Abstract
Rationale: Obliterative bronchiolitis (OB) after lung transplantation is triggered by alloimmunity, but is ultimately mediated by transforming growth factor (TGF)-β1–dependent airway fibrosis.
Objectives: Chronic alcohol use increases TGF-β1 expression and renders the lung susceptible to injury. Therefore, we hypothesized that donor alcohol abuse could prime the lung allograft for OB, as many organ donors have a history of alcohol abuse.
Methods: Tracheas from control and alcohol-fed rats (8 wk) were heterotopically transplanted into recipients with varying degrees of alloimmune mismatch and analyzed for obliterative airway disease severity on Postoperative Day 21.
Measurements and Main Results: Although donor alcohol ingestion did not increase the number of antigen-presenting cells or infiltrating lymphocytes, it nevertheless increased allograft lumenal collagen content fourfold compared with allografts from control donors. In parallel, alcohol increased TGF-β1 and α-smooth muscle actin expression in allografts. Alcohol amplified airway disease even in isografts with minor alloimmune mismatches. In contrast, it did not cause any airway disease in isografts in a pure isogenic background, suggesting that a minimal alloimmune response is necessary to trigger alcohol-induced airway fibrosis.
Conclusions: Although alloimmune inflammation is required to initiate airway disease, alcohol primes the allograft for greater TGF-β1 expression, myofibroblast transdifferentiation, and fibrosis than by alloimmune inflammation alone. This has serious clinical implications, as many lung donors have underlying alcohol abuse that may prime the allograft recipient for subsequent OB.
Keywords: fibrosis, lung transplant, obliterative bronchiolitis, transforming growth factor-β1
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Obliterative bronchiolitis (OB) is the main long-term cause of graft dysfunction after lung transplantation. Although some risk factors have been implicated in the development of OB, predicting which graft recipients develop OB has been as yet unreliable.
What This Study Adds to the Field
Donor alcohol abuse amplifies the severity of OB in the recipient.
Obliterative bronchiolitis (OB) is a histological diagnosis of small airway fibrosis and occlusion for which no effective treatment exists. OB is the major long-term cause of allograft dysfunction after lung transplantation (1, 2). OB is believed to result from immunologically mediated airway epithelial destruction and subsequent lumenal fibrosis. Although a variety of risk factors have been implicated in the development of OB (3), predicting which transplant recipients develop OB has been as yet unreliable. Several studies have demonstrated a correlation between the presence of a variety of biomarkers and the development of OB (4–10). Studies have shown that acute rejection is a risk factor for long-term graft dysfunction (3, 11, 12); however, others have failed to establish this correlation (13, 14).
Using heterotopic tracheal transplantation (HTT) in animals to induce obliterative airway disease (OAD) as an experimental model of OB, recent studies have demonstrated that alloimmunity represents but one early component of OAD pathophysiology (15, 16). Rather, OAD development appears to be mediated via two mechanisms. First, an alloimmune trigger leads to inflammation and subsequent destruction of the airway epithelium. Second, the alloimmune response wanes, and is superseded by tissue remodeling pathways. Taken together, the majority of experimental evidence suggests that therapies designed to prevent or treat OB by attenuating the alloimmune response may not be clinically viable. This contention is supported by the fact that progression of OB is refractory to augmented immunosuppressive therapy (17).
Using animal models of alcoholism, studies have shown that alcohol ingestion primes the lung for injury (18–20) and increases transforming growth factor (TGF)-β1 in the lung (21, 22). Clinically, subjects with alcoholism increased risk of acute respiratory distress syndrome after stresses, such as sepsis or trauma (23, 24). Young and otherwise healthy subjects with alcoholism have impaired alveolar epithelial barrier function despite having normal airway physiology (25). According to the United Network for Organ Sharing, 19% of deceased organ donors have a history of alcohol abuse. In addition, donor alcohol abuse increases the risk of graft dysfunction after heart transplantation (26, 27). Overall, these studies suggest that clinically silent, alcohol-mediated lung dysfunction in the allograft donor could promote development of OB.
In this study, we used the HTT model to test the hypothesis that alcohol ingestion by donors amplifies development of OAD in the recipient. To test this hypothesis, we transplanted airways from control or ethanol-fed rats into recipients with varying degrees of alloimmune mismatch. The data reported in this study support the contention that alcohol potentiates the development of OAD. In addition, we show that alcohol mediates its effects only when a sufficient alloimmune trigger is present. The data presented here are relevant in the context of lung transplantation, as many donors are otherwise young, healthy individuals who die in alcohol-related accidents. Some of the results of this study have been previously published in the form of abstracts (28, 29).
METHODS
HTT of Tracheas in Rats
All procedures involving animals were performed with approval by the Veterans Affairs Medical Center institutional animal care and use committee, and in accordance with National Institutes of Health guidelines, the Animal Welfare Act, and applicable Veterans Affairs Medical Center policies. Male Sprague Dawley (SD; Charles River Laboratories, Wilmington, MA) or Fischer 344 (F344; Harlan, Indianapolis, IN) donor rats (age, 3–5 mo) were fed the Lieber-DeCarli isocaloric liquid diet, containing either ethanol (36% total calories) or Maltin-Dextrin as substitute (control diet) for 8 weeks. HTT was performed as described previously (16). Briefly, isografts or allografts were obtained by transplanting tracheas from control or alcohol-fed donor rats subcutaneously into recipient rats. Tracheas were harvested before transplant or at 3, 7, 14, or 21 days after transplant, and either cut into 6-μm-thick sections for histological and immunohistological analyses, or homogenized for hydroxyproline assays. We used three transplant paradigms to determine alloimmune involvement in the development of OAD. First, to minimize alloimmunity, isogeneic transplants were performed between inbred F344 rats. Second, to elicit a mild alloimmune response, isogeneic transplants were performed using outbred SD rats. The RT1 haplotype of SD rats is not monitored; however, SD rats would be expected to be heterozygous between each animal across the genome. Third, to model clinically relevant transplantation whereby a strong alloimmune response follows transplantation, we performed allogeneic transplants between SD or F344 rats as donors and F344 or SD rats as recipients, respectively. All recipients were fed normal rodent chow and water.
Hydroxyproline Assay
Collagen content was assayed biochemically by determining hydroxyproline content according to Woessner (30). Additional details on hydroxyproline determination are provided in the online supplement.
Immunohistochemistry
Anti–TGF-β1 and anti–α-smooth muscle actin (SMA) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and LabVision Corp. (Fremont, CA), respectively. All other primary antibodies were obtained from BD Pharmingen (San Jose, CA), including OX-6 (major histocompatibility complex [MHC] II), anti-CD54 (intercellular adhesion molecule [ICAM]-1), OX-38 (CD4), and OX-8 (CD8a). Biotinylated donkey anti-mouse and anti-rabbit antibodies were obtained from Jackson Immunoresearch (West Grove, PA). Immunohistochemical staining was performed as described previously (31). Additional details on immunohistochemical methods are provided in the online supplement.
Terminal Deoxynucleotidyl Transferase–Mediated dUPT Nick-End Labeling
Tracheal sections from control and ethanol-fed SD donor rats were evaluated pretransplant (1 and 8 wk of ethanol) using an in situ cell death detection kit (POD; Roche Diagnostics, Indianapolis, IN) to assess tracheal epithelial apoptosis. Additional details on terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining are provided in the online supplement.
Histochemical Evaluation of OAD
Allograft injury, as determined by epithelial loss, cellular infiltration, and collagen deposition within the lumen of the airway, was assessed at 3, 7, 14, and 21 days after transplantation, as described previously (16). Image analyses were performed as described by Schacker and colleagues (32). Additional details on histological evaluation of OAD are provided in the online supplement.
Statistical Analyses
To determine significance between two groups, comparisons were made using Student's t test. Analyses of multiple groups were performed using one-way analysis of variance with Bonferonni's posttest using GraphPad Prism version 4.0a (GraphPad Software, San Diego, CA). For all statistical tests, P less than 0.05 was accepted for statistical significance. All graphical data are displayed as means (± SEM).
RESULTS
Donor Alcohol Ingestion Exacerbates OAD Depending on the Degree of Alloimmune Mismatch
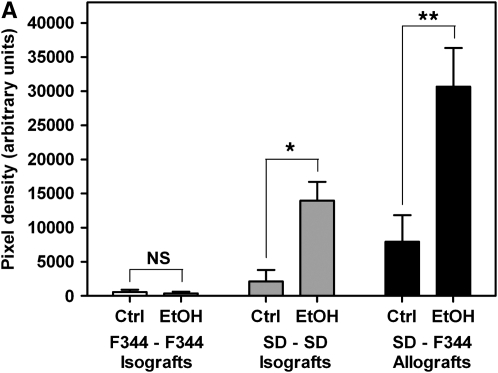
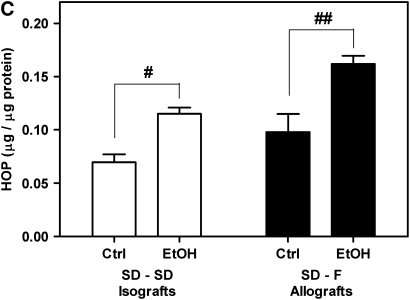
To determine the effect of donor alcohol ingestion on OAD pathology, allogeneic transplants using control or alcohol-fed SD donors and F344 recipients were performed (SD–F344). Allografts were analyzed histochemically and biochemically at 21 days after transplant. Quantification of trichrome-stained sections revealed significantly increased collagen deposition (fourfold) within the airway lumen in allografts from alcohol-fed donors compared with control donors (Figures 1A and 1B, bottom panels). All allografts (from control and alcohol-fed donors) displayed complete obliteration at 21 days after transplantation. To rule out the possibility that strain differences contributed to the amplified pathology, F344–SD allogeneic transplants were also performed. This transplant paradigm similarly resulted in a fourfold (3.911 ± 0.7268) increase in lumenal collagen in allografts originating from alcohol-fed donors. Determination of hydroxyproline content in allografts also revealed a significant increase in collagen content in allografts from alcohol-fed donors (Figure 1C). These results indicated that OAD in the recipient was amplified by donor alcohol ingestion, and that these effects were independent of which strain was used as donors versus recipients.
Figure 1.
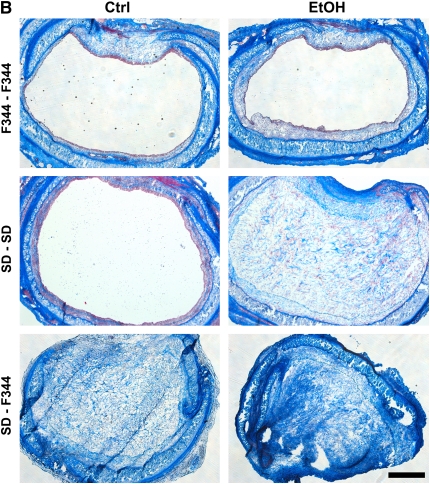
Donor alcohol ingestion exacerbates obliterative airway disease, depending on the degree of alloimmune mismatch. (A) Quantification of Masson's trichrome nlue staining of 21-day isografts and allografts from control (Ctrl) and alcohol-fed (EtOH) donor rats. No significant difference was observed between control (n = 4) and alcohol (n = 4) groups for Fischer 344 (F344)–F344 isografts. Statistical significance was observed between control (n = 8) and ethanol (n = 7) groups for Sprague Dawley (SD)–SD isografts (*P < 0.05), and control (n = 10) and ethanol (n = 12) SD–F344 allografts (**P < 0.05). (B) Masson's Trichrome Blue histology staining of F344–F344 isografts (top panels), SD–SD isografts (middle panels), and SD–F344 allografts (bottom panels). Scale bar = 500 μm. (C) Hydroxyproline (HOP) content (indicative of collagen content) is significantly increased in SD–SD isografts (#P < 0.05) and SD–F344 allografts (##P < 0.05) from ethanol-fed donors.
Because OB pathology has been attributed to alloimmune-dependent and alloimmune-independent mechanisms, we sought to determine the contribution of the alloimmune response to alcohol-mediated exacerbation of OAD. To assess alloimmune involvement, we performed two types of syngeneic transplants: F344–F344 and SD–SD, using control and alcohol-fed donor rats. When alcohol-fed donors were used for F344–F344 transplants, we did not observe increased OAD pathology compared with isografts originating from control donors (Figures 1A and 1B; top panels). All F344–F344 isografts were free from OAD pathology. However, when SD–SD transplants were performed, isografts from alcohol-fed donors displayed significant OAD pathology (as assessed by trichrome staining and hydroxyproline content) and had no airway epithelium present (Figures 1A and 1B, middle panels; and Figure 1C). As expected, the majority of isografts from control donors did not exhibit OAD, and had a fully regenerated and intact airway epithelium. Of seven SD–SD control animals, only one isograft had OAD pathology, whereas all eight SD–SD alcohol isografts displayed OAD pathology. These data support the contention that prior alcohol ingestion by donors exacerbates OAD pathology in the recipient via a process that requires a threshold alloimmune trigger.
Airway Epithelial Viability Pretransplant Is Not Compromised in Alcohol-Fed Donors
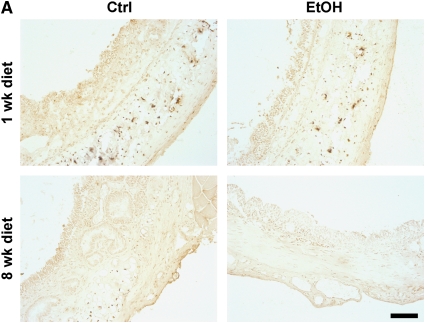
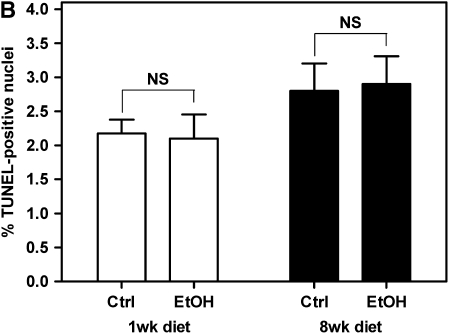
Using the HTT model, we have shown that alcohol ingestion by donors increases OAD pathology in recipients depending on the degree of alloimmune mismatch. To investigate mechanisms by which alcohol ingestion by donors increased OAD, we first examined the possibility that alcohol ingestion by donors exacerbates OAD in allografts by decreasing the viability of the tracheal epithelium. To test this hypothesis, we analyzed pretransplant airway from control or alcohol-fed SD donors at 1 and 8 weeks of control/alcohol diet for the presence of TUNEL-positive cells. We did not observe an increase in the percentage of apoptotic epithelial or submucosal cells between control and alcohol-fed donors at either time point (Figures 2A and 2B). In fact, TUNEL-positive cells were not observed in the airway epithelium in sections from any experimental group. These results suggest that alcohol does not mediate its effects via heightened airway epithelium injury due to increased apoptosis in the tracheal epithelium.
Figure 2.
Pretransplant airway epithelium viability is not compromised in alcohol-fed SD donors. (A) Terminal deoxynucleotidyl transferase–mediated dUTP nick-end labeling (TUNEL) staining of pretransplant tracheal sections at 1-week ethanol diet (top panels) and 8-week ethanol diet (bottom panels). TUNEL-positive nuclei were not observed in the airway epithelium of any groups. Scale bar = 100 μm. (B) Quantification of TUNEL staining in the airway subepithelial layers. No significant differences were observed in the prevalence of TUNEL-positive cells between control (n = 4) and alcohol-fed (n = 4) donors at either 1 or 8 weeks of dietary ethanol (P > 0.05). NS = not significant.
Pretransplant Airways from Alcohol-Fed Donors Do Not Appear to Be Primed for Heightened Alloimmune Injury
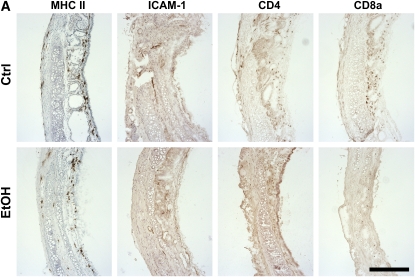
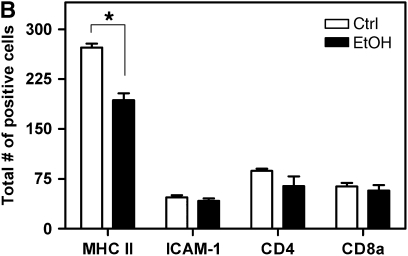
As another putative mechanism by which donor alcohol ingestion exacerbates OAD, we sought to determine if alcohol primes the transplanted tissue for heightened alloimmune activity by increasing numbers of antigen-presenting cells. Alternatively, alcohol may cause lymphocytic infiltration in untransplanted tracheal tissue. To characterize the immunological state of the alcohol-fed tissue before transplantation, tracheae from control and alcohol-fed SD donors (8 wk of dietary alcohol) were analyzed for the presence of antigen-presenting cells and T lymphocytes. Sections from pretransplant tracheae were immunostained for the presence of MHC II–, ICAM-1–, CD4-, and CD8a-positive cells. Tracheae from 8-week alcohol-fed rats show a significant decrease in the prevalence of MHC II–positive cells; however, no significant differences were noted for ICAM-1, CD4, or CD8a expression (Figures 3A and 3B). Thus, tracheae from alcohol-fed donor rats do not appear to be primed to induce a heightened alloimmune response.
Figure 3.
Pretransplant airways from alcohol-fed SD donors do not appear to be primed to heightened alloimmune injury. (A) Untransplanted tracheal sections (8-wk dietary ethanol) were analyzed by immunohistochemical staining for major histocompatibility complex (MHC) II, intercellular adhesion molecule (ICAM)-1, CD4, and CD8a expression. Scale bar = 250 μm. (B) Quantification of MHC II–, ICAM-1–, CD4-, and CD8a-positive cells in untransplanted tracheal sections. MHC II expression is decreased approximately 50% in sections from alcohol-fed donors (*P < 0.05); however, no significant differences were noted between control (open bars) and alcohol (closed bars) groups for ICAM-1, CD4, or CD8a (n = 4 for each group).
Donor Alcohol Ingestion Exacerbates Epithelial Injury after Transplantation
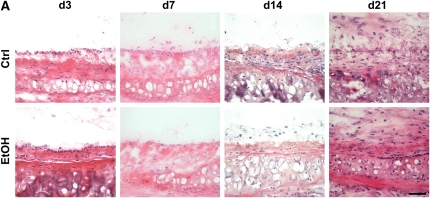
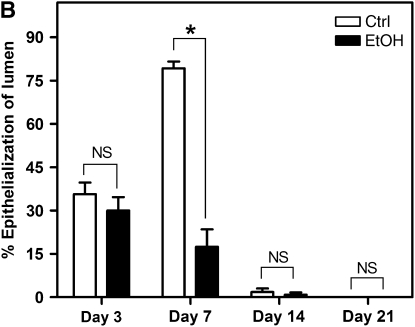
Destruction of the epithelium appears to be a critical step in the development of OAD (15, 33). To investigate the possibility that donor alcohol ingestion renders the airway epithelium more susceptible to injury, we analyzed the degree of epithelialization in SD–F344 allografts from control and alcohol-fed donors at 3, 7, 14, and 21 days after transplantation. In allografts from control donors, considerable epithelial loss occurred by Day 3, followed by regeneration by Day 7, and permanent loss by Day 14 (Figures 4A and 4B). In contrast, allografts from alcohol-fed donors displayed significantly reduced epithelialization at Day 7 (Figures 4A and 4B), suggesting either that regeneration of the epithelium is inhibited, or that permanent loss of the epithelium occurs earlier.
Figure 4.
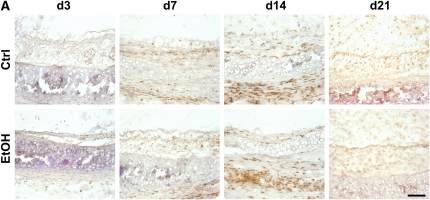
Donor alcohol ingestion exacerbates epithelial injury post-transplantation. (A) Sections from 3-, 7-, 14-, and 21-day allografts originating from control (top panels) or alcohol-fed (bottom panels) donors were stained with H&E. Areas highlighting the epithelium are shown in representative H&E images from each group. Scale bar = 50 μm. (B) Quantification of the degree of epithelialization of allografts from control (open bars) or alcohol-fed (closed bars) donors at Days 3, 7, 14, and 21 post-transplantation (n = 4–5 for each group; *P < 0.05). NS = not significant.
Lymphocytic Infiltration Is Not Increased in Allografts Originating from Alcohol-Fed Donors
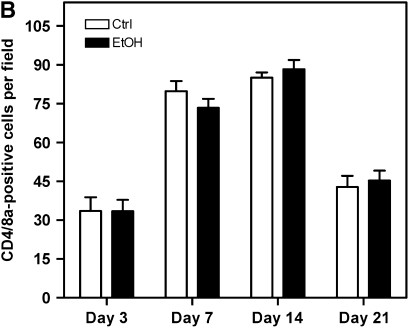
Because infiltrating T lymphocytes are indicative of increased alloimmune activity, we determined if allografts from alcohol-fed donors displayed augmented alloimmune infiltration relative to control animals at 3, 7, 14, and 21 days after transplantation. Allografts were analyzed for the presence of infiltrating CD4- and CD8a-positive T lymphocytes in the subepithelial layers between the cartilage and airway epithelium/epithelial remnants. Representative images are of CD8a-positive lymphocytes, as this subset of cells was significantly more prevalent (Figure 5A). No significant differences were observed in the total number of CD4- and CD8a-positive cells between allografts originating from control or alcohol-fed SD donors at the time points analyzed (Figure 5B). These data suggest that alcohol does not mediate its deleterious pathological effects via increased infiltration of CD4- and CD8a-positive cells.
Figure 5.
Lymphocytic infiltration is not increased in allografts originating from alcohol-fed donors. (A) Sections from 3-, 7-, 14-, and 21-day allografts originating from control or alcohol-fed donors were immunostained for CD8a. Representative CD8a (brown staining) images from each group show the allograft lumen/epithelial/subepithelial region. Scale bar = 100 μm. (B) Quantification of CD4- and CD8a-positive cells reveals no difference in the percentage of infiltrating T lymphocytes in allografts from control (open bars) or alcohol-fed (closed bars) donors (n = 4 each group).
Allografts from Alcohol-Fed Donors Display Increased TGF-β1 and α-SMA Expression
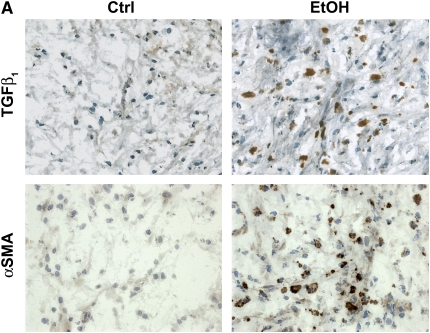
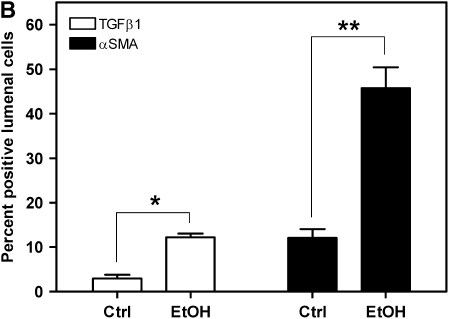
Because the alloimmune-independent component of OAD pathology is dependent on increased fibrogenic activity, we investigated the role of TGF-β1 and α-SMA in alcohol-mediated exacerbation of OAD. Previous studies have demonstrated that OAD pathology in allografts is TGF-β1–dependent (16, 34, 35). To determine if components of the TGF-β1 fibrogenic pathway are increased in alcohol-mediated exacerbation of OAD, 21-day allografts from control and alcohol-fed SD donors were immunostained for TGF-β1. TGF-β1 expression is significantly increased in allograft sections from alcohol-fed donors (Figure 6A, top panels; and Figure 6B).
Figure 6.
Allografts originating from alcohol-fed donors display increased transforming growth factor (TGF)-β1 and α-smooth muscle actin (SMA) expression. (A) TGF-β1 (top panels) and α-SMA (bottom panels) immunohistochemical stain (brown) and hematoxylin counterstaining (blue) of 21-day allografts from control and alcohol-fed rats. Note the increased TGF-β1 and α-SMA staining in allografts from alcohol-fed versus control rats. Scale bar = 50 μm. (B) Quantification of TGF-β1– and α-SMA–positive cells reveals a significant increase in the percentage of TGF-β1– (open bars; *P < 0.05) and α-SMA (closed bars; **P < 0.05)–positive cells within the lumen of allografts (fourfold and threefold increase, respectively) (n = 4 each group).
Fibrosis, one of the hallmarks of OAD, is characterized by excessive synthesis of collagen and accumulation of myofibroblasts. TGF-β1, a potent inducer of collagen synthesis, and myofibroblast transdifferentiation, are implicated in fibrosis (for review, see Reference 36). First, pretransplant tracheae from control and alcohol-fed rats were examined for the presence of α-SMA–positive cells. No differences in TGF-β1 and α-SMA expression were observed between pretransplant control and alcohol tracheae (data not shown). Allografts from control and alcohol-fed SD donors were assayed immunohistochemically at Day 21 after transplant for the presence of myofibroblast transdifferentiation. α-SMA–positive cells, indicative of increased myofibroblast transdifferentiation, were significantly increased in the allograft lumen from alcohol-fed donors (Figure 6A, bottom panels; and Figure 6B). These data show that alcohol-mediated amplification of OAD is associated with increased TGF-β1 and α-SMA expression, and suggest that alcohol mediates its effects via increased fibrogenesis.
DISCUSSION
In the current study, we determined that donor alcohol ingestion amplifies OAD in the recipient. Using a variety of different transplant paradigms to elicit varying degrees of alloimmune mismatch, we showed that alcohol mediates its effects only when a threshold alloimmune trigger is present. Specifically, we demonstrated that a slight alloimmune trigger (HTT between outbred rats) is sufficient to elicit amplified OAD pathology if the airway is exposed to alcohol before transplantation. In the more clinically relevant transplant paradigm (HTT between different strains of rats), donor alcohol ingestion amplifies OAD significantly. In experiments designed to test the mechanism by which prior alcohol ingestion amplifies OAD pathology, we determined that alcohol ingestion does not decrease airway epithelium viability before transplant. Moreover, alcohol did not appear to prime the alloimmune trigger in grafted tissue, as no increase in the number of antigen-presenting cells was observed. In addition, allografts from alcohol-fed donors did not display increased lymphocytic infiltration. However, epithelial integrity at Post-Transplantation Day 7 was decreased in allografts originating from alcohol-fed donors. Finally, allografts from alcohol-fed donors had increased numbers of lumenal TGF-β1– and α-SMA–positive cells, indicating that tissue remodeling/fibrotic pathways are amplified.
Development of OAD pathophysiology after transplantation occurs via two distinct yet codependent mechanisms. First, an alloimmune trigger occurs in the grafted tissue, leading to oxidative stress and up-regulation of proinflammatory mediators. Subsequent injury to the airway epithelium likely represents one of the critical steps in OAD pathophysiology (15, 37). In support of this concept, denuded allografts develop severe OAD pathology, and replacement of epithelium in denuded allografts can prevent this (33).
Damage to the airway epithelium exposes subepithelial layers of cells, which further amplifies the alloimmune response, as well as activating profibrotic pathways in the underlying connective tissue. At some stage of OAD pathogenesis, alloimmune injury wanes and TGF-β1–dependent components of tissue remodeling are activated. Although TGF-β1 is thought to have desirable antiinflammatory properties, increased levels of TGF-β1 in patients with OB appear to correlate with the severity of the disease (38, 39). TGF-β1 is a critical factor known to induce myofibroblast transdifferentiation (40). Activation of this pathway leads to increased numbers of fibrogenic cells, migration of fibrotic cells into the airway lumen, and subsequent transdifferentiation into myofibroblasts. Myofibroblasts are a key source of extracellular matrix protein deposition that occurs in the airway lumen (34, 41). Thus, it is likely that factors that modulate components of the tissue remodeling pathway play a role in OAD pathogenesis.
Using the HTT model in rodents, King and colleagues demonstrated that, when alloimmunity is removed before permanent destruction of the airway epithelium, OAD does not develop (15). In contrast, OAD develops, regardless of whether alloimmunity is absent, if the integrity of the airway epithelium is abrogated. Thus, a “point of no return” exists for OAD development, whereby an alloimmune trigger alone is not sufficient to cause development of OAD. Ramirez and colleagues demonstrated the importance of the TGF-β1 pathway in OAD pathophysiology by showing that Smad3, an intracellular signal transducer for TGF-β1, is required to elicit OAD, despite the presence of a normal alloimmune response (16). In a subsequent study, it was shown that TGF-β1 stimulates myofibroblast transdifferentiation via Smad3-dependent and Smad3-independent pathways, contributing to the excessive matrix deposition that is characteristic of OAD (34). Taken together, the results from these studies show that alloimmunity represents but one early component of OAD pathophysiology.
The results presented in this study show that alcohol mediates its effects when a threshold alloimmune trigger is present. When transplants were performed between inbred F344 rats, a minimal alloimmune trigger was present. Predictably, isografts from alcohol-fed donors did not develop OAD pathology. These data are not surprising, as the majority of clinical and experimental evidence underscore the necessity for an alloimmune trigger to induce OAD pathogenesis. Transplants between the outbred SD rats provided a mild alloimmune mismatch. However, because a sufficient alloimmune trigger was present, isografts from alcohol-fed donors displayed OAD pathology. In the more clinically relevant transplant paradigm, transplants between different strains (SD–F344 and F344–SD), providing a significant alloimmune mismatch, predictably resulted in OAD pathology in allografts from control donors, but also led to exacerbated OAD pathology in allografts from alcohol-fed donors. By using this combination of syngeneic and allogeneic transplantation paradigms to vary the degree of alloimmune trigger, the experimental data presented here argue against ischemic injury being a possible source of the heightened OAD in grafts from alcohol-fed donors. Importantly, these results also demonstrate that the deleterious effects of alcohol occur independent of the strain of rat used for the donor.
However, the experimental evidence shown here suggests that alcohol does not prime the transplanted tissue to elicit an augmented alloimmune response. In this study, histological analyses show that untransplanted tracheal tissue from alcohol-fed donors appears grossly normal, with no obvious signs of immune infiltration. Immunohistochemical analyses of MHC II antigens failed to detect an increase in antigen presentation on the airway epithelium or submucosal layers. In fact, alcohol ingestion decreased the number of MHC II–positive cells in pretransplanted airway tissue, suggesting that the immunological state of the tissue is compromised. This contention is supported by previous studies showing that alcohol impairs immune function in the lung (for review, see Reference 42). Furthermore, we show that pretransplanted tracheae from alcohol-fed donors do not have increased expression of the costimulatory ligand, ICAM-1, or CD4/CD8a-positive lymphocytes. In 3-, 7-, 14-, and 21-day allografts, no differences were observed in the prevalence of CD4/CD8a cells in allografts from control or alcohol-fed donors, despite decreased airway epithelial integrity in 7-day allografts from alcohol-fed donors.
Thus, alcohol appears to amplify the tissue remodeling response that is considered to be a later event in the development of OAD. Specifically, alcohol ingestion by donors increases the number of TGF-β1– and α-SMA–positive cells in the airway, indicating that components of tissue remodeling are amplified in response to alcohol. These data suggest that prior alcohol ingestion results in increased numbers of lumenal myofibroblasts, and provides a possible explanation for the increased collagen deposition observed in allografts from alcohol-fed donors. In agreement with previous studies (15, 16), this work underscores the notion that modulation of fibrotic mechanisms is equally important as the initial alloimmune involvement in development of OAD pathophysiology. These data, taken together, suggest that prior alcohol ingestion by donors does not initiate the alloimmune trigger, but instead primes the grafted tissue for injury and subsequent development of OAD.
Previous studies have shown that, in the alcoholic lung, macrophage function is decreased (43) and other innate and adaptive immune functions are impaired (for review, see Reference 42). Expression of TGF-β1 is increased in the lungs of alcohol-fed rats (21). Alcohol ingestion also activates the renin–angiotensin system, and angiotensin II is capable of inducing oxidative stress by depleting pulmonary glutathione levels and increasing TGF-β1 expression (22). However, it is possible that other components of the alloimmune response may be increased in response to alcohol ingestion.
Despite the fact that several post-transplant risk factors for the development of OB have been identified (for review, see Reference 3), no effective therapies exist to prevent or attenuate the development of OB because of its progressive and irreversible nature. Comprehensive studies on how donor characteristics affect the success of lung transplantation in the recipient are lacking. Specifically, the effects of donor alcohol ingestion on the development of OB are unknown. This is an important issue to address, as many lung donors are likely to be young individuals who die in alcohol-related accidents. Importantly, this population is otherwise healthy, and does not display obvious clinical signs of lung dysfunction.
Although there is a lack of published, specific epidemiological data, we predict that conducting prospective (as well as retrospective) clinical studies on the effect of donor alcohol abuse on long-term success of lung transplants will result in the identification of alcohol as a risk factor for the development of OB. In support of our prediction, the now widely known link between alcohol abuse and susceptibility to acute lung injury was unrecognized until clinical studies published 10 years ago established such a link (23). The results presented here may have implications for the consideration of donor characteristics in regard to establishing criteria for determining what is considered “healthy” lung tissue for transplantation.
Supplementary Material
Acknowledgments
The authors thank Raena Garcia and Todd Mills for technical assistance with surgical procedures involving animals.
Supported by National Institutes of Health F32 NRSA fellowship AA016262-01 (P.O.M.) and Veterans Affairs Merit Review (D.M.G.).
This article has an online supplement, which is available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200702-255OC on August 23, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Boehler A, Kesten S, Weder W, Speich R. Bronchiolitis obliterans after lung transplantation: a review. Chest 1998;114:1411–1426. [DOI] [PubMed] [Google Scholar]
- 2.Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J 2003;22:1007–1018. [DOI] [PubMed] [Google Scholar]
- 3.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant 2002;21:271–281. [DOI] [PubMed] [Google Scholar]
- 4.Scholma J, Slebos DJ, Boezen HM, van den Berg JW, van der Bij W, de Boer WJ, Koeter GH, Timens W, Kauffman HF, Postma DS. Eosinophilic granulocytes and interleukin-6 level in bronchoalveolar lavage fluid are associated with the development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med 2000;162:2221–2225. [DOI] [PubMed] [Google Scholar]
- 5.DiGiovine B, Lynch JP III, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol 1996;157:4194–4202. [PubMed] [Google Scholar]
- 6.Riise GC, Andersson BA, Kjellstrom C, Martensson G, Nilsson FN, Ryd W, Schersten H. Persistent high BAL fluid granulocyte activation marker levels as early indicators of bronchiolitis obliterans after lung transplant. Eur Respir J 1999;14:1123–1130. [DOI] [PubMed] [Google Scholar]
- 7.Hauber HP, Mikkila A, Erich JM, Kroger N, Meyer A, Schoder V, Zander AR, Pforte A. TNFα, interleukin-10 and interleukin-18 expression in cells of the bronchoalveolar lavage in patients with pulmonary complications following bone marrow or peripheral stem cell transplantation: a preliminary study. Bone Marrow Transplant 2002;30:485–490. [DOI] [PubMed] [Google Scholar]
- 8.Reynaud-Gaubert M, Marin V, Thirion X, Farnarier C, Thomas P, Badier M, Bongrand P, Giudicelli R, Fuentes P. Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J Heart Lung Transplant 2002;21:721–730. [DOI] [PubMed] [Google Scholar]
- 9.Ward C, Whitford H, Snell G, Bao H, Zheng L, Reid D, Williams TJ, Walters EH. Bronchoalveolar lavage macrophage and lymphocyte phenotypes in lung transplant recipients. J Heart Lung Transplant 2001;20:1064–1074. [DOI] [PubMed] [Google Scholar]
- 10.Silkoff PE, Caramori M, Tremblay L, McClean P, Chaparro C, Kesten S, Hutcheon M, Slutsky AS, Zamel N, Keshavjee S. Exhaled nitric oxide in human lung transplantation: a noninvasive marker of acute rejection. Am J Respir Crit Care Med 1998;157:1822–1828. [DOI] [PubMed] [Google Scholar]
- 11.Bando K, Paradis IL, Similo S, Konishi H, Komatsu K, Zullo TG, Yousem SA, Close JM, Zeevi A, Duquesnoy RJ, et al. Obliterative bronchiolitis after lung and heart–lung transplantation: an analysis of risk factors and management. J Thorac Cardiovasc Surg 1995;110:4–13. [Discussion, 13–14.] [DOI] [PubMed] [Google Scholar]
- 12.Sharples LD, Tamm M, McNeil K, Higenbottam TW, Stewart S, Wallwork J. Development of bronchiolitis obliterans syndrome in recipients of heart–lung transplantation—early risk factors. Transplantation 1996;61:560–566. [DOI] [PubMed] [Google Scholar]
- 13.Khalifah AP, Hachem RR, Chakinala MM, Yusen RD, Aloush A, Patterson GA, Mohanakumar T, Trulock EP, Walter MJ. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. Am J Transplant 2005;5:2022–2030. [DOI] [PubMed] [Google Scholar]
- 14.Jackson CH, Sharples LD, McNeil K, Stewart S, Wallwork J. Acute and chronic onset of bronchiolitis obliterans syndrome (BOS): are they different entities? J Heart Lung Transplant 2002;21:658–666. [DOI] [PubMed] [Google Scholar]
- 15.King MB, Pedtke AC, Levrey-Hadden HL, Hertz MI. Obliterative airway disease progresses in heterotopic airway allografts without persistent alloimmune stimulus. Transplantation 2002;74:557–562. [DOI] [PubMed] [Google Scholar]
- 16.Ramirez AM, Takagawa S, Sekosan M, Jaffe HA, Varga J, Roman J. Smad3 deficiency ameliorates experimental obliterative bronchiolitis in a heterotopic tracheal transplantation model. Am J Pathol 2004;165:1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine SM, Bryan CL. Bronchiolitis obliterans in lung transplant recipients: the “thorn in the side” of lung transplantation. Chest 1995;107:894–897. [DOI] [PubMed] [Google Scholar]
- 18.Holguin F, Moss I, Brown LA, Guidot DM. Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest 1998;101:761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lois M, Brown LA, Moss IM, Roman J, Guidot DM. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am J Respir Crit Care Med 1999;160:1354–1360. [DOI] [PubMed] [Google Scholar]
- 20.Velasquez A, Bechara RI, Lewis JF, Malloy J, McCaig L, Brown LA, Guidot DM. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res 2002;26:1245–1251. [DOI] [PubMed] [Google Scholar]
- 21.Bechara RI, Brown LA, Roman J, Joshi PC, Guidot DM. Transforming growth factor β1 expression and activation is increased in the alcoholic rat lung. Am J Respir Crit Care Med 2004;170:188–194. [DOI] [PubMed] [Google Scholar]
- 22.Bechara RI, Pelaez A, Palacio A, Joshi PC, Hart CM, Brown LA, Raynor R, Guidot DM. Angiotensin II mediates glutathione depletion, transforming growth factor-β1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol 2005;289:L363–L370. [DOI] [PubMed] [Google Scholar]
- 23.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA 1996;275:50–54. [PubMed] [Google Scholar]
- 24.Moss M, Parsons PE, Steinberg KP, Hudson LD, Guidot DM, Burnham EL, Eaton S, Cotsonis GA. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003;31:869–877. [DOI] [PubMed] [Google Scholar]
- 25.Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med 2000;161:414–419. [DOI] [PubMed] [Google Scholar]
- 26.Freimark D, Aleksic I, Trento A, Takkenberg JJ, Valenza M, Admon D, Blanche C, Queral CA, Azen CG, Czer LS. Hearts from donors with chronic alcohol use: a possible risk factor for death after heart transplantation. J Heart Lung Transplant 1996;15:150–159. [PubMed] [Google Scholar]
- 27.Houyel L, Petit J, Nottin R, Duffet JP, Mace L, Neveux JY. Adult heart transplantation: adverse role of chronic alcoholism in donors on early graft function. J Heart Lung Transplant 1992;11:1184–1187. [PubMed] [Google Scholar]
- 28.Mitchell PO, Guidot DM. Chronic alcohol ingestion by donors amplifies obliterative bronchiolitis in a rodent model of lung transplantation [abstract]. J Heart Lung Transplant 2007;26(2 Suppl 1):S197. [Google Scholar]
- 29.Mitchell PO, Guidot DM. Chronic ethanol ingestion by donors predisposes the lung to injury in a rat model of lung transplantation [abstract]. Alcohol Clin Exp Res 2006;30(6 Suppl 1):224A. [Google Scholar]
- 30.Woessner JF Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys 1961;93:440–447. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell PO, Pavlath GK. Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol Cell Physiol 2004;287:C1753–C1762. [DOI] [PubMed] [Google Scholar]
- 32.Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, Haase AT. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest 2002;110:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams BF, Brazelton T, Berry GJ, Morris RE. The role of respiratory epithelium in a rat model of obliterative airway disease. Transplantation 2000;69:661–664. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: TGF-β signaling through Smad3-dependent and -independent pathways. Am J Transplant 2006;6:2080–2088. [DOI] [PubMed] [Google Scholar]
- 35.Zhou H, Latham CW, Zander DS, Margolin SB, Visner GA. Pirfenidone inhibits obliterative airway disease in mouse tracheal allografts. J Heart Lung Transplant 2005;24:1577–1585. [DOI] [PubMed] [Google Scholar]
- 36.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol 2003;200:500–503. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez FG, Jaramillo A, Chen C, Liu DZ, Tung T, Patterson GA, Mohanakumar T. Airway epithelium is the primary target of allograft rejection in murine obliterative airway disease. Am J Transplant 2004;4:319–325. [DOI] [PubMed] [Google Scholar]
- 38.El-Gamel A, Sim E, Hasleton P, Hutchinson J, Yonan N, Egan J, Campbell C, Rahman A, Sheldon S, Deiraniya A, et al. Transforming growth factor β (TGF-β) and obliterative bronchiolitis following pulmonary transplantation. J Heart Lung Transplant 1999;18:828–837. [DOI] [PubMed] [Google Scholar]
- 39.Charpin JM, Valcke J, Kettaneh L, Epardeau B, Stern M, Israel-Biet D. Peaks of transforming growth factor-β mRNA in alveolar cells of lung transplant recipients as an early marker of chronic rejection. Transplantation 1998;65:752–755. [DOI] [PubMed] [Google Scholar]
- 40.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-β–induced α-smooth muscle actin expression. Am J Respir Cell Mol Biol 2003;29:397–404. [DOI] [PubMed] [Google Scholar]
- 41.Neuringer IP, Mannon RB, Coffman TM, Parsons M, Burns K, Yankaskas JR, Aris RM. Immune cells in a mouse airway model of obliterative bronchiolitis. Am J Respir Cell Mol Biol 1998;19:379–386. [DOI] [PubMed] [Google Scholar]
- 42.Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc 2005;2:428–432. [DOI] [PubMed] [Google Scholar]
- 43.Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte–macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol 2005;175:6837–6845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.