35). In lung tissue from human infants who died from BPD, the typical patterns of alveolar simplification with “dysmorphic” microvasculature is associated with reduced lung VEGF and VEGFR-1 mRNA and protein expression, as well as decreased expression of the endothelial marker PECAM-1 and the angiopoietin receptor, Tie-2 (32).
Angiogenic Growth Factor Therapy for Restoring Lung Structure—Koch's Modified Postulate 4
The data described previously here form the rationale to test the therapeutic potential for angiogenic growth factor modulation in experimental lung diseases characterized by alveolar damage. Recombinant human VEGF treatment of newborn rats during or after exposure to hyperoxia enhances vessel growth and improves alveolarization (40, 41). Likewise, postnatal intratracheal adenovirus-mediated VEGF gene therapy improves survival, promotes lung capillary formation, preserves alveolar development, and regenerates new alveoli in this same model of irreversible lung injury (20). In both animal studies, VEGF induced immature and leaky capillaries and lung edema. Indeed, despite its central role in vascular formation, VEGF works in concert with other factors—notably, angiopoietins. Angiopoietin-1 is required to stabilize the vessel wall by maximizing interactions between endothelial cells and their surrounding support cells and matrix (59). Accordingly, combined lung VEGF and angiopoietin-1 gene transfer preserves alveolarization and enhances angiogenesis with more mature capillaries that are less permeable, reducing the vascular leakage seen in VEGF-induced capillaries (20). Given that VEGF-induced angiogenesis is in part mediated by NO, some of these findings may explain the beneficial effects of early and prolonged, low-dose, inhaled NO seen in three recent, randomized, controlled trials to prevent BPD (60–62). Accordingly, sildenafil, which acts downstream to enhance NO-induced cGMP levels, prevents hyperoxia-induced alterations of lung structure in neonatal rats (63).
These experiments provide proof of concept for the crucial role of the lung vasculature in what is traditionally thought of as an airspace disease, and open new therapeutic avenues to protect or regenerate new alveoli. These observations also highlight the tightly orchestrated process of angiogenesis and points toward the need to closely recapitulate this process to warrant efficient and safe angiogenesis. Hypoxia-inducible factor (HIF) is a master transcription factor modulating O2-sensitive gene expression (including VEGF and angiopoietin-1) and vessel growth (64). HIF is activated in hypoxia and inhibited by increased O2 levels (65, 66). However, because HIF deficiency is embryonically or immediately postnatally lethal, the role of HIF during alveolarization remains unknown. Nonetheless, HIF activation via inhibition of prolyl hydroxylase domain–containing proteins prevents lung injury in the premature baboon model of BPD, and further supports a potential role for angiogenic growth factor in promoting alveolar development (67).
EPC and Lung Diseases—the Cohnheim Hypothesis
If angiogenic growth factors and the lung vasculature contribute to integrity of the lung, then vascular progenitor cells are appealing candidate cells, likely to be involved in the same mechanisms. The current promise of stem cells to repair injured organs, and the availability of new technology to explore resident and circulating stem cells, have very recently sparked the interest in EPCs. EPCs are a specific subtype of hematopoietic stem cell that has been isolated from the peripheral blood of humans (68). Purified CD34+ hematopoietic progenitor cells could differentiate ex vivo to an endothelial phenotype, express several endothelial markers, and incorporate into neovessels at sites of ischemia. EPCs can migrate from the bone marrow to the peripheral circulation, where they contribute to the repair of injured endothelium and to the formation of new blood vessels (69). Levels of circulating EPCs may be a prognostic biological marker for vascular function and cumulative cardiovascular risk in male subjects without a history of cardiovascular disease (70). These observations corroborate Cohnheim's hypothesis, which suggested that all of the cells come from the bloodstream and, therefore, in light of subsequent observations, from the bone marrow (71).
Until recently, little information was available on the role of circulating EPCs during lung injury and repair. LPS-induced murine lung injury causes a rapid release of EPCs into the circulation, and contributes, together with other bone marrow-derived progenitor cells, to lung repair (72). In elastase-induced emphysema, cells derived from the bone marrow develop characteristics of endothelial cells and contribute to repair the alveolar capillary wall (73, 74). Patients with acute lung injury have twofold higher numbers of circulating EPCs than healthy control subjects (75), suggesting some biological role for the mobilization of these cells in lung disease. More interestingly, improved survival has been correlated with increased circulating EPCs, even after correcting for differences in age, sex, and severity of illness (75). Likewise, the number of circulating EPCs is significantly increased in patients with pneumonia, and patients with low EPC counts tend to have persistent fibrotic changes in their lungs even after their recovery from pneumonia (76). Finally, EPCs are also decreased in patients with restrictive and chronic lung disease (77). In this study by Fadini and colleagues, circulating EPCs were only defined by the surface expression of CD34, CD133, and VEGFR-2. In both diseases, there was a correlation between EPC count and disease-severity (77). Recent findings suggest that circulating, lung, and bone marrow EPC levels are reduced in an experimental model of BPD in hyperoxic neonatal mice (78). These observations suggest that EPCs migrate from the bone marrow to the peripheral circulation and the lung, where they contribute to the repair of injured endothelium and help restore lung integrity. These observations are consistent with the beneficial effect of angiogenic growth factors in experimental BPD, and underscore the therapeutic potential of promoting lung angiogenesis to repair the lung.
CONCLUSIONS
In summary, lung angiogenesis, via the secretion of angiogenic growth factors, such as VEGF and NO, contribute to normal alveolar development. Impaired alveolar development in BPD is associated with arrested and dysmorphic vascular growth and decreased lung angiogenic growth factor expression. Exogenous VEGF, NO, or activation of HIF preserve alveolar development, suggesting new therapeutic avenues for preserving or enhancing alveolar structure. However, aside from the mechanism of action, major gaps in our knowledge remain before the safe translation of angiogenic growth factor modulation into the clinical setting. It will be crucial to determine the appropriate dosing and timing of angiogenesis stimulation to avoid the formation of angiomas, abnormal vascular proliferation, pulmonary hemorrhage or edema, and other problems. Cell therapy using EPCs or transfected EPCs for gene therapy that modify their microenvironment and are, themselves, instructed by the microenvironment to restore organ integrity may be an appealing alternative. However, stem/progenitor cell therapy is still in its infancy, and many questions remain to be answered: a crucial one is how to define an EPC? Overall, there is consensus that EPC can derive from the bone marrow, and that CD133/VEGFR-2 cells represent a population with endothelial progenitor capacity. However, increasing evidence suggests that there are additional bone marrow and non–bone marrow–derived cell populations (e.g., myeloid cells) within the blood, which also can give rise to endothelial cells. Are these circulating EPCs retained within the lungs, and what is their fate? Do they participate in the repair of the lungs and, if so, by which mechanism (i.e., replacement of dying cells and/or paracrine effect)? What are the homing signals produced by the lung to attract and retain these cells?
Much like irrigation enhancing crop growth in agriculture, enhancing angiogenesis promotes alveolar development, maintenance, and regeneration. If current limitations and knowledge gaps can be overcome, enhancing angiogenesis holds promise for new therapeutic options for diseases that were traditionally thought of as airway diseases.
Supported by the Canadian Institutes for Health Research, the Canada Foundation for Innovation, the Alberta Heritage Foundation for Medical Research, the Alberta Lung Association, the Stollery Children's Hospital Foundation (B.T.), and by the National Institute of Health (NIH RO1HL68702) and the Thrasher Foundation (S.H.A.). B.T. is a Canada Research Chair in Translational Lung and Vascular Developmental Biology.
Originally Published in Press as DOI: 10.1164/rccm.200611-1660PP on February 1, 2007
Conflict of Interest Statement: B.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.A. serves as a scientific advisor to INO Therapeutics and receives less than $10,000/yr. A grant from Bayer Pharmaceuticals is pending.
References
- 1.Goldenberg RL, Jobe AH. Prospects for research in reproductive health and birth outcomes. JAMA 2001;285:633–639. [DOI] [PubMed] [Google Scholar]
- 2.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, Simon NP, Wilson DC, Broyles S, Bauer CR, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 2000;105:1216–1226. [DOI] [PubMed] [Google Scholar]
- 3.Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med 1967;276:357–368. [DOI] [PubMed] [Google Scholar]
- 4.McLeod A, Ross P, Mitchell S, Tay D, Hunter L, Hall A, Paton J, Mutch L. Respiratory health in a total very low birthweight cohort and their classroom controls. Arch Dis Child 1996;74:188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shapiro SD. Vascular atrophy and VEGFR-2 signaling: old theories of pulmonary emphysema meet new data. J Clin Invest 2000;106:1309–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med 2001;164:1755–1756. [DOI] [PubMed] [Google Scholar]
- 7.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600–L607. [DOI] [PubMed] [Google Scholar]
- 8.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warburton D, Tefft D, Mailleux A, Bellusci S, Thiery JP, Zhao J, Buckley S, Shi W, Driscoll B. Do lung remodeling, repair, and regeneration recapitulate respiratory ontogeny? Am J Respir Crit Care Med 2001;164:S59–S62. [DOI] [PubMed] [Google Scholar]
- 10.Burri PH, Dbaly J, Weibel ER. The postnatal growth of the rat lung: I. Morphometry. Anat Rec 1974;178:711–730. [DOI] [PubMed] [Google Scholar]
- 11.Burri PH, Moschopulos M. Structural analysis of fetal rat lung development. Anat Rec 1992;234:399–418. [DOI] [PubMed] [Google Scholar]
- 12.Parera MC, van Dooren M, van Kempen M, de Krijger R, Grosveld F, Tibboel D, Rottier R. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol 2005;288:L141–L149. [DOI] [PubMed] [Google Scholar]
- 13.deMello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am J Respir Cell Mol Biol 1997;16:568–581. [DOI] [PubMed] [Google Scholar]
- 14.Hall SM, Hislop AA, Pierce CM, Haworth SG. Prenatal origins of human intrapulmonary arteries: formation and smooth muscle maturation. Am J Respir Cell Mol Biol 2000;23:194–203. [DOI] [PubMed] [Google Scholar]
- 15.Burri P. Lung development and pulmonary angiogenesis. In: Gaultier C, Bourbon JR, Post M, editors. Lung development. New York: Oxford University Press; 1999. pp. 122–151.
- 16.Djonov V, Schmid M, Tschanz SA, Burri PH. Intussusceptive angiogenesis: its role in embryonic vascular network formation. Circ Res 2000;86:286–292. [DOI] [PubMed] [Google Scholar]
- 17.Coalson JJ. Pathology of chronic lung disease of early infancy. In: Bland R, Coalson JJ, editors. Lung Biology in health and disease: chronic lung disease in early infancy. New York: Marcel Dekker; 2000. pp. 85–124.
- 18.Le Cras TD, Kim DH, Markham NE, Abman AS. Early abnormalities of pulmonary vascular development in the fawn-hooded rat raised at Denver's altitude. Am J Physiol Lung Cell Mol Physiol 2000;279:L283–L291. [DOI] [PubMed] [Google Scholar]
- 19.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 2002;283:L555–L562. [DOI] [PubMed] [Google Scholar]
- 20.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005;112:2477–2486. [DOI] [PubMed] [Google Scholar]
- 21.Tomashefski JF Jr, Oppermann HC, Vawter GF, Reid LM. Bronchopulmonary dysplasia: a morphometric study with emphasis on the pulmonary vasculature. Pediatr Pathol 1984;2:469–487. [DOI] [PubMed] [Google Scholar]
- 22.Wilson WL, Mullen M, Olley PM, Rabinovitch M. Hyperoxia-induced pulmonary vascular and lung abnormalities in young rats and potential for recovery. Pediatr Res 1985;19:1059–1067. [DOI] [PubMed] [Google Scholar]
- 23.De Paepe ME, Mao Q, Powell J, Rubin SE, DeKoninck P, Appel N, Dixon M, Gundogan F. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med 2006;173:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csak K, Szabo V, Szabo A, Vannay A. Pathogenesis and genetic basis for retinopathy of prematurity. Front Biosci 2006;11:908–920. [DOI] [PubMed] [Google Scholar]
- 25.Hislop AA, Haworth SG. Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol 1990;9:152–161. [DOI] [PubMed] [Google Scholar]
- 26.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2004;170:1006–1013. [DOI] [PubMed] [Google Scholar]
- 27.Grover TR, Parker TA, Balasubramaniam V, Markham NE, Abman SH. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 2005;288:L648–L654. [DOI] [PubMed] [Google Scholar]
- 28.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res 2005;57:38R–46R. [DOI] [PubMed] [Google Scholar]
- 29.Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 2005;67:623–661. [DOI] [PubMed] [Google Scholar]
- 30.Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D'Amore PA, DeMello DE. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol 2002;27:194–203. [DOI] [PubMed] [Google Scholar]
- 31.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 1999;126:1149–1159. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt AJ, Amin SB, Chess PR, Watkins RH, Maniscalco WM. Expression of vascular endothelial growth factor and Flk-1 in developing and glucocorticoid-treated mouse lung. Pediatr Res 2000;47:606–613. [DOI] [PubMed] [Google Scholar]
- 33.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971–1980. [DOI] [PubMed] [Google Scholar]
- 34.Lassus P, Ristimaki A, Ylikorkala O, Viinikka L, Andersson S. Vascular endothelial growth factor in human preterm lung. Am J Respir Crit Care Med 1999;159:1429–1433. [DOI] [PubMed] [Google Scholar]
- 35.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 2001;164:1981–1987. [DOI] [PubMed] [Google Scholar]
- 36.Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol 1997;16:557–567. [DOI] [PubMed] [Google Scholar]
- 37.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 2002;282:L811–L823. [DOI] [PubMed] [Google Scholar]
- 38.Roberts RJ, Weesner KM, Bucher JR. Oxygen-induced alterations in lung vascular development in the newborn rat. Pediatr Res 1983;17:368–375. [DOI] [PubMed] [Google Scholar]
- 39.Asikainen TM, Waleh NS, Schneider BK, Clyman RI, White CW. Enhancement of angiogenic effectors through hypoxia-inducible factor in preterm primate lung in vivo. Am J Physiol Lung Cell Mol Physiol 2006;291:L588–L595. [DOI] [PubMed] [Google Scholar]
- 40.Kunig A, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L1068–L1078. [DOI] [PubMed] [Google Scholar]
- 41.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol 2005;289:L529–L535. [DOI] [PubMed] [Google Scholar]
- 42.Liebow AA. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis 1959;80:67–93. [DOI] [PubMed] [Google Scholar]
- 43.Lammert E, Cleaver O, Melton D. Role of endothelial cells in early pancreas and liver development. Mech Dev 2003;120:59–64. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996;380:439–442. [DOI] [PubMed] [Google Scholar]
- 45.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996;380:435–439. [DOI] [PubMed] [Google Scholar]
- 46.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 1995;376:66–70. [DOI] [PubMed] [Google Scholar]
- 47.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1–deficient mice. Nature 1995;376:62–66. [DOI] [PubMed] [Google Scholar]
- 48.Pierce RA, Michael Shipley J. Retinoid-enhanced alveolization: identifying relevant downstream targets. Am J Respir Cell Mol Biol 2000;23:137–141. [DOI] [PubMed] [Google Scholar]
- 49.Acarregui MJ, Penisten ST, Goss KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am J Respir Cell Mol Biol 1999;20:14–23. [DOI] [PubMed] [Google Scholar]
- 50.Ng YS, Rohan R, Sunday ME, Demello DE, D'Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn 2001;220:112–121. [DOI] [PubMed] [Google Scholar]
- 51.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Cool C, Wood K, Tuder RM, Burns N, Kasper M, Voelkel NF. Simvastatin causes endothelial cell apoptosis and attenuates severe pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2006;291:L668–L676. [DOI] [PubMed] [Google Scholar]
- 52.Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest 2006;116:2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L119–L127. [DOI] [PubMed] [Google Scholar]
- 54.Han RN, Babaei S, Robb M, Lee T, Ridsdale R, Ackerley C, Post M, Stewart DJ. Defective lung vascular development and fatal respiratory distress in endothelial NO synthase–deficient mice: a model of alveolar capillary dysplasia? Circ Res 2004;94:1115–1123. [DOI] [PubMed] [Google Scholar]
- 55.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase–deficient mouse. Am J Physiol Lung Cell Mol Physiol 2003;284:L964–L971. [DOI] [PubMed] [Google Scholar]
- 56.DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, et al. Loss of PECAM-1 function impairs alveolarization. J Biol Chem 2006;281:8724–8731. [DOI] [PubMed] [Google Scholar]
- 57.Maniscalco WM, Watkins RH, Finkelstein JN, Campbell MH. Vascular endothelial growth factor mRNA increases in alveolar epithelial cells during recovery from oxygen injury. Am J Respir Cell Mol Biol 1995;13:377–386. [DOI] [PubMed] [Google Scholar]
- 58.Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH. Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 2004;287:L1178–L1185. [DOI] [PubMed] [Google Scholar]
- 59.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999;286:2511–2514. [DOI] [PubMed] [Google Scholar]
- 60.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 2006;355:343–353. [DOI] [PubMed] [Google Scholar]
- 61.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, Sekar KC, Auten RL, Bhutani VK, Gerdes JS, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med 2006;355:354–364. [DOI] [PubMed] [Google Scholar]
- 62.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 2003;349:2099–2107. [DOI] [PubMed] [Google Scholar]
- 63.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med 2005;172:750–756. [DOI] [PubMed] [Google Scholar]
- 64.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992;359:843–845. [DOI] [PubMed] [Google Scholar]
- 65.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Loss of HIF-2α and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 2002;8:702–710. [DOI] [PubMed] [Google Scholar]
- 66.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA 1997;94:4273–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asikainen TM, Chang LY, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, Shannon JM, Winter VT, Grubb P, Clyman RI, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. FASEB J 2006;20:1698–1700. [DOI] [PubMed] [Google Scholar]
- 68.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 69.Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 2003;9:702–712. [DOI] [PubMed] [Google Scholar]
- 70.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 2003;348:593–600. [DOI] [PubMed] [Google Scholar]
- 71.Cohnheim J. Über Entzündung und Eiterung. Virchows Arch Pathol Anat Physiol Klin Med 1867;40:1–79. [Google Scholar]
- 72.Yamada M, Kubo H, Kobayashi S, Ishizawa K, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow–derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol 2004;172:1266–1272. [DOI] [PubMed] [Google Scholar]
- 73.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Numasaki M, Ueda S, Suzuki T, Sasaki H. Bone marrow–derived cells contribute to lung regeneration after elastase-induced pulmonary emphysema. FEBS Lett 2004;556:249–252. [DOI] [PubMed] [Google Scholar]
- 74.Ishizawa K, Kubo H, Yamada M, Kobayashi S, Suzuki T, Mizuno S, Nakamura T, Sasaki H. Hepatocyte growth factor induces angiogenesis in injured lungs through mobilizing endothelial progenitor cells. Biochem Biophys Res Commun 2004;324:276–280. [DOI] [PubMed] [Google Scholar]
- 75.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med 2005;172:854–860. [DOI] [PubMed] [Google Scholar]
- 76.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax 2005;60:410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fadini GP, Schiavon M, Cantini M, Baesso I, Facco M, Miorin M, Tassinato M, Kreutzenberg SV, Avogaro A, Agostini C. Circulating progenitor cells are reduced in patients with severe lung disease. Stem Cells 2006;24:1806–1813. [DOI] [PubMed] [Google Scholar]
- 78.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol [online ahead of print] Jan 1, 2007; DOI: 10.1152/ajplung.00347.2006. Most recent version available from: http://dx.doi.org/10.1152/ajplung.00347.2006. [DOI] [PubMed]