Abstract
Rationale: Although pulmonary hypertension (PH) is a common complication of sickle cell disease (SCD) associated with high mortality, there exist few data characterizing hemodynamics and cardiopulmonary function in this population.
Objectives: To characterize hemodynamics and cardiopulmonary function in patients with SCD with and without PH.
Methods: Patients with SCD with PH (n = 26) were compared with control subjects with SCD but without PH (n = 17), matched for age, hemoglobin levels, and fetal hemoglobin levels.
Measurements and Main Results: Upon catheterization, 54% of the patients with PH had pulmonary arterial hypertension, and 46% had pulmonary venous hypertension. When compared with control subjects, patients with PH exhibited lower six-minute-walk distance (435 ± 31 vs. 320 ± 20 m, p = 0.002) and oxygen consumption (50 ± 3% vs. 41 ± 2% of predicted, p = 0.02), and also had mild restrictive lung disease and more perfusion abnormalities on radionuclide lung scans. The six-minute-walk distance in this population inversely correlated with tricuspid regurgitant jet velocity (r = −0.55, p < 0.001), and mean pulmonary artery pressure (r = −0.57, p < 0.001), and directly correlated with maximal oxygen consumption (r = 0.49, p = 0.004), even after adjustment for hemoglobin, supporting an independent contribution of increasing pulmonary artery pressures to loss of exercise capacity.
Conclusions: Patients with SCD-associated PH have both pulmonary arterial and venous PH associated with severe limitations in exercise capacity, likely compounded by interstitial lung fibrosis and severe anemia. These data support the use of the six-minute-walk distance as an index of PH and cardiopulmonary function in patients with SCD.
Keywords: sickle cell disease, pulmonary hypertension, six-minute walk, hemodynamics, echocardiogram
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Pulmonary hypertension is an emerging complication of sickle cell disease with high mortality. There are few data characterizing hemodynamics and cardiopulmonary function in this population.
What This Study Adds to the Field
Patients with sickle cell disease–associated pulmonary hypertension have both pulmonary arterial and venous pulmonary hypertension associated with severe limitations in exercise capacity, likely compounded by interstitial lung fibrosis and severe anemia.
Pulmonary arterial hypertension is an increasingly recognized complication of chronic hereditary and acquired hemolytic anemias, including sickle cell disease (SCD), thalassemia intermedia and major, paroxysmal nocturnal hemoglobinuria, hereditary spherocytosis and stomatocytosis, microangiopathic hemolytic anemias, pyruvate kinase deficiency, alloimmune hemolytic anemia, and possibly malaria (1, 2). In addition, certain conditions are associated with intravascular hemolysis, and consequently there is the potential risk for the development of pulmonary hypertension, such as schistosomiasis (3, 4), and iatrogenic hemolysis from mechanical heart valves (5, 6), left ventricular assist devices, and cardiopulmonary bypass procedures (7–11). Importantly, there are no case reports of pulmonary hypertension complicating iron deficiency anemia, the most common anemia in the world, suggesting a requirement for intravascular hemolysis for the development of this complication. The prevalence of pulmonary hypertension in adult patients with both SCD (using echocardiography, and defining pulmonary hypertension as a tricuspid regurgitant jet velocity ⩾ 2.5 m/s) and thalassemia intermedia (using echocardiography, and defining pulmonary hypertension defined as Doppler peak systolic tricuspid gradient > 30 mm Hg) approaches 30% (12, 13), and recent autopsy studies suggest that up to 75% of patients with SCD have histologic evidence of pulmonary arterial hypertension at the time of death (14). Considering the fact that SCD is the most common monogenetic disease in the world, with an estimated 30 million affected persons, hemolytic anemia represents a major emerging cause of pulmonary hypertension. However, only a few hemodynamic studies have been published and no functional characterization of these patients has been performed.
Although the degree of pulmonary hypertension in SCD is moderate, with mean pulmonary artery pressures of approximately 35 mm Hg, patients with severe anemia do not appear to tolerate this pulmonary vascular disease, because 2-year mortality rates range from 16 to 50% (12, 15). The high mortality rate in this population despite mild to moderate pulmonary hypertension with low pulmonary vascular resistance raises important questions about whether pulmonary hypertension is a marker of systemic vasculopathy and risk of death, rather than a direct cause of death, and whether the pulmonary hypertension occurs secondarily to intrinsic pulmonary vasculopathy (pulmonary arterial hypertension) or secondarily to left ventricular systolic or diastolic dysfunction (pulmonary venous hypertension) (16, 17). In addition, very little is known about the comorbidities and cardiopulmonary function of patients with hemolysis-associated pulmonary hypertension. Therefore, to better understand the pathophysiology of pulmonary hypertension in patients with SCD, we performed right-heart catheterization and detailed clinical evaluations of cardiopulmonary function in 26 patients with SCD with pulmonary hypertension compared with 17 age-, hemoglobin-, and fetal hemoglobin–matched control subjects with SCD without pulmonary hypertension.
Some of the data in this article were presented previously in abstract form (18).
METHODS
Patient Characteristics
All patients with SCD (n = 43) were enrolled in a National Heart, Lung, and Blood Institute (NHLBI)–approved human subjects protocol, and all subjects provided written, informed consent. These subjects were identified from a NHLBI-approved human subjects screening protocol that has evaluated 250 patients with SCD (12, 19, 20). All patients were consecutively recruited from 2002 through 2006, had SCD, and were homozygous for hemoglobin S, measured by high-pressure liquid chromatography. Transthoracic echocardiography was performed at least twice in all patients, and those with tricuspid regurgitant jet velocity of 2.5 m/second or greater were catheterized (n = 35). Subjects with SCD were determined to have pulmonary hypertension based on a mean pulmonary artery pressure of 25 mm Hg or greater, measured by right-heart catheterization (n = 26). Control subjects with SCD but without pulmonary hypertension (n = 17) were compared with subjects with pulmonary hypertension (n = 26) based on age, fetal hemoglobin, and total hemoglobin levels (Table 1). The control subjects without pulmonary hypertension were defined as having a mean pulmonary artery pressure of less than 25 mm Hg (n = 9) measured by right-heart catheterization, or in those in whom a right-heart catheterization was not performed, a peak tricuspid regurgitant jet velocity that was less than 2.5 m/second measured on at least two occasions (n = 8).
TABLE 1.
HEMATOLOGIC, ECHOCARDIOGRAPHIC, AND HEMODYNAMIC MEASUREMENTS IN PATIENTS WITH SICKLE CELL DISEASE WITHOUT AND WITH PULMONARY HYPERTENSION
| Without PHT | With PHT | p Value | |
|---|---|---|---|
| No. of patients | 17 | 26 | |
| Age, yr | 41 ± 2.2 (27, 60) | 43 ± 2.1 (23, 62) | 0.39 |
| Sex, M/F | 8/9 | 11/15 | |
| Hb, g/dl | 8.1 ± 0.3 (4.7, 10) | 8.3 ± 0.2 (5.2, 11.0) | 0.60 |
| Hb F, % | 6.8 ± 1.2 (0.7, 18.2) | 7.3 ± 1.2 (1.2, 21.8) | 0.78 |
| TR jet, m/s | 2.4 ± 0.1 (2, 3) | 3.4 ± 0.1 (2.8, 4.2) | < 0.001 |
| Right atrial area, cm2 | 21.5 ± 1.4 (13.1, 41.8) | 22.5 ± 1.1 (13, 35) | 0.57 |
| Left atrial size, mm | 40.4 ± 1.6 (30, 56) | 43.4 ± 1.3 (31, 62) | 0.15 |
| Ejection fraction | 65 ± 2.2 (47, 77) | 61 ± 1.8 (39, 77) | 0.16 |
| RAP, mm Hg | 6.2 ± 0.5 (4, 9) | 10.7 ± 1.1 (4, 34) | 0.02 |
| mPAP, mm Hg* | 21.1 ± 0.4 (19, 23) | 36.6 ± 1.5 (25, 51.7) | < 0.001 |
| PCWP, mm Hg | 12.1 ± 0.6 (10, 14) | 16.6 ± 1 (7, 27) | 0.009 |
| CO, L/min | 10.9 ± 0.6 (7.6, 13.7) | 8.6 ± 0.5 (4, 13) | 0.017 |
| PVR, dyn · s · cm−5 | 68.3 ± 6.2 (43, 94) | 206.1 ± 22.8 (57.4, 421) | 0.001 |
| TPR, dyn · s · cm−5 | 157 ± 10 (116, 231) | 370 ± 26 (192, 612) | < 0.001 |
| Pulmonary capacitance, ml/mm Hg | 8.5 ± 0.8 (4.8, 13.5) | 4.3 ± 0.5 (1.6, 13.8) | < 0.001 |
| Sv̇O2, % | 73 ± 1.5 (68, 84) | 66 ± 1.3 (47, 78) | 0.010 |
Definition of abbreviations: CO = cardiac output; Hb = hemoglobin; Hb F = fetal hemoglobin; M/F = male/female; mPAP = mean pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure; Sv̇O2= mixed venous oxygen saturation; TPR = total pulmonary resistance; TR jet = tricuspid regurgitant jet velocity;
Data are presented as mean ± SEM (min, max).
All patients with TR jet ⩾ 2.5 m/second were catheterized (n = 35).
Echocardiography
Transthoracic echocardiography was performed in all patients using the Acuson Sequoia (Siemens-Acuson, Inc., Mountain View, CA) and the Sonos 5500 (Philips, Inc., Andover, MA). Tricuspid regurgitation was assessed in the parasternal right ventricular inflow, parasternal short axis, and apical four-chamber views, and a minimum of five sequential complexes were recorded. Continuous wave Doppler sampling of the peak regurgitant jet velocity was used to estimate the right ventricular to right atrial systolic pressure gradient using the modified Bernoulli equation (4 × [tricuspid regurgitant jet velocity] × [tricuspid regurgitant jet velocity]). This measurement has been validated to correlate with pulmonary artery systolic pressure in the absence of right ventricular outflow obstruction and pulmonic stenosis (21). We have recently validated this measurement in patients with SCD (12).
Diastolic function was evaluated using Doppler peak E and A velocities, E/A ratio, deceleration time, and tissue Doppler imaging of the septal and lateral mitral annulus as previously described (22).
Right-Heart Catheterization
Standard right-heart catheterizations were performed in 35 patients and cardiac output was measured by thermodilution (Baxter Healthcare, Deerfield, IL). Pulmonary arterial capacitance (stroke volume/pulmonary artery pulse pressure) was calculated as previously described (23). The mean hemodynamic data from a subgroup of these patients have been previously reported (12).
Pulmonary Function Studies and Cardiopulmonary Exercise Testing
Patients were exercised on a bicycle ergometer and a computerized metabolic cart (Vmax 229 Cardiopulmonary Exercise System; Sensormedics, Yorba Linda, CA) using standard incremental protocols (24, 25), as previously reported (26). SaO2 was measured using a pulse oximeter (model 295; Nellcor Puritan Bennett, Pleasanton, CA). Tests were stopped when the patient reached an oxygen uptake plateau, when SaO2 fell below 88%, or when the patient complained of dyspnea, leg weakness, or severe fatigue. V̇o2max was defined as the highest oxygen uptake observed during any 30-second measurement period. Tests were supervised by a physician. Predicted values for V̇o2max were calculated from standard equations (27).
Lung volumes, flow rates, and diffusion capacity of carbon monoxide (DlCO) were measured using a computerized system (Master Screen PFT; Erich Jaeger, Wurzburg, Germany) according to American Thoracic Society recommendations (28, 29).
The six-minute-walk test was performed in accordance with standard practice (30, 31). Briefly, patients were asked to walk at an unhurried pace for 6 minutes along a premeasured path in the NIH Clinical Center. A single practice walk was performed first.
Ventilation–Perfusion Lung Scan
Each patient underwent a Xe 133 ventilation/Tc 99 MAA (macroaggregated albumin) perfusion scan in the National Institutes of Health Department of Nuclear Medicine. For these studies, patients were administered up to 30 mCi Xe-133 gas and 4 mCi Tc-99m MAA containing approximately 100,000 to 150,000 particles for each perfusion scan. Current recommendations are that MAA doses be limited to 100,000 to 200,000 particles in patients with severe pulmonary hypertension. To achieve this, we prepared the MAA kits in a manner specifically intended to yield approximately 100,000 to 150,000 particles in a 4-mCi dose. This required deviation from the manufacturer's instructions for preparation of the kit. Particle size was checked for each lot before use. Labeling efficiency was checked for each dose before use.
High-Resolution Computed Tomography Scans of the Lungs
Standard computed tomography (CT) and high-resolution CT (HRCT) of the chest were performed with GE Hispeed Advantage and Lightspeed scanners (GE Medical Systems, Waukesha, WI). HRCT scores were determined blindly by an investigator (N.A.A.) using a modification of a previously described technique (32). The CT findings were graded according to the severity and extent of pulmonary interstitial disease as follows: grade 0, normal findings; grade 1, minimal disease (thickened interalveolar septa, ground-glass opacities); grade 2, mild disease (more extensive thickening of the interalveolar septa and ground-glass opacities, reticular disease, and subpleural cysts); grade 3, moderate disease (findings of grade 2 in addition to traction bronchiectasis); and grade 4, severe disease (findings in grade 3 involving the lung parenchyma diffusely).
Statistical Analysis
Significant differences between patients with SCD with and without pulmonary hypertension were evaluated by unpaired t test. Comparisons of the relationship between tricuspid regurgitant jet velocities and perfusion scan and CT scan abnormalities were performed using one-way analysis of variance with a Dunnett's multiple comparison test for comparing differences between two groups. Bivariate correlations were assessed using Pearson correlations. A p value of less than 0.05 was considered statistically significant. Associations between six-minute-walk distance values and hemodynamic and exercise results were also analyzed by Pearson correlation analysis, with adjustment for hemoglobin levels evaluated by including hemoglobin levels in the regression model. Note that the study was designed to eliminate the hemoglobin effect by selection of control subjects with similar hemoglobin levels; this design allowed for exploration of other factors associated with pulmonary hypertension (i.e., exercise limitation independent of anemia). Analysis was done using NCSS 2004 software (Number Cruncher Statistical Systems, Kaysville, UT).
RESULTS
Patient Characteristics and Hemodynamics
As shown in Table 1, the patients defined as having pulmonary hypertension had significantly increased tricuspid regurgitant jet velocity, mean pulmonary artery pressure, pulmonary vascular resistance, right atrial pressure, and pulmonary capillary wedge pressure, and lower cardiac output. As previously reported (12, 33), patients with SCD without pulmonary hypertension have low pulmonary vascular resistance values (68.3 dyne · s · cm−5), whereas this value is 206.1 dyne · second · cm−5 in subjects with SCD and pulmonary hypertension.
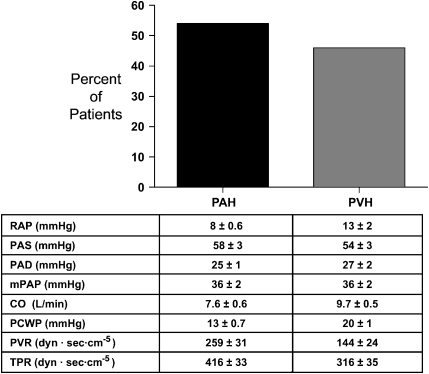
Among the 26 patients with pulmonary hypertension, defined by right-heart catheterization, 14 had pulmonary capillary wedge pressures of 15 mm Hg or less and 12 had pulmonary capillary wedge pressures greater than 15 mm Hg (Figure 1). As such, pulmonary arterial hypertension (defined by a mean pulmonary artery pressure ⩾ 25 mm Hg and a wedge pressure ⩽ 15 mm Hg) was present in 54% of catheterized patients and pulmonary venous hypertension (defined by a mean pulmonary artery pressure ⩾ 25 mm Hg and a wedge pressure > 15 mm Hg) was present in 46% of patients. These findings suggest that the etiology of pulmonary hypertension in patients with SCD is multifactorial, with half of the catheterized patients having pulmonary arterial hypertension with intrinsic pulmonary arteriopathy and half having pulmonary venous hypertension. It is, however, important to point out that, even though patients with pulmonary hypertension and normal wedge pressures have pulmonary vascular resistance greater than three times the reported values of chronically anemic patients with SCD (33), some individuals may not fit the traditional definition of pulmonary arterial hypertension (i.e., mean pulmonary artery pressure ⩾ 25 mm Hg, wedge pressure ⩽ 15 mm Hg, and pulmonary vascular resistance > 240 dyne · s · cm−5).
Figure 1.
Pulmonary arterial hypertension (PAH) is the most common cause of high pulmonary artery pressures in patients with sickle cell disease. Mean values ± SEM of hemodynamic variables for patient subgroups with PAH and pulmonary venous hypertension (PVH) are shown. CO = cardiac output; mPAP = mean pulmonary artery pressure; PAD = pulmonary artery diastolic pressure; PAS = pulmonary artery systolic pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure; TPR = total pulmonary resistance.
To gain insight into the etiology of pulmonary venous hypertension in the patients with elevated pulmonary capillary wedge pressure, we evaluated diastolic function using Doppler peak E and A velocities, E/A ratio, deceleration time, and tissue Doppler imaging of the septal and lateral mitral annulus. Four patients had moderate mitral or aortic valve regurgitation (which prevented adequate evaluation of diastolic function), four patients had normal diastolic function, three had mild diastolic dysfunction, and one had moderate diastolic dysfunction.
Effect of Anemia on Hemodynamics in Catheterized Patients
To investigate the role of a high-output state associated with chronic anemia and the presence of pulmonary hypertension, we evaluated the correlation between hemoglobin level and hemodynamic parameters in our patients with pulmonary hypertension. Consistent with the association between anemia and a high-output state, cardiac output correlated inversely with hemoglobin levels (r = −0.40, p = 0.033). However, hemoglobin levels directly correlated with mean pulmonary artery pressure (r = 0.48, p = 0.011) and pulmonary vascular resistance (r = 0.68, p = 0.001), and inversely with pulmonary capillary wedge pressure (r = −0.42, p = 0.030). These results suggest that, although the presence of anemia contributes to a high-output state and high left ventricular filling pressures, it does not seem to contribute to the elevations in pulmonary artery pressures and pulmonary arterial hypertension seen in our patients.
Cardiopulmonary Exercise Capacity and Six-Minute-Walk Distance
As shown in Table 2, the six-minute-walk distance and maximal oxygen consumption were significantly reduced in the patients with pulmonary hypertension compared with control subjects with SCD. The six-minute-walk distance was inversely correlated with pulmonary vascular resistance (r = −0.37, p = 0.029) and mean pulmonary artery pressure (r = −0.57, p < 0.001), and directly correlated with maximal oxygen consumption (r = 0.49, p = 0.003), supporting the contribution of increasing pulmonary artery pressures to loss of exercise capacity (Table 3 and Figure 2). We also observed strong correlation between six-minute-walk distance and tricuspid regurgitant jet velocity (r = −0.55, p < 0.001). All of these associations were adjusted for the levels of hemoglobin and fetal hemoglobin and remained statistically significant, supporting the contention that pulmonary hypertension impairs six-minute-walk distance independent of degree of anemia (Table 3).
TABLE 2.
SIX-MINUTE-WALK DISTANCE AND EXERCISE CAPACITY IN PATIENTS WITH SICKLE CELL DISEASE WITHOUT AND WITH PULMONARY HYPERTENSION
| Without PHT | With PHT | p Value | |
|---|---|---|---|
| No. of patients | 16 | 25 | |
| Six-minute walk, m | 435 ± 31 (249, 620) | 320 ± 20 (126, 507) | 0.002 |
| V̇o2max, ml/kg/min* | 14.49 ± 1.05 (9.4, 23) | 11.62 ± 0.73 (6.9, 19) | 0.027 |
| V̇o2max, % pred | 50 ± 3 (33, 70) | 41 ± 2 (29, 66) | 0.024 |
| HR max, beat/min | 142 ± 7 (103, 177) | 133 ± 5 (94, 185) | 0.29 |
| HR max, % pred | 80 ± 3 (58, 99) | 77 ± 2 (57, 107) | 0.51 |
| SBP rest, mm Hg | 121 ± 3.5 (99, 148) | 116 ± 3.3 (100, 132) | 0.29 |
| DBP rest, mm Hg | 72 ± 2.4 (57, 94) | 64 ± 1.9 (55, 77) | 0.042 |
| SBP max, mm Hg | 136 ± 3.0 (117, 149) | 148 ± (121, 197) | 0.11 |
| DBP max, mm Hg | 71 ± 2.6 (59, 83) | 76 ± 3.5 (53, 103) | 0.28 |
| O2 pulse,† ml/beat | 6.7 ± 0.5 (4.3, 9.4) | 6.4 ± 0.4 (4.1, 11.2) | 0.63 |
| V̇o2-AT, L/min | 0.70 ± 0.04 (0.44, 0.97) | 0.71 ± 0.05 (0.37, 1.10) | 0.77 |
| V̇o2-AT, % max | 35 ± 1.8 (26, 49) | 34 ± 2.3 (20, 60) | 0.65 |
| BR, % | 54 ± 2 (43, 77) | 41 ± 3 (8, 73) | 0.012 |
| RQ max | 1.32 ± 0.16 (1.11, 1.70) | 1.26 ± 0.04 (0.85, 1.77) | 0.32 |
| Work max, W | 85 ± 6.8 (46, 126) | 71 ± 4.9 (41, 123) | 0.11 |
| Work max, % pred | 62 ± 5.4 (30, 88) | 47 ± 4.6 (22, 91) | 0.044 |
| VeCO2 AT | 31.6 ± 1.5 (27, 46) | 39.2 ± 1.6 (26, 54) | 0.035 |
| VeCO2 max | 32.2 ± 1.5 (26, 46) | 37.8 ± 1.4 (28, 54) | 0.017 |
| SaO2 (rest), % | 96 ± 0.7 (92, 100) | 95 ± 0.8 (87, 100) | 0.36 |
| SaO2 (max), % | 95 ± 0.9 (90, 100) | 93 ± 1.2 (83, 100) | 0.21 |
Definition of abbreviations: BR = breathing reserve; DBP = systemic diastolic blood pressure; HR max = heart rate at peak exercise; PHT = pulmonary hypertension; RQ max = respiratory quotient at peak exercise; SBP = systemic systolic blood pressure; Vo2- AT = oxygen uptake at anaerobic threshold; VeCO2 AT = ventilatory equivalent for CO2 at anaerobic threshold; VeCO2 max = ventilatory equivalent for CO2 at peak exercise.
Data are presented as mean ± SEM (min, max).
Thirteen patients without PHT and 21 patients with PHT performed exercise test.
Oxygen pulse = V̇o2/HR max.
TABLE 3.
CORRELATION ANALYSIS OF TR JET VELOCITY, FUNCTIONAL CAPACITY, AND HEMODYNAMIC PARAMETERS IN PATIENTS WITH SICKLE CELL DISEASE
| Unadjusted
|
Adjusted
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | r | p | r | p | ||||
| Pearson correlation of six-minute-walk distance with TR jet velocity, hemodynamic measurements, and exercise capacity* | ||||||||
| TR jet velocity | −0.55 | < 0.001 | −0.55 | < 0.001 | ||||
| PVR | −0.37 | 0.029 | −0.25 | 0.16 | ||||
| mPAP | −0.57 | < 0.001 | −0.51 | 0.003 | ||||
| V̇o2max | 0.49 | 0.003 | 0.51 | 0.003 | ||||
| Pearson correlation of TR jet velocity with six-minute-walk distance, hemodynamic measurements, and exercise capacity* | ||||||||
| Six-minute walk | −0.55 | < 0.001 | −0.55 | < 0.001 | ||||
| PVR | 0.55 | < 0.001 | 0.57 | < 0.001 | ||||
| mPAP | 0.76 | < 0.001 | 0.77 | < 0.001 | ||||
| V̇o2max | −0.43 | 0.011 | −0.42 | 0.014 | ||||
Definition of abbreviations: mPAP = mean pulmonary artery pressure; PVR = pulmonary vascular resistance; TR jet velocity = tricuspid regurgitant jet velocity.
Unadjusted and adjusted for hemoglobin.
Figure 2.
Six-minute-walk distance (A) is significantly lower in patients with sickle cell disease and pulmonary hypertension (PHT) compared with those without PHT, (B) is inversely correlated with tricuspid regurgitant jet velocity (n = 40), and (C) is inversely correlated with mean pulmonary artery pressure (mPAP) (n = 35). Six-minute-walk distance is directly correlated with (D) maximal oxygen consumption (V̇o2max) (n = 34).
The V̇o2max and peak work rate were severely reduced in all patients with SCD, but the severity of the impairment was significantly more pronounced in patients with pulmonary hypertension (Table 2). Patients with pulmonary hypertension also demonstrated abnormal ventilatory responses to exercise as evidenced by lower breathing reserve and higher ventilatory equivalent for CO2 at anaerobic threshold when compared with patients without pulmonary hypertension. In addition, correlation analysis of V̇o2max revealed a significant association between these values and both tricuspid regurgitant jet velocity and six-minute-walk distance, even after adjustment for hemoglobin levels (Table 3).
Pulmonary Function
In contrast to children with SCD, who have an apparent increased prevalence of obstructive lung disease (34), the pulmonary function studies in adults with SCD reveal a restrictive pattern, especially in those with pulmonary hypertension (Table 4). There was also an observed reduction in DlCO between patients with and without pulmonary hypertension (Table 4). We performed methacholine testing in all patients without clinically active asthma (defined as requiring daily inhaled controller medications). No significant differences in airway reactivity was observed between patients with and without pulmonary hypertension, whereas approximately 20% of all adult patients had either methacholine-induced bronchospasm or clinically active asthma (Table 4).
TABLE 4.
PULMONARY FUNCTION STUDIES IN PATIENTS WITH SICKLE CELL DISEASE WITHOUT AND WITH PULMONARY HYPERTENSION
| Without PHT | With PHT | p Value | |
|---|---|---|---|
| No. of patients | 15 | 24 | |
| FEV1, L | 2.4 ± 0.1 (1.6, 3) | 1.8 ± 0.1 (0.8, 2.6) | < 0.001 |
| FEV1, % pred | 85.1 ± 3.2 (68, 108) | 70.6 ± 3.6 (27, 113) | 0.008 |
| FVC, L | 3.0 ± 0.1 (2, 3.9) | 2.4 ± 0.1 (0.9, 3.8) | 0.005 |
| FVC, % pred | 81.6 ± 2.3 (67.4, 103) | 70.2 ± 3.6 (24, 108) | 0.02 |
| FEV1/FVC ratio | 0.80 ± 0.02 (0.67, 0.91) | 0.78 ± 0.02 (0.65, 0.87) | 0.54 |
| TLC, L | 4.6 ± 0.2 (3.3, 5.7) | 4.0 ± 0.2 (2.2, 6.4) | 0.07 |
| TLC, % pred | 85.3 ± 2.9 (69.4, 106) | 79.9 ± 3.6 (38, 116) | 0.30 |
| FRC, L | 2.3 ± 0.1 (1.8, 3.1) | 2.8 ± 0.8 (1, 3.4) | 0.65 |
| FRC, % pred | 74.1 ± 3.6 (45, 93) | 67.8 ± 4.2 (31, 102) | 0.31 |
| RV, L | 1.5 ± 0.1 (0.7, 2.3) | 1.5 ± 0.1 (0.7, 2.6) | 0.78 |
| RV, % pred | 90.7 ± 6.7 (38, 132) | 97.5 ± 4.9 (59, 156) | 0.41 |
| RV/TLC ratio | 1.09 ± 0.06 (0.61, 1.46) | 1.29 ± 0.03 (0.98, 1.84) | 0.06 |
| DlCO, ml/min/mm Hg | 16.5 ± 1.2 (10.5, 27.2) | 13.7 ± 0.9 (4.9, 24.8) | 0.07 |
| DlCO, % pred | 65.8 ± 4.5 (48, 106) | 55.5 ± 3.3 (23.7, 85) | 0.07 |
| DlCOC, ml/min/mm Hg/L/Hb | 23.0 ± 2.0 (13, 41) | 18.5 ± 1.4 (6.4, 32) | 0.07 |
| DlCOC, % pred | 90.7 ± 6.5 (58, 135) | 71.9 ± 4.7 (31.6, 110.5) | 0.02 |
| Patients with positive methacholine test or clinically active asthma, n (%) | 3 (20%) | 4 (16.6%) |
Definition of abbreviations: DlCO = diffusion capacity for carbon monoxide; DlCOC = diffusion capacity for carbon monoxide corrected for hemoglobin level.
Data are presented as mean ± SEM (min, max).
Lung Parenchymal Fibrosis and Perfusion Abnormalities
Consistent with the results of pulmonary function testing, HRCT scans revealed mild basilar focal fibrosis, with fibrosis scores trending to increase with increased pulmonary artery systolic pressures (Figures 3A and 3B). V̇/Q̇ scans revealed that patients with pulmonary hypertension had significantly increased patchy pulmonary perfusion defects (Figures 3C and 3D). Most of these defects had low or indeterminate probability for pulmonary embolus. Six patients, three of whom had a clinical history of pulmonary embolism, had high probability V̇/Q̇ scans but had helical spiral CT scans that were negative for thromboemboli. Three of the scans suggested chronic thromboembolic pulmonary hypertension (CTEPH) (as read by L.J.R. and colleagues at the University of California, San Diego). Because pulmonary angiography was not performed in these patients, at this time we cannot conclusively rule out the presence of surgically accessible CTEPH. It also is not known if these defects represent vascular occlusion from episodic “sickling,” obliterative changes secondary to pulmonary hypertension, or in situ thrombosis secondary to pulmonary arterial hypertension.
Figure 3.
Mild interstitial lung disease and lung perfusion defects in patients with sickle cell disease and pulmonary hypertension (PHT). (A and B) Patients with PHT have more abnormalities on high-resolution computed tomography (CT) scans graded as mild to moderate interstitial lung disease, compared with patients without PHT. (C and D) Patients with PHT have more lung perfusion scans graded as low, intermediate, and high probability compared with patients without PHT whose scans are largely normal. *p < 0.05 when compared with patients with normal perfusion scans.
DISCUSSION
Although pulmonary hypertension is a common complication of chronic hemolytic diseases, little is known about the relationship between this vasculopathy and cardiopulmonary function and about factors that contribute to its development and associated mortality. We herein show that patients with SCD and pulmonary hypertension have both pulmonary arterial and pulmonary venous hypertension, mild restrictive interstitial lung disease, abnormalities on lung perfusion scans, and significant reductions in exercise capacity. Furthermore, in this study the six-minute-walk distance inversely correlated with the severity of pulmonary hypertension and directly with peak oxygen consumption, suggesting that this test can be used as a noninvasive measure of severity of pulmonary hypertension and functional capacity in this population. Despite mild to moderate pulmonary hypertension and a low pulmonary vascular resistance compared with that seen in idiopathic pulmonary arterial hypertension, the six-minute-walk distance and oxygen consumption are severely and equivalently impaired in both diseases. These results suggest that both pulmonary hypertension and severe anemia independently contribute to loss of functional capacity, potentially explaining the marked morbidity and mortality associated with pulmonary hypertension in this anemic population.
A major question that has arisen from the study of pulmonary hypertension in patients with SCD and thalassemia is how such mild pulmonary hypertension, and mild elevations in pulmonary vascular resistance, can be associated with risk of sudden death (12, 16, 17). Sudden death typically occurs as a consequence of progressive right-heart failure and hemodynamic collapse in patients with idiopathic pulmonary arterial hypertension. In our recently completed prospective study of mortality in patients with SCD and pulmonary hypertension, only 13 of 195 patients had evidence of right-heart failure (right atrial pressure > 15 mm Hg), and many of the patients who died had tricuspid regurgitant jet velocities of 2.5 to 3.0 m/second (12). It is also important to point out that, although the degree of increase in pulmonary vascular resistance is relatively mild, it is greater than two times higher than the values observed in our patients without pulmonary hypertension and in two previous series of patients with severe anemia (33, 35).
The current studies begin to help us understand this observation. Patients with idiopathic pulmonary arterial hypertension are young, have no comorbid organ dysfunction, and do not have critical anemia. Patients with SCD have multiorgan complications, such as pulmonary fibrosis and renal insufficiency, and perhaps more importantly, severe anemia. Despite mild pulmonary hypertension, our patients had maximal oxygen consumption values and six-minute-walk distances more severely reduced than patients with idiopathic pulmonary arterial hypertension. For comparison, the placebo group of patients with primary pulmonary arterial hypertension in a recently completed trial of sitaxsentan had a mean pulmonary vascular resistance of 911 dynes · second · cm−5, a mean pulmonary artery pressure of 52 mm Hg, a mean six-minute-walk distance of 413 m, and a V̇o2max of 48% of predicted (36), whereas our patients had values of 206 (ranging from 57 to 421) dyne · second · cm−5, 36 mm Hg, 320 m, and a V̇o2max of 41% of predicted, respectively. We speculate that the combined effects of anemia, increased pulmonary artery pressures, and central venous pressures and multiorgan dysfunction (pulmonary fibrosis, mild renal insufficiency, and diastolic cardiac dysfunction secondary to iron overload and anemia) all lead to significant functional impairment and increased risk of sudden death from dysrhythmia and acute right-heart failure during stress (vasoocclusive crisis, acute chest syndrome, or exercise).
Another question of importance, in light of the elevated pulmonary capillary wedge pressures and dilated right and left cardiac chambers observed (Table 1, Figure 1), is the potential contribution of left-sided heart disease, in particular diastolic dysfunction and high-output biventricular failure, to the development of pulmonary arterial hypertension. Here we show that in the majority of patients with SCD, pulmonary arterial hypertension is the cause of elevated pulmonary artery pressures, but also that pulmonary venous hypertension (in some cases due to valvular disease or diastolic dysfunction) does contribute to pulmonary hypertension in a subgroup of patients. Interestingly, echocardiographic evidence of diastolic dysfunction is common in patients with SCD; it contributes to pulmonary hypertension in only one-third of patients with a tricuspid regurgitant jet velocity of 2.5 m/second or greater, and represents an independent predictor of prospective mortality in these patients above and beyond a high tricuspid regurgitant jet velocity (22).
The observation of increasing perfusion defects on V̇/Q̇ scans as pulmonary pressures increase in this population is interesting. In situ thrombosis is observed in patients with idiopathic pulmonary arterial hypertension and in patients with SCD at autopsy (37–39). Thromboembolism is a reported cause of death in the sickle cell population; however, most of these data derive from autopsy studies, and a discrimination between in situ versus embolic etiology of vascular thrombosis was rarely considered. Recent autopsy studies suggest that much of the thrombosis is in situ, similar to what occurs in idiopathic pulmonary arterial hypertension (14). Indeed, the perfusion abnormalities observed in the current study are consistent with patchy perfusion defects typically observed in idiopathic pulmonary arterial hypertension, and in only three cases did the perfusion scans have the classic appearance of CTEPH. However, regardless of whether thrombosis occurs in situ or secondarily to thromboembolism, the findings in the present study, that patients with elevated pulmonary artery pressures have more perfusion defects, suggest that these patients could benefit from systemic anticoagulation similarly to those with idiopathic pulmonary arterial hypertension (40).
CTEPH can occur in patients with SCD and has been treated surgically with success (41); patients with SCD and pulmonary hypertension should therefore undergo imaging studies and, if suggestive of CTEPH, they should undergo more invasive studies (i.e., angiography) to exclude this potentially surgically treatable condition. Restrictive pulmonary function abnormalities in this setting may represent prior areas of infarction (42). Alternatively, mild restriction has been observed in idiopathic pulmonary hypertension as well, for reasons that are not entirely clear (43). Interestingly, although conventional wisdom holds that pulmonary fibrosis develops in patients with SCD as a consequence of repeated episodes of the acute chest syndrome, we have observed no association between the development of pulmonary hypertension and history of acute chest syndrome in two separate populations of patients with SCD (12, 19). Pulmonary fibrosis also develops in patients with thalassemia, who do not suffer from the acute chest syndrome and who frequently develop pulmonary hypertension.
In conclusion, despite mild to moderate elevations in pulmonary pressures, mild pulmonary fibrosis, and mild restrictive lung disease, patients with SCD have severe impairments in aerobic exercise capacity and six-minute-walk distance that correlates with the severity of pulmonary hypertension. On hemodynamic assessment, 54% of the patients with pulmonary hypertension had pulmonary arterial hypertension, and 46% had pulmonary venous hypertension. Finally, these data support the use of both the tricuspid regurgitant jet velocity and the six-minute-walk distance as noninvasive indices of pulmonary hypertension and cardiopulmonary function in patients with SCD, and may be useful as endpoints in clinical trials targeting this emerging complication.
Acknowledgments
The authors thank Bill Blackwelder for assistance with statistical analysis and Stanislav Sidenko for technical assistance with echo measurements.
Supported by the Intramural Research Division of the National Institutes of Health.
Originally Published in Press as DOI: 10.1164/rccm.200610-1498OC on March 22, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Machado RF, Gladwin MT. Chronic sickle cell lung disease: new insights into the diagnosis, pathogenesis and treatment of pulmonary hypertension. Br J Haematol 2005;129:449–464. [DOI] [PubMed] [Google Scholar]
- 2.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 2005;293:1653–1662. [DOI] [PubMed] [Google Scholar]
- 3.Strauss E, Da Costa Gayotto LC, Antonelli R, Deperon S, Cabral GL, Raia S. Systemic surgical shunts and splenomegaly as causes of haemolysis in portal hypertension in Mansonic schistosomiasis: evaluation through serum levels of haptoglobin, hemopexin and bilirubins. J Hepatol 1986;2:340–350. [DOI] [PubMed] [Google Scholar]
- 4.de Cleva R, Herman P, Pugliese V, Zilberstein B, Saad WA, Rodrigues JJ, Laudanna AA. Prevalence of pulmonary hypertension in patients with hepatosplenic Mansonic schistosomiasis:–prospective study. Hepatogastroenterology 2003;50:2028–2030. [PubMed] [Google Scholar]
- 5.Kyllonen K, Mattila T, Hartikainen M, Tala P. Mitral valve replacement with ball and tilting disc valve prosthesis: a clinical and haemodynamic study. Scand J Thorac Cardiovasc Surg 1976;10:15–20. [DOI] [PubMed] [Google Scholar]
- 6.Iwaki H, Kuraoka S, Tatebe S. Hemolytic anemia due to aortic valve regurgitation after mitral valve replacement [in Japanese]. Kyobu Geka 2003;56:124–128. [PubMed] [Google Scholar]
- 7.Takami Y, Makinouchi K, Nakazawa T, Benkowski R, Glueck J, Ohara Y, Nose Y. Hemolytic characteristics of a pivot bearing supported Gyro centrifugal pump (C1E3) simulating various clinical applications. Artif Organs 1996;20:1042–1049. [DOI] [PubMed] [Google Scholar]
- 8.Chukwuemeka AO, Turtle MR, Trivedi UH, Venn GE, Chambers DJ. A clinical evaluation of platelet function, haemolysis and oxygen transfer during cardiopulmonary bypass comparing the Quantum HF-6700 to the HF-5700 hollow fibre membrane oxygenator. Perfusion 2000;15:479–484. [DOI] [PubMed] [Google Scholar]
- 9.Pierangeli A, Masieri V, Bruzzi F, De Toni E, Grillone G, Boni P, Delnevo A. Haemolysis during cardiopulmonary bypass: how to reduce the free haemoglobin by managing the suctioned blood separately. Perfusion 2001;16:519–524. [DOI] [PubMed] [Google Scholar]
- 10.Gerrah R, Shargal Y, Elami A. Impaired oxygenation and increased hemolysis after cardiopulmonary bypass in patients with glucose-6-phosphate dehydrogenase deficiency. Ann Thorac Surg 2003;76:523–527. [DOI] [PubMed] [Google Scholar]
- 11.Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res 2004;94:119–126. [DOI] [PubMed] [Google Scholar]
- 12.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 2004;350:886–895. [DOI] [PubMed] [Google Scholar]
- 13.Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, Joussef J, Rombos J, Loukopoulos D. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood 2001;97:3411–3416. [DOI] [PubMed] [Google Scholar]
- 14.Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol 2002;33:1037–1043. [DOI] [PubMed] [Google Scholar]
- 15.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood 2003;101:1257–1261. [DOI] [PubMed] [Google Scholar]
- 16.Hassoun PM, Krishnan JA. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease [letter to the editor]. N Engl J Med 2004;350:2521–2522; author reply 2521–2. [PubMed] [Google Scholar]
- 17.Klings ES, Farber HW. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease [letter to the editor]. N Engl J Med 2004;350:2521–2522; author reply 2521–2. [DOI] [PubMed] [Google Scholar]
- 18.Machado RF, Martyr SE, Anthi A, Kato GJ, Hunter LA, Coles WA, Nichols JS, Castro OL, Gladwin MT. Pulmonary hypertension in sickle cell disease: cardiopulmonary evaluation and response to chronic phosphodiasterase 5 inhibitor therapy. Blood 2004;104:71a. [Google Scholar]
- 19.Machado RF, Anthi A, Steinberg MH, Bonds D, Sachdev V, Kato GJ, Taveira-DaSilva AM, Ballas SK, Blackwelder W, Xu X, et al. N-terminal pro-brain natriuretic peptide levels and risk of death in sickle cell disease. JAMA 2006;296:310–318. [DOI] [PubMed] [Google Scholar]
- 20.Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 2005;294:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol 1985;6:359–365. [DOI] [PubMed] [Google Scholar]
- 22.Sachdev N, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, St Peter M, Coles W, Rosing DR, Blackwelder W, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol 2007;49:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahapatra S, Nishimura RA, Sorajja P, Cha S, McGoon MD. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2006;47:799–803. [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003;167:211–277. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman K, Hansen J, Sue DY, Casaburi R, Whipp BJ. Principles of exercise testing and interpretation. Baltimore, MD: Lippincott, Williams & Wilkins; 1999.
- 26.Taveira-DaSilva AM, Stylianou MP, Hedin CJ, Kristof AS, Avila NA, Rabel A, Travis WD, Moss J. Maximal oxygen uptake and severity of disease in lymphangioleiomyomatosis. Am J Respir Crit Care Med 2003;168:1427–1431. [DOI] [PubMed] [Google Scholar]
- 27.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis 1984;129:S49–S55. [DOI] [PubMed] [Google Scholar]
- 28.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor): recommendations for a standard technique—1995 update. Am J Respir Crit Care Med 1995;152:2185–2198. [DOI] [PubMed] [Google Scholar]
- 30.American Thoracic Society. ATS statement: guidelines for the six-minute-walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 31.Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute-walk test. Am J Respir Crit Care Med 2003;167:1287. [DOI] [PubMed] [Google Scholar]
- 32.Avila NA, Brantly M, Premkumar A, Huizing M, Dwyer A, Gahl WA. Hermansky-Pudlak syndrome: radiography and CT of the chest compared with pulmonary function tests and genetic studies. AJR Am J Roentgenol 2002;179:887–892. [DOI] [PubMed] [Google Scholar]
- 33.Leight L, Snider TH, Clifford GO, Hellems HK. Hemodynamic studies in sickle cell anemia. Circulation 1954;10:653–662. [DOI] [PubMed] [Google Scholar]
- 34.Minter KR, Gladwin MT. Pulmonary complications of sickle cell anemia: a need for increased recognition, treatment, and research. Am J Respir Crit Care Med 2001;164:2016–2019. [DOI] [PubMed] [Google Scholar]
- 35.Roy SB, Bhatia ML, Mathur VS, Virmani S. Hemodynamic effects of chronic severe anemia. Circulation 1963;28:346–356. [DOI] [PubMed] [Google Scholar]
- 36.Barst RJ, Langleben D, Frost A, Horn EM, Oudiz R, Shapiro S, McLaughlin V, Hill N, Tapson VF, Robbins IM, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med 2004;169:441–447. [DOI] [PubMed] [Google Scholar]
- 37.Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med 2001;125:1436–1441. [DOI] [PubMed] [Google Scholar]
- 38.Manci EA, Culberson DE, Yang YM, Gardner TM, Powell R, Haynes J Jr, Shah AK, Mankad VN. Causes of death in sickle cell disease: an autopsy study. Br J Haematol 2003;123:359–365. [DOI] [PubMed] [Google Scholar]
- 39.Vichinsky EP. Pulmonary hypertension in sickle cell disease. N Engl J Med 2004;350:857–859. [DOI] [PubMed] [Google Scholar]
- 40.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 1992;327:76–81. [DOI] [PubMed] [Google Scholar]
- 41.Yung GL, Channick RN, Fedullo PF, Auger WR, Kerr KM, Jamieson SW, Kapelanski DP, Moser KM. Successful pulmonary thromboendarterectomy in two patients with sickle cell disease. Am J Respir Crit Care Med 1998;157:1690–1693. [DOI] [PubMed] [Google Scholar]
- 42.Morris TA, Auger WR, Ysrael MZ, Olson LK, Channick RN, Fedullo PF, Moser KM. Parenchymal scarring is associated with restrictive spirometric defects in patients with chronic thromboembolic pulmonary hypertension. Chest 1996;110:399–403. [DOI] [PubMed] [Google Scholar]
- 43.Sun XG, Hansen JE, Oudiz RJ, Wasserman K. Pulmonary function in primary pulmonary hypertension. J Am Coll Cardiol 2003;41:1028–1035. [DOI] [PubMed] [Google Scholar]