Abstract
Rationale: Mechanisms that impair angiogenesis in neonatal persistent pulmonary hypertension (PPHN) are poorly understood.
Objectives: To determine if PPHN alters fetal pulmonary artery endothelial cell (PAEC) phenotype and impairs growth and angiogenesis in vitro, and if altered vascular endothelial growth factor–nitric oxide (VEGF–NO) signaling contributes to this abnormal phenotype.
Methods: Proximal PAECs were harvested from fetal sheep that had undergone partial ligation of the ductus arteriosus in utero (PPHN) and age-matched control animals. Growth and tube formation ± VEGF and NO stimulation and inhibition were studied in normal and PPHN PAECs. Western blot analysis was performed for VEGF, VEGF receptor-2 (VEGF-R2), and endothelial NO synthase (eNOS) protein content. NO production with VEGF administration was measured in normal and PPHN PAECs.
Measurements and Main Results: PPHN PAECs demonstrate decreased growth and tube formation in vitro. VEGF and eNOS protein expression were decreased in PPHN PAECs, whereas VEGF-R2 protein expression was not different. VEGF and NO increased PPHN PAEC growth and tube formation to values achieved in normal PAECs. VEGF inhibition decreased growth and tube formation in normal and PPHN PAECs. NOS inhibition decreased growth in normal and PPHN PAECs, but tube formation was only reduced in normal PAECs. NO reversed the inhibitory effects of VEGF-R2 inhibition on tube formation in normal and PPHN PAECs. VEGF increased NO production in normal and PPHN PAECs.
Conclusions: PPHN in utero causes sustained impairment of PAEC phenotype in vitro, with reduced PAEC growth and tube formation and down-regulation of VEGF and eNOS protein. VEGF and NO enhanced growth and tube formation of PPHN PAECs.
Keywords: persistent pulmonary hypertension of the newborn, angiogenesis, vascular endothelial growth factor, nitric oxide, endothelial cells
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
In severe neonatal pulmonary hypertension, lung vascular growth is impaired. Mechanisms that regulate vascular growth during normal lung development and impair angiogenesis in severe neonatal persistent pulmonary hypertension (PPHN) are poorly understood.
What This Study Adds to the Field
Impaired VEGF–NO signaling within the endothelial cell itself appears to contribute to abnormal vascular growth in PPHN.
Persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome characterized by elevated pulmonary vascular resistance (PVR) after birth, which causes extrapulmonary right-to-left shunting across the foramen ovale and ductus arteriosus (DA), leading to severe hypoxemia (1). Although PPHN is associated with diverse cardiopulmonary disorders, mechanisms contributing to high PVR include elevated pulmonary vascular tone, hypertensive remodeling of the vessel wall, and in severe cases, decreased vascular growth due to impaired angiogenesis (2).
Laboratory studies of experimental models of PPHN suggest that endothelial cell dysfunction, including decreased production of vasodilators, such as nitric oxide (NO), and increased release of vasoconstrictors, such as endothelin (ET)-1, contributes significantly to the pathophysiology of high PVR (3–9, 17, 18). Although treatment with inhaled NO is effective in improving outcomes of many newborns with PPHN, some infants fail to respond to inhaled NO, and require extracorporeal membrane oxygenation (ECMO) therapy with increased morbidity and mortality (10–13). PPHN associated with poor responsiveness to inhaled NO is often seen in the setting of impaired vascular growth, as seen in patients with lung hypoplasia, such as congenital diaphragmatic hernia and primary lung hypoplasia (12). In the setting of lung hypoplasia, decreased arterial number plays a prominent role in maintaining high PVR that is refractory to therapy. Due to the lack of developmentally relevant in vivo and in vitro models, mechanisms responsible for endothelial cell dysfunction and impaired angiogenesis in severe PPHN remain poorly understood.
Past studies using partial ligation of the DA in utero in late-gestation fetal sheep provide a useful animal model for studying the pathogenesis and treatment of PPHN (3, 14–16). In this model, partial ligation of the DA increases pulmonary artery pressure without causing sustained elevations of pulmonary blood flow or hypoxemia (3). After chronic intrauterine DA constriction, PVR remains elevated at birth with elevated pulmonary artery pressure and reduced pulmonary blood flow, despite ventilation with high levels of supplemental oxygen (3). Endothelial cell dysfunction, as defined by altered production of vasoactive mediators, contributes to elevated vascular tone in PPHN, but may also decrease lung vascular growth. Recent studies in this model have demonstrated that chronic intrauterine pulmonary hypertension impairs lung angiogenesis and causes lung hypoplasia (19). Mechanisms through which fetal pulmonary hypertension inhibits lung vascular growth are uncertain, but they may be related to decreased production of proangiogenic growth factors, such as vascular endothelial growth factor (VEGF), or altered responsiveness to mitogenic stimuli (20, 21, 38).
We recently reported that impaired alveolarization and vessel growth in chronic intrauterine pulmonary hypertension is associated with decreased VEGF protein expression (19) and that VEGF treatment improved hemodynamics and lung vascular growth (20). PPHN in vivo is further characterized by decreased endothelial NO synthase (eNOS) protein content and impaired NO production (5, 6). Decreased NO production has been implicated in contributing to elevated vascular tone as well as hypertensive remodeling in PPHN (6, 35). Alterations in proangiogenic growth factors may be responsible for impaired angiogenesis in PPHN; however, the mechanisms by which hemodynamic stress (hypertension) impairs endothelial cell function and angiogenesis in the developing lung remain unknown.
Therefore, we hypothesized that chronic intrauterine pulmonary hypertension directly alters endothelial cell phenotype, impairing VEGF–NO signaling within the endothelial cell, and decreasing the ability of the developing endothelium to proliferate and form new vessels.
METHODS
Isolation and Culture of Fetal Ovine Pulmonary Arterial Endothelial Cells
All procedures and protocols were reviewed and approved by the Animal Care and Use Committee at the University of Colorado Health Sciences Center (Denver, CO). The left and right pulmonary arteries were isolated from late-gestation normal fetal sheep (mixed-breed Columbia-Rambouillet pregnant ewes at 135 d gestation (n = 4), term = 147 d) and from fetal sheep that had undergone partial ligation of the DA in utero 7 to 10 days before being killed (PPHN) (n = 4), as previously described (3). Proximal pulmonary artery endothelial cells (PAECs) were isolated as previously described (7) and endothelial cell phenotype confirmed by positive immunostaining for standard endothelial cell markers. Cells from passage 4 and 5 were used for each of the study experiments, and cells from each animal were kept separate throughout all passages and for all experiments.
Cell Growth
Fetal PAECs from normal and PPHN lambs were plated at 2 × 105 cells/well and allowed to adhere overnight. Cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum under 3% oxygen conditions. Daily cell counts were performed for 5 days using a Beckman Coulter cell counter (Beckman Coulter, Inc., Fullerton, CA), after removing cells from wells by 0.25% trypsin/0.53 mM ethylenediaminetetraacetic acid digestion.
The effects of VEGF (50 ng/ml), S-nitroso-N-acetylpenicillamine (SNAP; 1 mM; NO donor) (Calbiochem, San Diego, CA), NO gas (10 ppm), SU5416 (a VEGF receptor inhibitor, 10 μM), nitro-l-arginine (LNA; an NOS inhibitor; 4 mM), and combinations of these agents on cell growth and tube formation were compared between PAECs from normal and PPHN fetal sheep. Doses for each agent were based on preliminary experiments (VEGF, NO gas, SNAP, and LNA) and previously published data (SU5416) (31). For each agent, the lowest dose of agent for which a given effect was seen was used. NO gas was used for the growth studies because preliminary experiments revealed NO gas at 10 ppm to be more effective than SNAP at stimulating cell growth in PPHN PAECs. SNAP, at various doses (1 mM, 5 mM, 10 mM) with single treatment or with repeated administrations every 24 hours, was less effective than NO gas at stimulating PAEC growth in PPHN cells (data no shown). SNAP (1 μm) was used for the tube formation studies because NO gas was less effective at enhancing tube formation than SNAP when continuously administered at 10 ppm (data not shown). Growth studies with treatment were performed in DMEM supplemented with 5% serum, because this was the lowest serum concentration that supported fetal PAEC proliferation.
Tube Formation Assay
The ability of fetal PAECs to form vascular structures in vitro was assayed by plating PAECs on EHS Matrigel (BD Pharmingen, San Jose, CA). PAECs from normal and PPHN animals were seeded at a density of 5 × 104 cells/well in serum-free DMEM supplemented with and without VEGF (50 ng/ml), SNAP (1 μm), LNA (4 mM), SU5416 (10 μm), and SU5416 (10 μm) with SNAP (1 μm) in combination. PAECs were incubated for 6 hours under 3% oxygen conditions. Branch-point counting was performed in blinded fashion under ×10 magnification from each of four wells, as previously described (23).
Western Blot Analysis
PAECs from normal and PPHN animals were grown on 150-mm cloning dishes in DMEM supplemented with 10% serum. At 90% confluence, cell lysates were collected as previously described (22). Protein content was determined by the BCA assay (catalog no. 23225; Pierce Biotechnology, Inc., Rockford, IL), using bovine serum albumin as the standard. A 20-μg protein sample per lane was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and proteins from the gel were transferred to nitrocellulose membrane. VEGF (catalog no. SC152; Santa Cruz Biotech, Santa Cruz, CA), VEGF receptor 2 (VEGF-R2) (catalog no. SC504; Santa Cruz Biotech), eNOS (catalog no. BD610297) (eNOS/NOS III), and β-actin (catalog no. A5316; Sigma, St. Louis, MO) were detected as previously described (46, 47) using appropriate controls and molecular weight as identified by the manufacturer for the protein of interest. Densitometry was performed using NIH Image (version 1.61; National Institutes of Health, Bethesda, MD). Changes in protein expression were analyzed after normalizing for β-actin expression.
NO Assay
NO production by normal and PPHN PAECs was determined using the DAF-FM Nitric Oxide indicator (no. D-23844; Molecular Probes, Eugene, OR). NO was measured by fluorescence after a 1-hour VEGF (50 ng/ml) exposure, as previously described (36, 37).
Proliferation/apoptosis was assessed in normal and PPHN PAECs. Normal and PPHN PAECs were plated on chamber slides and immunostaining performed for Ki67 (1:250, SC15402; Santa Cruz Biotech) and active caspase-3 (1:250, AB2362; Chemicon, Temecula, CA). Comparison was made between normal and PPHN PAECs.
Statistical Analysis
Data are presented as means ± SEM, with data from all four animals in the normal and PPHN groups combined for analysis. Statistical analysis was performed with the Prism 4 software package (GraphPad Software, San Diego, CA). Statistical comparisons were made using analysis of variance for growth and tube formation assays with Bonferroni post test analysis. An unpaired t test was used for Western blot analysis. P values less than 0.05 were considered significant.
RESULTS
Decreased Growth and Tube Formation in Fetal PAECs from PPHN Lambs
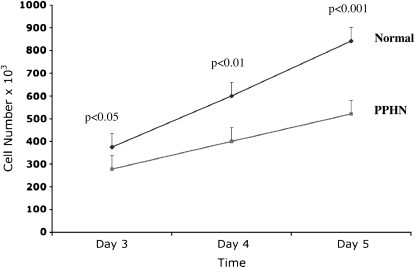
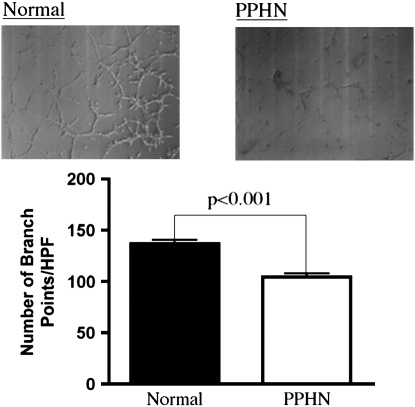
In comparison with control animals, PAEC numbers from PPHN lambs were decreased by 28% (P < 0.05) and 35% (P < 0.001) on Days 3 and 5, respectively (Figure 1). The ability of PAECs to spontaneously form vascular networks was also decreased in PPHN cells (Figure 2). In comparison with normal fetal PAECs, the number of branch points was decreased by 24% (P < 0.001) in PAECs harvested from PPHN lambs (Figure 2).
Figure 1.
Decreased growth in fetal pulmonary artery endothelial cells (PAECS) from sheep with persistent pulmonary hypertension of the newborn (PPHN). Fetal ovine PAECs from normal and PPHN lambs were plated in 10% fetal bovine serum under 3% oxygen conditions and counted daily for 5 days. In comparison with normal PAECs, proliferation of endothelial cells from PPHN lambs was decreased over time in culture from Day 3 to Day 5. Error bars represent SD from mean.
Figure 2.
Decreased tube formation in fetal pulmonary artery endothelial cells (PAECS) from sheep with persistent pulmonary hypertension of the newborn (PPHN). Fetal ovine PAECs from normal and PPHN lambs were plated on Matrigel in serum-free media under 3% oxygen conditions. The ability of PPHN cells to form vascular networks is markedly impaired when compared with normal controls. Error bars represent SD from mean. HPF = high power field.
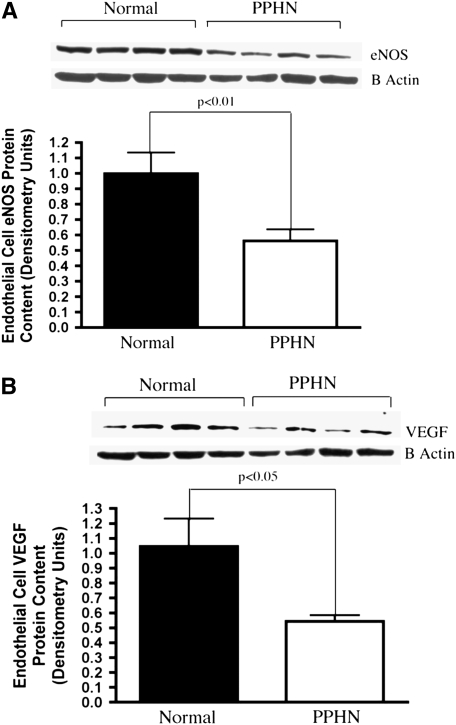
Decreased eNOS and VEGF Protein Expression in Fetal PAECs from PPHN Lambs
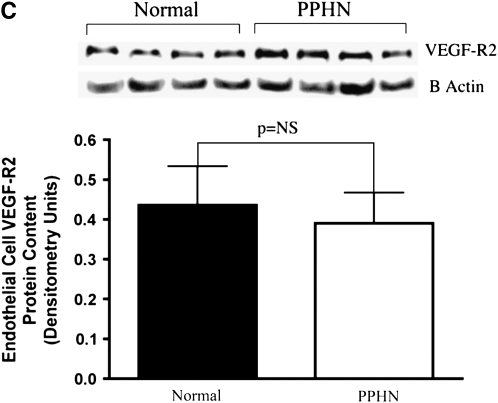
Western blot analysis of PAEC cell lysates from normal and PPHN lambs demonstrated decreased eNOS and VEGF protein. In comparison with controls, eNOS protein expression was decreased by 38% in PPHN PAECs (P < 0.01) (Figure 3A) and VEGF protein expression by 48% in PPHN PAECs (P < 0.05) (Figure 3B). VEGF-R2 expression was not different between normal and PPHN PAECs (Figure 3C).
Figure 3.
Decreased endothelial NO synthase (eNOS) and vascular endothelial growth factor (VEGF) protein expression in fetal pulmonary artery endothelial cells (PAECs) from sheep with persistent pulmonary hypertension of the newborn (PPHN). Western blot analysis on cell lysates from PAECs from PPHN sheep demonstrate decreased eNOS (A) and VEGF (B) protein when compared with normal controls. There was no difference in kinase domain receptor (C) expression. Error bars represent SD from mean.
Effect of VEGF and SU5416 on Endothelial Cell Growth
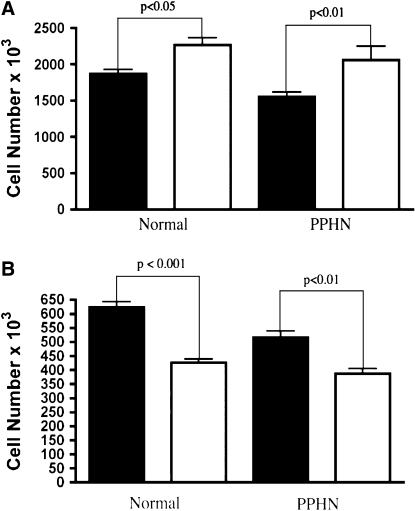
VEGF treatment increased growth in PAECs harvested from both normal and PPHN lambs. VEGF treatment of normal PAECs increased cell number by 21% (P < 0.05). VEGF treatment of PPHN PAECs increased cell number by 33% (P < 0.01), achieving values similar to that measured in the normal PAECs (Figure 4A). Treatment with the VEGF receptor blocker SU5416 decreased cell number by 32% (P < 0.001) in normal and 25% (P < 0.05) in the PPHN PAECs (Figure 4B).
Figure 4.
Effect of VEGF and SU5416 on endothelial cell growth. Ovine pulmonary artery endothelial cells (PAECs) from normal lambs and lambs with persistent pulmonary hypertension of the newborn (PPHN) were grown in 5% fetal bovine serum under 3% oxygen conditions with and without VEGF (50 ng/ml) and SU5416 (10 μM). (A) VEGF treatment stimulated growth of PAECs in both normal and PPHN cells (solid bars, control; open bars, VEGF). (B) VEGF receptor blockade with SU5416 decreased growth in both normal and PPHN PAECs (solid bars, control; open bars, SU5416). Error bars represent SD from mean.
Effect of NO Gas and LNA on Endothelial Cell Growth
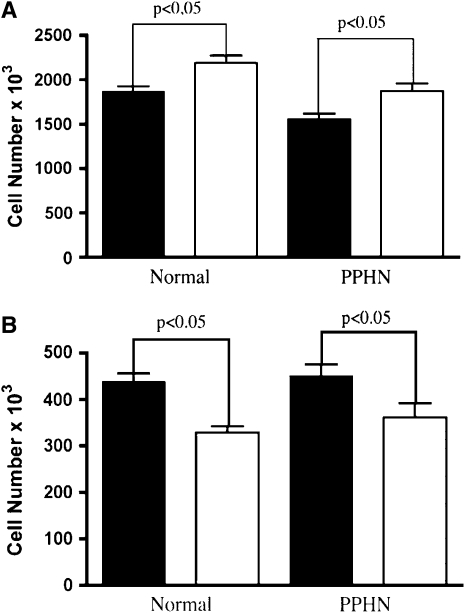
NO gas (10 ppm) treatment increased endothelial PAEC number by 17% (P < 0.05) in normal and by 21% (P < 0.05) in PPHN PAECs (Figure 5A). NOS inhibition with LNA treatment decreased cell number by 25 and by 29% in normal and PPHN PAECs, respectively (P < 0.05, for each comparison) (Figure 5B). NO gas did not rescue growth of control and PPHN PAECs from the inhibitory effects of SU5416 treatment. Growth remained decreased by 32% (P < 0.001) in normal and 31% (P < 0.01) in PPHN PAECs with combined treatment with SU5416 and NO gas (not shown).
Figure 5.
Effect of NO gas and nitro-l-arginine (LNA) on endothelial cell growth. Fetal ovine pulmonary artery endothelial cells (PAECs) from normal lambs and lambs with persistent pulmonary hypertension of the newborn (PPHN) were grown in 5% fetal bovine serum under 3% oxygen conditions with and without NO gas (10 ppm) and LNA (4 mM). (A) As shown, NO treatment stimulated growth (solid bars, control; open bars, NO gas); and (B) NO synthase inhibition with LNA decreased growth in normal and PPHN PAECs (solid bars, control; open bars, LNA). Error bars represent SD from mean.
Effect of VEGF and SU5416 on Tube Formation
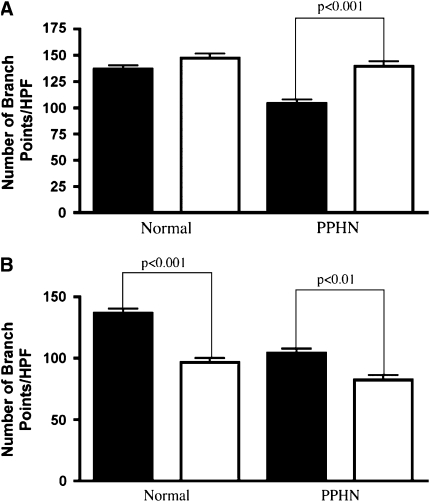
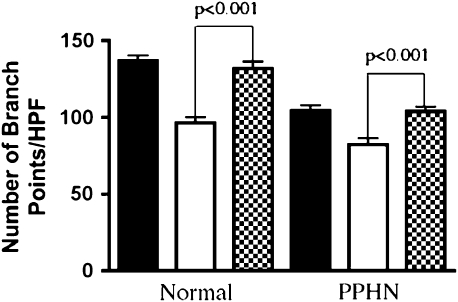
VEGF treatment had no effect on the number of branch points in normal PAECs, but increased the number of branch points by 31% in the PPHN cells (P < 0.001; Figure 6A). VEGF treatment fully reversed the decreased angiogenesis seen in vitro in PPHN PAECs to values achieved by control PAECs. VEGF receptor blockade with SU5416 decreased the number of branch points in both normal and PPHN PAECs. The number of branch points was decreased by 29% in normal PAECs (P < 0.001; Figure 6B). The reduction in tube formation by normal PAECs after SU5416 treatment was similar to the untreated values measured in PAECs from PPHN lambs. In PAECs from PPHN lambs, SU5416 further decreased tube formation by 21% (P < 0.01; Figure 6B).
Figure 6.
Effect of VEGF and SU5416 on tube formation. Fetal ovine pulmonary artery endothelial cells (PAECs) from normal sheep and sheep with persistent pulmonary hypertension of the newborn (PPHN) were plated on Matrigel in serum-free media under 3% oxygen conditions with and without VEGF treatment (50 ng/ml) and SU5416 (10 μM) treatment. (A) VEGF treatment increased the number of branch points in PPHN but not normal cells, rescuing the abnormal phenotype (solid bars, control; open bars, VEGF). (B) VEGF receptor blockade with SU5416 decreased tube formation in both normal and PPHN cells (solid bars, control; open bars, SU5416). HPF = high power field.
Effect of SNAP and LNA on Tube Formation
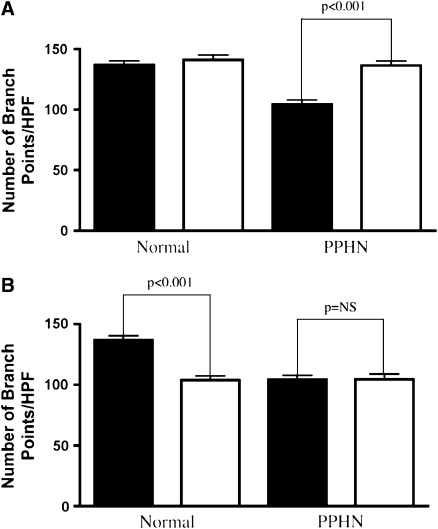
The addition of SNAP as an NO donor increased the number of branch points by 34% in PPHN PAECs (P < 0.001; Figure 7A). SNAP had no effect on the number of branch points in normal PAECs. NOS inhibition with LNA decreased the number of branch points by 24% (P < 0.001) in normal PAECs, but did not decrease tube formation further in PPHN PAECs (Figure 7B).
Figure 7.
Effect of S-nitroso-N-acetylpenicillamine (SNAP) and nitro-l-arginine (LNA) on tube formation. Fetal ovine pulmonary artery endothelial cells (PAECs) from normal sheep and sheep with persistent pulmonary hypertension of the newborn (PPHN) were plated on Matrigel in serum-free media under 3% oxygen conditions with and without SNAP (1 μM) treatment and LNA (4 mM) treatment. (A) SNAP increased the number of branch points in PPHN but not normal cells, rescuing the abnormal phenotype (solid bars, control; open bars, SNAP). (B) NO synthase inhibition with LNA decreased tube formation in normal but not PPHN PAECs (solid bars, control; open bars, LNA).
NO Rescue of SU5416-induced Reduction of Tube Formation
Blockade of VEGF receptor activity with SU5416 treatment decreased PAEC tube formation in vitro. The addition of SNAP as an NO donor completely reversed the effects of SU5416 on tube formation, as demonstrated by the increase in tube formation to control values in both normal and PPHN PAECs (Figure 8).
Figure 8.
NO rescue of SU5416-induced reduction of tube formation. Fetal ovine pulmonary artery endothelial cells (PAECs) from normal sheep and sheep with persistent pulmonary hypertension of the newborn (PPHN) were plated on Matrigel in serum-free media under 3% oxygen conditions with and without S-nitroso-N-acetylpenicillamine (SNAP) (1 μM) and SU5416 (10 μM) treatment in combination. In both normal and PPHN cells, as previously shown, blockade of VEGF activity with SU5416 treatment decreased tube formation in vitro. The addition of SNAP as an NO donor completely reversed the decrease in tube formation in both normal and PPHN cells. Solid bars, control; open bars, SU5416; cross-hatched bars, SU5416 + SNAP.
Effect of VEGF Treatment on NO Production
VEGF treatment (50 ng/ml) increased NO production in both normal and PPHN PAECs by 36% (P < 0.01) and 33% (P < 0.01), respectively.
Proliferation and Apoptosis in Normal and PPHN PAECs
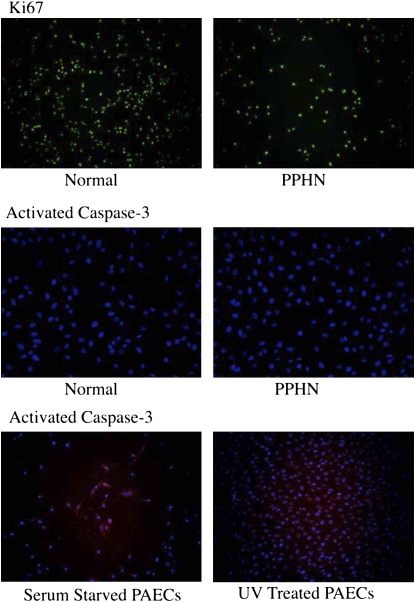
Normal and PPHN PAECs were uniformly positive for Ki67 by immunostaining when studied at both 50 and 90% confluence. In contrast, normal and PPHN PAECs were negative for active caspase-3 protein. Serum-starved and ultraviolet light–treated normal PAECs were used as positive controls and were strongly positive for active caspase-3. In addition to negative active caspase-3 staining, no condensed nuclei were seen after DAPI (4′, 6-diamidino-2-phenylindole) staining in normal and PPHN PAECs (Figure 9).
Figure 9.
Proliferation and apoptosis were measured in normal and PPHN pulmonary arterial endothelial cells (PAECs) by immunostaining. Both normal and persistent pulmonary hypertension of the newborn (PPHN) PAECs were uniformly Ki67 positive and active caspase-3 negative, demonstrating decreased proliferation rather than increased apoptosis in PPHN PAECs. Serum-starved and ultraviolet (UV) light–treated normal PAECs were used as controls for apoptosis and were strongly positive for activated caspase-3.
DISCUSSION
In addition to high PVR due to increased vascular tone and hypertensive remodeling, severe PPHN is often characterized by reduced vascular growth. Previous studies have shown that pulmonary hypertension during late gestation impairs fetal pulmonary vascular growth in vivo (19), but mechanisms by which high pressure inhibits angiogenesis in the developing lung are unknown. Whether PPHN causes functional abnormalities of endothelial cells, such as changes in proliferation and angiogenesis, and whether these changes in an endothelial cell phenotype persist in vitro have not been previously studied. We found that fetal PAECs harvested from lambs with experimental PPHN maintain an abnormal phenotype in vitro, as characterized by decreased PAEC growth and tube formation when compared with normal gestational age–matched control lambs. Because VEGF and NO signaling can regulate endothelial cell growth and angiogenesis, we measured VEGF, eNOS, and VEGFR-2 protein expression in normal and PPHN PAECs and demonstrated that VEGF and eNOS protein expression are markedly reduced in PAECs from PPHN lambs. VEGF and NO treatment with either NO gas or SNAP rescue the abnormal in vitro phenotype of the PPHN PAECs and restore their growth rate and ability to form vascular networks on Matrigel to normal. In addition, inhibition of endogenous VEGF receptor and NOS activities decreased growth and tube formation in normal PAECs to values seen in PPHN PAECs. NO treatment with SNAP rescued the effects of VEGF receptor inhibition on tube formation in both groups. These findings are the first data to demonstrate that endothelial cells from an experimental model of PPHN maintain an abnormal phenotype in vitro, including impaired proliferation and tube formation, as well as dysfunctional VEGF–NO signaling, suggesting that this abnormal endothelial cell phenotype directly contributes to decreased vascular growth in severe PPHN.
Previous in vivo studies in this model have demonstrated that increased vascular tone and impaired vasoreactivity are partly due to endothelial dysfunction (3, 15). More recently, we reported that prolonged intrauterine pulmonary hypertension also impairs angiogenesis, decreases alveolarization, and reduces lung–to–body weight ratios in fetal sheep, suggesting that pulmonary hypertension itself impairs angiogenesis in the developing lung and causes lung hypoplasia (19). Our studies parallel and extend these in vivo observations by demonstrating persistent abnormalities of endothelial cell growth and tube formation in vitro, and suggesting that impaired endothelial cell function likely mediates the reduction in vascular growth observed in severe PPHN. This study also further supports the hypothesis that decreased VEGF and NO signaling contributes to persistent abnormalities in PAEC phenotype in the fetal sheep model of PPHN.
VEGF is a potent endothelial cell mitogen and regulator of angiogenesis. As well as being characterized by impaired angiogenesis and lung hypoplasia, this model of PPHN also demonstrates decreased VEGF protein expression (20). VEGF treatment improves hemodynamics and lung vascular growth in this experimental model of PPHN (21). In vivo inhibition of VEGF receptors in normal fetal sheep results in impaired vascular growth and pulmonary hypertension (20). Rodent and rabbit models of hyperoxia-induced lung injury are characterized by decreased blood vessel growth and alveolar simplification (32–35). Associated with the lung injury is decreased VEGF protein expression. In this model of hyperoxia-induced lung injury, treatment with recombinant human VEGF protein (rhVEGF) or, more recently, intratracheal adenovirus-mediated VEGF gene therapy has been shown to improve lung vascular growth and alveolarization (28, 30). VEGF treatment was also able to reverse the abnormal in vitro phenotype in PPHN PAECs. These findings suggest that VEGF is an important regulator of PAEC growth and angiogenesis in the developing fetal and postnatal lung and that disruption of VEGF signaling pharmacologically, by hyperoxia or by hypertension, reduces distal lung vascular and alveolar growth.
In addition to regulating vascular tone, NO, which is produced by eNOS, also plays an important role in angiogenesis and has been shown to mediate many of the downstream functions of VEGF (24, 25). This model of PPHN is characterized by marked down-regulation of eNOS protein and impaired NO production (5, 6). Previous studies in this model have emphasized the role of impaired NO production or activity, with a reduction in pulmonary vasodilation to oxygen, pharmacologic agents, and birth-related stimuli in fetal sheep (3, 39, 40). NO is also an important regulator of smooth muscle cell proliferation (41, 42). Decreased NO production has been associated with smooth muscle cell hyperplasia, which contributes to hypertensive remodeling in PPHN (6, 35). More recently, the importance of NO in regulating vascular and alveolar growth in the developing lung has been demonstrated in eNOS-deficient mice. Lungs from eNOS−/− mice exhibit marked disruption of lung vascular and alveolar structures (27). Other studies further demonstrate the susceptibility of eNOS-deficient mice to hypoxia, because mild hypoxia reduces vascular and alveolar growth in neonatal eNOS−/− mice but not in wild-type mice (43). Treatment with inhaled NO has been shown to improve alveolarization and angiogenesis in experimental models of bronchopulmonary dysplasia due to hyperoxia or SU5416 administration in vivo (26, 29). Intratracheal adenovirus-mediated eNOS gene therapy has recently been shown to preserve alveolar development and promote lung capillary formation in neonatal rat pups exposed to hyperoxia (30, 45). Treatment of PAECs harvested from PPHN lambs with NO (either SNAP or NO gas) reversed the abnormal growth and tube formation in vitro, providing direct cellular evidence to support this previous in vivo work.
This is the first study to show persistent abnormalities in PAECs from PPHN fetal sheep and suggests that decreased VEGF and eNOS protein expression within the endothelial cell itself may contribute to this abnormal phenotype. Our finding of decreased eNOS protein expression in PPHN PAECs differs from Konduri and colleagues (44), who reported decreased eNOS protein from isolated arteries (7), but not from PAECs from fetal sheep (44). Differences between the results of these studies are unclear but may be due to differences in oxygen tension (3% in our studies vs. room air) as well as other methodologies. We chose 3% oxygen for our studies on the basis of preliminary data as well as past studies from our lab that have shown improved PAEC function in low-oxygen conditions that mimic the intrauterine environment (22). Tirosh and coworkers showed decreased NO production in fetal PAECs from PPHN sheep in response to increased oxygen tension but did not report on eNOS content (40). Our study is the only work to measure VEGF protein in PPHN PAECs and demonstrate marked decreases in VEGF content. Thus, these findings suggest that decreased VEGF and eNOS protein expression may account for abnormal VEGF–NO signaling within the endothelial cell itself, which may be responsible for impaired vascular growth in PPHN.
Differences seen in PAEC growth were due to decreased proliferation rather than increased apoptosis because PPHN PAECs demonstrated positive Ki67 staining and negative staining for markers of apoptosis (activated caspase-3 and condensed nuclei with DAPI staining). Together with these findings, cell viability was always in excess of 95% when assessed by trypan blue exclusion on a Beckman-Coulter cell counter, thus supporting the finding of decreased proliferation in PPHN PAECs. Although similar effects were seen with respect to PAEC growth in normal and PPHN PAECs with VEGF and NO stimulation and SU5416 and LNA inhibition, differences were seen in the responses of normal and PPHN PAECs to stimulation and inhibition with respect to tube formation. VEGF and NO treatment with SNAP increased tube formation in only PPHN PAECs, while VEGF receptor inhibition decreased tube formation in both normal and PPHN PAECs. NOS inhibition decreased tube formation in only normal PAECs. We speculate that the lack of further impairment of tube formation in PPHN PAECs with NOS inhibition is due to already down-regulated eNOS expression. Treatment with SNAP as an NO donor rescued the angiogenic capacity of SU5416-treated normal and hypertensive PAECs, demonstrating an intact VEGF–eNOS signaling pathway in both normal and PPHN PAECs. By demonstrating increased NO release in response to VEGF stimulation in normal and PPHN PAECs, we further confirm intact VEGF–NO signaling within both normal and PPHN PAECs. Angiogenesis is a complex process that involves the formation of new blood vessels from preexisting vessels. For new blood vessels to form in the developing lung, endothelial cells must proliferate and form vascular tubes. Our current study demonstrates distinctive yet overlapping roles for VEGF–NO regulation of angiogenesis within the developing lung as well as the critical role that VEGF–NO signaling pathway plays in stimulating endothelial cells to proliferate and form new blood vessels within the developing lung.
Potential limitations of this study include the concern that these studies used fetal PAECs harvested from large vessels, and differences may exist in the behavior of these cells as compared with microvascular PAECs. Because microvascular PAECs may primarily be involved in lung angiogenesis during development in vivo, future studies are needed to compare and contrast microvascular PAEC function from normal and PPHN fetal sheep in parallel fashion to these current studies. These studies looked exclusively at in vitro endothelial cell function. In vivo, the mesenchyme and epithelium are important regulators of endothelial cell growth and tube formation. The effects of the mesenchyme and epithelium on the endothelium were not assessed in this in vitro model.
In conclusion, we found that chronic intrauterine pulmonary hypertension directly alters endothelial cell phenotype, which persists in vitro, as characterized by decreased endothelial cell growth and tube formation. On the basis of these findings, PAECs harvested from fetal lambs with chronic pulmonary hypertension provide a unique in vitro model for studying basic mechanisms of endothelial dysfunction in PPHN. In addition, this study demonstrates that impaired VEGF and NO signaling contribute to the abnormal PAEC phenotype in vitro. Because exogenous VEGF and NO treatment (either SNAP or NO gas) rescues the abnormal PPHN PAEC phenotype in vitro, we speculate that treatment strategies that up-regulate VEGF and NO signaling pathways may enhance angiogenesis in vivo in patients with severe PPHN, and may be especially important in the setting of lung hypoplasia.
Supplementary Material
Supported by an iNO therapeutics grant for advancing newborn medicine (NIH R01 HL068702-05A2, Role of VEGF in Perinatal Pulmonary Hypertension).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200705-750OC on September 6, 2007
Conflict of Interest Statement: J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.J.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.B. received a $75,000 grant from INO Therapeutics for studies not directly related to this manuscript. N.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H.A. is a scientific advisor for INO Therapeutics, receiving less than $10,000 per year, and received a $25,000 grant in 2007 from Bayer.
References
- 1.Levin DL, Heymann MA, Kitterman JA, Gregory GA, Phibbs RH, Rudolph AM. Persistent pulmonary hypertension of the newborn infant. J Pediatr 1976;89:626–630. [DOI] [PubMed] [Google Scholar]
- 2.Geggel RL, Reid LM. The structural basis of PPHN. Clin Perinatol 1984;11:525–549. [PubMed] [Google Scholar]
- 3.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest 1989;83:1849–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villanueva ME, Zaher FM, Svinarich DM, Konduri G. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res 1998;44:338–343. [DOI] [PubMed] [Google Scholar]
- 5.Villamor E, Le Cras TD, Horan MP, Halbower AC, Tuder RM, Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 1997;16:L1013–L1020. [DOI] [PubMed] [Google Scholar]
- 6.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 1997;16:L1005–L1012. [DOI] [PubMed] [Google Scholar]
- 7.Konduri G, Yang Shi JO, Pritchard KA Jr. Decreased association of HSP90 impairs endothelial nitric oxide synthase in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 2003;285:H204–H211. [DOI] [PubMed] [Google Scholar]
- 8.Murata T, Sato K, Hori M, Ozaki H, Karaki H. Decreased endothelial nitric-oxide synthase (eNOS) activity resulting from abnormal interaction between eNOS and its regulatory proteins in hypoxia-induced pulmonary hypertension. J Biol Chem 2002;277;44085–44092. [DOI] [PubMed]
- 9.Wedgwood S, Black SM. Endothelin-1 decreases endothelial NOS expression and activity through ETA receptor-mediated generation of hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 2005;288:L480–L487. [DOI] [PubMed] [Google Scholar]
- 10.Kinsella JP, Neish SR, Shaffer E, Abman SH. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 1992;340:819–820. [DOI] [PubMed] [Google Scholar]
- 11.Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, Rhines J, Chang CT. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose–response, multicenter study. The I-NO/PPHN Study Group. Pediatrics 1998;101:325–334. [DOI] [PubMed] [Google Scholar]
- 12.Goldman AP, Tasker RC, Haworth SG, Sigston PE, Macrae DJ. Four patterns of response to inhaled nitric oxide for persistent pulmonary hypertension of the newborn. Pediatrics 1996;98:706–713. [PubMed] [Google Scholar]
- 13.Farrow KN, Fliman P, Steinhorn RH. The diseases treated with ECMO: focus on PPHN. Semin Perinatol 2005;29:8–14. [DOI] [PubMed] [Google Scholar]
- 14.Wild LM, Nickerson PA, Morin FC III. Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res 1989;25:251–257. [DOI] [PubMed] [Google Scholar]
- 15.Morin FC III. Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 1989;25:245–250. [DOI] [PubMed] [Google Scholar]
- 16.Belik J, Keeley FW, Baldwin F, Rabinovitch M. Pulmonary hypertension and vascular remodeling in fetal sheep. Am J Physiol 1994;266:H2303–H2309. [DOI] [PubMed] [Google Scholar]
- 17.Ivy D, Parker TA, Ziegler JW, Galan HL, Kinsella JP, Tuder RM, Abman SH. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Invest 1997;99:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivy D, Ziegler JW, Dubus MF, Fox J, Kinsella JP, Abman SH. Chronic intrauterine pulmonary hypertension alters endothelin receptor activity in the ovine fetal lung. Pediatr Res 1996;39:435–442. [DOI] [PubMed] [Google Scholar]
- 19.Grover TR, Parker TA, Balasubramaniam V, Markham NE, Abman SH. Pulmonary hypertension impairs alveolarization and reduces lung growth in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 2005;288:L648–L654. [DOI] [PubMed] [Google Scholar]
- 20.Grover TR, Parker TA, Zenge JP, Markham NE, Kinsella JP, Abman SH. Intrauterine hypertension decreases lung VEGF expression and VEGF inhibition causes pulmonary hypertension in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 2003;284:L508–L517. [DOI] [PubMed] [Google Scholar]
- 21.Grover TR, Parker TA, Abman SH. Vascular endothelial growth factor improves pulmonary vascular reactivity and structure in an experimental model of chronic pulmonary hypertension in fetal sheep. Chest 2005;128(Suppl):614S. [DOI] [PubMed] [Google Scholar]
- 22.Balasubramaniam V, Maxey AM, Fouty BW, Abman SH. Nitric oxide augments fetal pulmonary artery endothelial cell angiogenesis in vitro. Am J Physiol Lung Cell Mol Physiol 2006;290:L1111–L1116. [DOI] [PubMed] [Google Scholar]
- 23.Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro human ‘angiogenesis’ assays with capillaries formed in vitro. Angiogenesis 2001;4:113–121. [DOI] [PubMed] [Google Scholar]
- 24.Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997;100:3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol 1996;270:H411–H415. [DOI] [PubMed] [Google Scholar]
- 26.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled Nitric Oxide Enhances Distal Lung Growth after Exposure to Hyperoxia in Neonatal Rats. Pediatr Res 2005;58:22–29. [DOI] [PubMed] [Google Scholar]
- 27.Han RN, Stewart DJ. Defective lung vascular development in endothelial nitric oxide synthase-deficient mice. Trends Cardiovasc Med 2006;16:29–34. [DOI] [PubMed] [Google Scholar]
- 28.Kunig AM, Balasubramaniam V, Markham NE, Seedorf G, Gien J, Abman SH. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L1068–L1078. [DOI] [PubMed] [Google Scholar]
- 29.Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 2004;287:L344–L351. [DOI] [PubMed] [Google Scholar]
- 30.Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005;112:2477–2486. [DOI] [PubMed] [Google Scholar]
- 31.Ma L, Francia G, Viloria-Petit A, Hicklin DJ, du Manoir J, Rak J, Kerbel RS. In vitro procoagulant activity induced in endothelial cells by chemotherapy and antiangiogenic drug combinations: modulation by lower-dose chemotherapy. Cancer Res 2005;65:5365–5373. [DOI] [PubMed] [Google Scholar]
- 32.Klekamp JG, Jarzecka K, Perkett EA. Exposure to hyperoxia decreases the expression of vascular endothelial growth factor and its receptors in adult rat lungs. Am J Pathol 1999;154:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol 2003;285:L161–L168. [DOI] [PubMed] [Google Scholar]
- 34.Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am J Respir Cell Mol Biol 1997;16:557–567. [DOI] [PubMed] [Google Scholar]
- 35.Ambalavanan N, Mariani G, Bulger A, Philips JB III. Role of nitric oxide in regulating neonatal porcine pulmonary artery smooth muscle cell proliferation. Biol Neonate 1999;76:291–300. [DOI] [PubMed] [Google Scholar]
- 36.Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett 1998;427:263–266. [DOI] [PubMed] [Google Scholar]
- 37.Berkels R, Dachs C, Roesen R, Klaus W. Simultaneous measurement of intracellular Ca2+ and nitric oxide: a new method. Cell Calcium 2000;27:281–286. [DOI] [PubMed] [Google Scholar]
- 38.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006;290:L209–L221. [DOI] [PubMed] [Google Scholar]
- 39.McQueston JA, Kinsella JP, Ivy DD, McMurtry IF, Abman SH. Chronic pulmonary hypertension in utero impairs endothelium-dependent vasodilation. Am J Physiol 1995;268:H288–H294. [DOI] [PubMed] [Google Scholar]
- 40.Tirosh R, Resnik ER, Herron J, Sukovich DJ, Hong Z, Weir EK, Cornfield DN. Acute normoxia increases fetal pulmonary artery endothelial cell cytosolic Ca2+ via Ca2+-induced Ca2+ release. Pediatr Res 2006;60:258–263. [DOI] [PubMed] [Google Scholar]
- 41.Garg UC, Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 1989;83:1774–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomae KR, Nakayama DK, Billiar TR, Simmons RL, Pitt BR, Davies P. The effect of nitric oxide on fetal pulmonary artery smooth muscle growth. J Surg Res 1995;59:337–343. [DOI] [PubMed] [Google Scholar]
- 43.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol 2003;284:L964–L971. [DOI] [PubMed] [Google Scholar]
- 44.Konduri GG, Bakhutashvili I, Eis A, Pritchard KA. Oxidant stress from uncoupled nitric oxide synthase impairs vasodilation in fetal lambs with persistent pulmonary hypertension. Am J Physiol Heart Circ Physiol 2007;292:H1812–H1820. [DOI] [PubMed] [Google Scholar]
- 45.Zhao YD, Courtman DW, Ng DS, Robb MJ, Deng YP, Trogadis J, Han RN, Stewart DJ. Microvascular regeneration in established pulmonary hypertension by angiogenic gene transfer. Am J Respir Cell Mol Biol 2006;35:182–189. [DOI] [PubMed] [Google Scholar]
- 46.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 2006;291:L119–L127. [DOI] [PubMed] [Google Scholar]
- 47.Le Cras, TD, Xue C, Rengasamy A, Johns RA. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am J Physiol Lung Cell Mol Physiol 1996;270:L166–L170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.