Abstract
Rationale: Associations between oligomeric isocyanate exposure, sensitization, and respiratory disease have received little attention, despite the extensive use of isocyanate oligomers.
Objectives: To investigate exposure–response relationships of respiratory symptoms and sensitization in a large population occupationally exposed to isocyanate oligomers during spray painting.
Methods: The prevalence of respiratory symptoms and sensitization was assessed in 581 workers in the spray-painting industry. Personal exposure was estimated by combining personal task-based inhalatory exposure measurements and time activity information. Specific IgE and IgG to hexamethylene diisocyanate (HDI) were assessed in serum by ImmunoCAP assay and enzyme immunoassays using vapor and liquid phase HDI–human serum albumin (HDI–HSA) and HSA conjugates prepared with oligomeric HDI.
Measurements and Main Results: Respiratory symptoms were more prevalent in exposed workers than among comparison office workers. Log–linear exposure–response associations were found for asthmalike symptoms, chronic obstructive pulmonary disease–like symptoms, and work-related chest tightness (prevalence ratios for an interquartile range increase in exposure of 1.2, 1.3 and 2.0, respectively; P ⩽ 0.05). The prevalence of specific IgE sensitization was low (up to 4.2% in spray painters). Nevertheless, IgE to N100 (oligomeric HDI)–HSA was associated with exposure and work-related chest tightness. The prevalence of specific IgG was higher (2–50.4%) and strongly associated with exposure.
Conclusions: The results provide evidence of exposure–response relationships for both work-related and non–work-related respiratory symptoms and specific sensitization in a population exposed to oligomers of HDI. Specific IgE was found in only a minority of symptomatic individuals. Specific IgG seems to be merely an indicator of exposure.
Keywords: oligomer, isocyanate, asthma, spray painter, sensitization
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Isocyanates are among the most common causes of occupational asthma. Oligomeric isocyanates are increasingly used. Associations between oligomeric isocyanate exposure, sensitization, and respiratory disease have received little attention.
What This Study Adds to the Field
There are exposure–response relationships for exposure to oligomeric isocyanates and respiratory symptoms and sensitization. Specific IgE plays a role in a minority of symptomatic individuals. Specific IgG seems merely a marker of exposure.
Isocyanates, low-molecular-weight compounds characterized by highly reactive NCO groups, are one of the most commonly identified causes of occupational asthma (1–3). Besides allergic asthma, isocyanate exposure may also induce irritant asthma, hypersensitivity pneumonitis, and possibly accelerated lung function decline (4). Diisocyanates are used as cross-linking agents in polyurethane (PU) products, such as foams, paints, lacquers, inks, insulating materials, varnishes, rubber modifiers, and bonding and vulcanizing agents (5). The PU industry continues to increase, together with the number of workers at risk for exposure (4). Toluene diisocyanate (TDI), diphenyl-methane diisocyanate (MDI), and hexamethylene diisocyanate (HDI) are the most frequently used diisocyanate monomers.
Despite a vast amount of studies on isocyanates, aspects of the association between health effects and isocyanate exposure remain unclear. Isocyanate monomers have been studied relatively well in large TDI manufacturing or foam production units. In the early years of the industry, annual occupational asthma incidence was as high as 5 to 6% (6). The reduction of average TDI concentrations below the 8-hour occupational exposure limit of 5 ppb (17 μg/m3 total NCO group mass concentration) led to a decline to an incidence of below 1% (6). Conversely, asthma symptom prevalences of up to 41% have been reported in TDI end-user industries with possibly less controlled exposures (6).
Recently, diisocyanate oligomers, mainly of HDI and MDI with considerably lower vapor pressures, have been increasingly used to reduce inhalation exposure (4). In the present article, all polymeric diisocyanates, which are indicated with different terms (polyisocyanates, oligomers, adducts) in the literature, will be referred to as oligomers. Isocyanate asthma does occur in workers exposed to oligomers and specific inhalation challenge testing of individual patients confirms that oligomers can cause asthma (7). Yet, despite the extensive use of isocyanate oligomers, exposure–response associations have hardly been investigated. Commercial products based on oligomeric isocyanates commonly contain a variable mixture of several different chemical structures. The complexity of exposure assessment of these mixtures contributes to the absence of exposure–response studies.
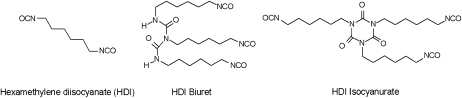
Spray painters, who are exposed to HDI oligomer mixtures, are among the occupational groups with the highest incidence of occupational asthma in industrialized countries (8–10). Figure 1 shows the chemical structures of HDI and two of its oligomers. This article describes a large cross-sectional study of isocyanate exposure and health effects in spray painters. Respiratory symptoms were recorded and specific IgE and IgG antibodies to various isocyanate conjugates were measured. Over 500 task-based exposure measurements to a wide range of isocyanates were used in combination with time activity information to estimate the exposure of each individual. We specifically aimed at establishing quantitative exposure–response relationships for a range of respiratory endpoints.
Figure 1.
Chemical structures of hexamethylene diisocyanate (HDI) and two HDI oligomers.
METHODS
Population and Study Design
The population consisted of 581 subjects working in various spray-painting industries in the Netherlands. Car body repair shops, furniture paint shops, and industrial paint shops specializing in ships and harbor equipment or airplanes were contacted by mail or telephone. Companies were visited between 2003 and 2006, and a workplace survey was performed. All workers were asked to complete a self-administered questionnaire and to provide a 20-ml blood sample. All participants were actively working at the time of the study and the study was performed on a working day. The Institutional Medical Ethical review board of University Centre Utrecht approved of the protocols, and written consent was obtained from all participants.
Questionnaire
Items included respiratory symptoms according to the Dutch version of the internationally accepted British Medical Research Council respiratory questionnaire (11), supplemented with questions on work-related symptoms. Symptoms were considered work related when they were reported to occur during or shortly after work. For statistical analyses, respiratory symptoms suggestive of chronic obstructive pulmonary disease (COPD) and asthma were combined: “COPD-like symptoms” included chronic cough, chronic phlegm, and shortness of breath; “asthmalike symptoms” included wheezing and chest tightness.
Additional items included smoking habits, job history, present job title, personal protective equipment use, and monthly task patterns. Workers reporting no tasks outside the office were classified as “office workers.” Workers involved in spray painting were classified as “spray painters,” and all other workers involved in tasks outside the office were classified as “others.” The latter category consisted of mostly mechanics and metal workers. In every company, all workers were working in the same building.
Personal Exposure Estimates
Personal exposure estimates were obtained by combining personal task-based inhalation measurements for 23 different isocyanate compounds (4 monoisocyanates, 5 aminoisocyanates, 6 diisocyanates and 8 diisocyanate oligomers) performed in the population under study (12) and time activity information:
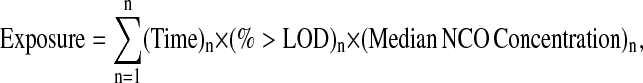 |
where Exposure = personal exposure expressed in μg NCO × m−3 × hour × month−1; n is an arbitrary value from 1 to 6 assigned to the following tasks: (1) spray painting; (2) mixing; (3) cleaning paint equipment; (4) assisting a spray painter; (5) sanding; and (6) welding; (Time)n = time task n was performed expressed in hours per month (on average, 82 h [SD, 89] out of a 161-h [SD, 26] working month was spent on exposed tasks); (% > LOD)n = percentage of samples above the limit of detection (LOD) for task n; (Median NCO concentration)n = median inhalatory isocyanate concentration during task n expressed in μg NCO/m3.
Separate task-based airborne exposure measurements were available for each combination of industry and task. The total isocyanate group (NCO) concentration and NCO from HDI and two HDI oligomers (biuret and isocyanurate) concentration were calculated.
More details are provided in the online supplement.
Serologic Analysis
Blood samples were processed within 8 hours and serum aliquots were stored at −20°C until serologic assays. HDI-specific IgE and IgG antibodies were analyzed using the ImmunoCAP assay (Phadia, Uppsala, Sweden) and specific IgE to common aeroallergens using the Phadiatop (Phadia) as a measure of atopy. Cutoff values of 0.35 kU/L for specific IgE and 5 mg/L for specific IgG were used.
Isocyanate-specific IgE and IgG were also assessed by enzyme immunoassay with HDI–HSA conjugates prepared in our own laboratories. HDI–HSA was prepared in liquid phase (HDIL–HSA) (13) and vapor phase (HDIV–HSA) (14) reactions essentially as described earlier. HDI oligomer–HSA conjugates were prepared with Desmodur N3300, a commercial product containing a low-viscosity isocyanurate oligomer of HDI, and Desmodur N100, a trimeric biuret structure (Bayer, Pittsburgh, PA). Table 1 gives an overview of immunoassays used. Cutoff values for HSA-corrected optical density values of 0.1 and 0.3 were used for IgE and IgG, respectively.
TABLE 1.
OVERVIEW OF CHARACTERISTICS OF ASSAYS USED IN SPECIFIC IgE AND IgG ANTI–HEXAMETHYLENE DIISOCYANATE ANALYSES
| Conjugate | Source* | Carrier | Phase Isocyanate† | Test System |
|---|---|---|---|---|
| HDI–ImmunoCAP | Phadia | ImmunoCAP (as solid phase) | Done by manufacturers | ImmunoCAP assay |
| HDIL–HSA | IRAS | HSA | Liquid | EIA |
| HDIV–HSA | Yale | HSA | Vapor | EIA |
| N3300–HSA | Yale | HSA | Liquid | EIA |
| N100–HSA | Yale | HSA | Liquid | EIA |
Definition of abbreviations: EIA = enzyme immunoassay; HDI = hexamethylene diisocyanate; HDIL–HSA = HDI–HSA liquid phase; HDIV–HSA = HDI–HSA vapor phase; HSA = human serum albumin; IRAS = Institute for Risk Assessment Sciences.
Source where the conjugate was prepared, technical details, and system in which the conjugate was used are shown.
Sources: Phadia, Sweden; IRAS, The Netherlands; Yale School of Medicine, Connecticut.
Phase of the isocyanate mixture during reaction.
Details of the enzyme immunoassay procedures and establishment of cutoff values are provided in the online supplement.
Physiological Testing
Bronchial hyperresponsiveness (BHR) was assessed in a subset of 229 workers. Selection of this subset is described in the online supplement. At least two maximal expiratory flow–volume maneuvers were obtained to assess baseline lung function. The largest FEV1 and FVC were recorded. Maximum midexpiratory flow (MMEF) was obtained from the maneuver with the largest sum of FEV1 + FVC as described by Miller and colleagues (15). BHR was assessed by methacholine challenge according to the European Respiratory Society guidelines (16). Methacholine was administered using a controlled tidal volume breathing dosimeter technique using the Aerosol Provocation System with a Medic-Aid nebulizer (Jaeger GmbH and Co KG, Wurzburg, Germany), starting with 0.019 mg methacholine after three quadrupling doses and one doubling dose up to a cumulative dose of 2.5 mg (short schedule). FEV1 was measured 30 and 90 seconds after challenge and the lowest FEV1 from a technically acceptable maneuver was used. After a fall in FEV1 of 5%, doubling doses were used (long schedule). The test was stopped when a fall of 20% in FEV1 was observed (BHR20) or the maximum cumulative dose was reached. Airway hyperresponsiveness was defined as a provocative dose of methacholine required to cause a 20% fall in FEV1 of 2.5 mg or less (∼10 μmol).
Statistical Analysis
SAS version 9.1 statistical software was used (SAS Institute, Cary, NC). Correlations between the exposure variables were assessed using Pearson correlation coefficients for log-transformed data. In cross-sectional studies, the prevalence ratio (PR) is often a more easily interpretable and meaningful measure of association than the odds ratio (17). Therefore, PRs and 95% confidence intervals (95% CI) were calculated by log-binomial regression (SAS GENMOD procedure) to describe associations for binary health outcomes. Log-transformed exposure data were used and PRs per unit increase were converted to PRs per interquartile range. Associations with exposure were further explored by nonparametric regression modeling (smoothing) using generalized additive models (SAS GAM procedure). Smoothing parameter degrees of freedom were selected by generalized cross-validation (18) but limited to three. Unless stated otherwise, all associations were adjusted for current smoking, age, sex, and atopy. Possible effect modification by atopy was explored as well.
RESULTS
Population Characteristics and Exposure
The 581 participating workers came from 128 companies: 88 car body repair shops; 33 furniture paint shops; and 7 industrial paint shops, of which 6 specialize in ships and harbor equipment and 1 in airplanes. Of all companies contacted through surface mail and telephone, 10 to 30% responded; the average worker participation rate per company was 67%. General characteristics of the study population are shown in Table 2.
TABLE 2.
GENERAL POPULATION CHARACTERISTICS, WORK HISTORY AND ISOCYANATE EXPOSURE OF 581 WORKERS IN SPRAY-PAINTING COMPANIES
| Office Workers | Spray Painters | Others | |
|---|---|---|---|
| No. | 50 | 241 | 290 |
| Sex, % male | 58 | 99 | 97 |
| Age, yr, AM (SD) | 40.1 (10.1) | 36.9 (10.4) | 39.0 (12.0) |
| Smoking status | |||
| Smoker, % | 23.4 | 42.8 | 35.6 |
| Stopped smoking within last year, % | 2.1 | 4.7 | 5.3 |
| Former smoker, % | 40.4 | 19.2 | 23.2 |
| Never smoked, % | 34.0 | 33.3 | 35.9 |
| Total pack-years, AM (SD) | 7.7 (10.4) | 8.2 (11.9) | 8.5 (13.4) |
| Branch type, % | |||
| Car body repair shop | 72 | 66 | 85 |
| Furniture paint shop | 6 | 16 | 11 |
| Boat/harbor equipment paint shop | 2 | 6 | 2 |
| Airplane paint shop | 20 | 12 | 2 |
| Work history, AM (SD) | |||
| No. of years worked | 15.6 (10.1) | 16.3 (9.7) | 19.2 (12.4) |
| No. of years in branch | 11.9 (10.7) | 15.7 (9.6) | 18.1 (12.4) |
| No. of years as spray painter | 2.0 (4.7) | 14.9 (9.6) | 3.4 (7.5) |
| Isocyanate exposure, μg NCO × m−3 × h × mo−1 | |||
| Total isocyanate, median (min–max) | 0 | 3,682 (4–66,464) | 8 (0–13,473) |
| HDI, median (min–max) | 0 | 27 (0.2–1,427) | 0.3 (0–1,920) |
| Biuret, median (min–max) | 0 | 269 (0.2–13,568) | 2 (0–1,587) |
| Isocyanurate, median (min–max) | 0 | 2,250 (6–87,623) | 6 (0–30,0006) |
Definition of abbreviations: AM = arithmetic mean; HDI = hexamethylene diisocyanate.
Estimated median total NCO exposure levels were higher in the “spray painters” category than among “others,” with a wide range in both categories. Exposure to HDI monomer represented only a very small fraction of total NCO. Of the HDI oligomers, which represented a larger fraction of total NCO, isocyanurate exposure was higher than biuret exposure.
Within the group of spray painters, those working in airplane paint shops were, on average, more highly exposed than those in furniture paint shops, ship and harbor equipment paint shops, and car body repair shops (median: 16,600 vs. 4,900, 4,700, and 3,300 μg NCO × m−3 × h × mo−1, respectively). The minimum, 25th percentile, median, 75th percentile, and maximum of the total exposure distribution were 0, 1.7, 165, 33,821, and 66,464 μg NCO × m−3 × hour × month−1, respectively. Pearson correlation coefficients among the exposure estimates for total NCO, HDI, biuret, and isocyanurate were very high (⩾0.95).
Prevalence of Symptoms and Positive Serology
Exposed workers more often reported respiratory symptoms than office workers (Table 3). Asthmalike symptoms were significantly (P ⩽ 0.05) more prevalent in both spray painters and other workers (adjusted PR [95% CI]: 2.8 [1.3–5.9] and 2.2 [1.0–4.8], respectively). Spray painters also reported more COPD-like symptoms (adjusted PR [95% CI], 2.9 [1.1–8.0]). No significant differences were found for any of the work-related symptoms.
TABLE 3.
PREVALENCE OF RESPIRATORY AND ALLERGIC SYMPTOMS AND SEROLOGIC OUTCOMES*
| Office Workers (n = 50) | Spray Painters (n = 241) | Others (n = 290) | |
|---|---|---|---|
| Respiratory symptoms, % | |||
| Chronic cough | 2.0 | 15.4† | 13.6† |
| Chronic phlegm | 4.0 | 13.3 | 10.8 |
| Shortness of breath | 4.0 | 8.8 | 8.4 |
| Wheezing | 12.0 | 29.1‡ | 22.6† |
| Frequent wheezing (>1 wk) | 4.0 | 12.5ठ| 4.9 |
| Shortness of breath during wheezing | 4.0 | 16.2† | 10.5 |
| Chest tightness | 14.0 | 18.3 | 14.4 |
| Chest tightness before start work | 10.0 | 8.8 | 7.1 |
| Clusters of symptoms, % | |||
| COPD-like symptoms | 8.0 | 26.1‡ | 20.6† |
| Asthmalike symptoms | 14.0 | 33.6‡ | 28.0‡ |
| Work-related symptoms, % | |||
| Work-related rhinitis | 14.3 | 19.8 | 15.0 |
| Work-related chest tightness | 2.0 | 8.3 | 4.0 |
| Work-related conjunctivitis | 12.0 | 16.0 | 10.4 |
| Positive serology, % | |||
| Atopy (Phadiatop) | 44.0 | 33.6‡‖ | 37.6 |
| Specific IgE | |||
| HDI–ImmunoCAP | 0 | 2.1 | 1.0 |
| HDIL–HSA | 0 | 2.9 | 3.5 |
| HDIV–HSA | 0 | 0.4 | 0.7 |
| N3300–HSA | 0 | 2.1 | 1.0 |
| N100–HSA | 0 | 4.2 | 2.1 |
| Specific IgG | |||
| HDI–ImmunoCAP | 4.0 | 9.5 | 7.2 |
| HDIL–HSA | 32.0 | 50.4‡ | 41.5 |
| HDIV–HSA | 2.0 | 20.0‡¶ | 9.3 |
| N3300–HSA | 10.0 | 23.3 | 15.1 |
| N100–HSA | 4.0 | 34.6‡ | 21.5‡ |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; HDI = hexamethylene diisocyanate; HDIL–HSA = HDI–HSA liquid phase; HDIV–HSA = HDI–HSA vapor phase; HSA = human serum albumin.
Atopy and specific IgE and IgG sensitization against HDI.
P < 0.10; significantly different from “office workers” category after adjustment for atopy, current smoking, age, and sex.
P < 0.05; significantly different from “office workers” category after adjustment for atopy, current smoking, age, and sex.
Adjusted for atopy, current smoking, and sex.
Adjusted for current smoking, age, and sex.
Adjusted for for atopy, current smoking, and age.
Despite the high symptom prevalence, specific IgE antibodies to isocyanates were found only in a small proportion of exposed workers (0.4–4.2% in spray painters, 0.7–3.5% in other workers, and none in the office group) (Table 3). The prevalence of elevated specific IgG antibody concentrations was much higher. Among spray painters, prevalences up to 50% were found. Antibodies to N100–HSA and HDIL–HSA were found most frequently, both for specific IgE and IgG.
Specific IgG to HDIL–HSA, HDIV–HSA, and N100–HSA was significantly (P ⩽ 0.05) more prevalent among spray painters compared with office workers (adjusted PR [95% CI]: 1.6 [1.0–2.6], 10.6 [1.5–75.2], and 7.8 [1.9–32.5], respectively). IgG antibodies to N100–HSA were also more often found in other workers than in office workers (adjusted PR [95% CI], 4.7 [1.1–19.4]).
Atopy was significantly (P ⩽ 0.05) less common among spray painters than office workers (adjusted PR [95% CI], 0.7 [0.5–1.0]).
Association between Symptoms and Serology
Table 4 shows the associations between symptoms and the presence of isocyanate-specific antibodies. A consistent pattern of significant positive associations was found for work-related rhinitis and specific IgE to each of the conjugates, with PRs between 1.8 and 2.8. All PRs for work-related chest tightness and specific IgE were positive, but showed much more variation, and only the association with IgE to N100–HSA was significant. Overall PRs for asthmalike and COPD-like symptoms were lower and, for most conjugates, were close to 1.0.
TABLE 4.
ASSOCIATION BETWEEN RESPIRATORY SYMPTOMS AND POSITIVE IgE AND IgG SENSITIZATION*
| COPD-like Symptoms | Asthmalike Symptoms | Work-related Chest Tightness | Work-related Rhinitis | Work-related Conjunctivitis | |
|---|---|---|---|---|---|
| IgE | |||||
| HDI–ImmunoCAP | 1.1 (0.3-3.6) | 0.8 (0.2-2.5) | 1.6 (0.2-10.3) | 2.6 (1.4-4.8) † | 1.6 (0.5-5.6) |
| HDIL–HSA | 1.2 (0.6-2.6) | 0.9 (0.4-1.9) | 1.8 (0.5-6.9) | 2.0 (1.1-3.6) † | 1.3 (0.4-3.7) |
| HDIV–HSA | 2.3 (0.9-5.5) | 1.6 (0.7-3.7) | 4.3 (0.8-23.1) | 2.8 (1.1-6.7) † | —‡ |
| N3300–HSA | 1.0 (0.3-3.4) | 0.7 (0.2-2.4) | 1.5 (0.2-10.2) | 2.1 (1.0-4.4) † | 0.8 (0.1-5.3) |
| N100–HSA | 1.6 (0.9-3.2) | 1.1 (0.6-2.2) | 3.7 (1.4-9.8) † | 1.8 (1.0-3.4) † | 1.2 (0.4-3.4) |
| IgG | |||||
| HDI–ImmunoCAP | 1.4 (0.8-2.4) | 1.2 (0.8-1.9) | 0.8 (0.2-3.2) | 1.5 (0.8-2.6) | 1.0 (0.5-2.2) |
| HDIL–HSA | 0.9 (0.6-1.2) | 1.0 (0.8-1.3) | 1.4 (0.7-3.0) | 1.4 (0.9-2.0) | 1.3 (0.8-2.0) |
| HDIV–HSA | 0.8 (0.5-1.4) | 1.2 (0.9-1.7) | 1.2 (0.5-3.0) | 1.2 (0.8-2.0) | 1.3 (0.7-2.3) |
| N3300–HSA | 1.0 (0.7-1.5) | 0.9 (0.7-1.3) | 1.0 (0.4-2.3) | 1.3 (0.3-1.9) | 1.1 (0.7-2.0) |
| N100–HSA | 1.4 (1.0-1.9)† | 1.1 (0.8-1.5) | 1.7 (0.8-3.5) | 1.5 (1.1-2.2)† | 1.6 (1.0-2.5)† |
For definition of abbreviations, see Table 3.
Prevalence ratio (95% confidence interval) adjusted for age, sex, current smoking, and atopy.
P ⩽ 0.05.
Too few positives to calculate a prevalence ratio.
Statistically significant associations were found for COPD-like symptoms and work-related rhinitis and conjunctivitis with IgG to N100–HSA. However, for the other conjugates, PRs for the association between specific IgG and symptoms were close to 1. Exclusion of workers with a high IgG background reaction to HSA did not alter the associations (data not shown).
Associations with Exposure
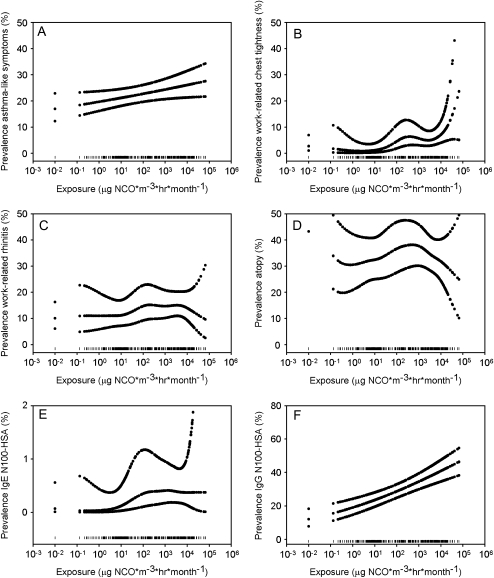
PRs were calculated based on log-transformed exposure data and expressed for an interquartile range increase in exposure (1.7–3,382 μg NCO × m−3× h × mo−1, or an approximate difference in exposure of a factor of 2,000) (Table 5). Significant positive log-linear associations with exposure were found for asthmalike symptoms, COPD-like symptoms, work-related chest tightness, and work-related conjunctivitis (Table 5). Only the association between work-related conjunctivitis and exposure differed between atopic and nonatopic individuals (P interaction term ⩽ 0.1). Surprisingly, the association was stronger in nonatopic than in atopic subjects (adjusted PR [95% CI]: 2.1 [1.2–3.9] and 1.1 [0.7–1.8], respectively). For asthmalike symptoms (Figure 2A) and COPD-like symptoms (plot not shown) the smoothed plots corroborate log-linear relations. For work-related chest tightness (Figure 2B), the smoothed plot suggests a steeper increase at high exposure levels (P spline ⩽ 0.05). No statistically significant association between rhinitis and exposure was found (Figure 2C).
TABLE 5.
ASSOCIATION BETWEEN RESPIRATORY SYMPTOMS AND SPECIFIC IgE AND IgG SENSITIZATION AND EXPOSURE
| PR (95% CI) | |
|---|---|
| Symptoms | |
| Asthmalike symptoms | 1.2 (1.01.5)* |
| COPD-like symptoms | 1.3 (1.0–1.7)* |
| Work-related chest tightness | 2.0 (1.0–3.9)* |
| Work-related rhinitis | 1.3 (0.9–1.7) |
| Work-related conjunctivitis | 1.5 (1.0–2.1)* |
| Specific IgE | |
| HDI–ImmunoCAP | 2.2 (0.6–8.2) |
| HDIL–HSA | 1.4 (0.7–3.0)† |
| HDIV–HSA | 0.6 (0.1–3.9)‡ |
| N3300–HSA | 1.8 (0.5–7.2) ‡ |
| N100–HSA | 3.0 (1.1–8.4)*§ |
| Specific IgG | |
| HDI–ImmunoCAP | 1.2 (0.71.9) |
| HDIL–HSA | 1.2 (1.1–1.4)* |
| HDIV–HSA | 2.2 (1.5–3.4)* |
| N3300–HSA | 1.6 (1.2–2.2)* |
| N100–HSA | 2.0 (1.5–2.6)* |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; HDI = hexamethylene diisocyanate; HDIL–HSA = HDI–HSA liquid phase; HDIV–HSA = HDI–HSA vapor phase; HSA = human serum albumin.
Shown are prevalence ratios (95% CI) for an interquartile range increase in exposure adjusted for age, sex, smoking, and atopy.
P ⩽ 0.05.
Adjusted for age, smoking, and atopy.
Adjusted for age, smoking, and sex.
Adjusted for age, sex, and atopy.
Figure 2.
Association between log-transformed exposure to isocyanates (μg NCO × m−3 × h × mo−1) and selected health endpoints. Penalized smoothed spline plots are given with smoothed 95% confidence intervals for (A) asthmalike symptoms (spline: P > 0.10); (B) work-related chest tightness (spline: P ⩽ 0.05); (C) work-related rhinitis (spline: P > 0.10); (D) atopy (spline: P ⩽ 0.05); (E) IgE N100–HSA (spline: P > 0.10); (F) IgG N100–HSA (spline: P > 0.10). Data rugs at the bottom of each graph indicate the distribution of data points. HSA = human serum albumin.
Interestingly, the prevalence of atopy was lower at high exposure levels. Figure 2D shows a sharp reduction for the prevalence of atopy at isocyanate exposures above approximately 1,000 μg NCO × m−3 × hour × month−1 (P spline ⩽ 0.05). Atopic subjects were significantly less exposed than nonatopic subjects (geometric mean: 24.1 and 57.9 μg NCO × m−3 × h × mo−1, respectively; P ⩽ 0.05).
Exposure was also associated with N100–HSA-specific IgE. The smoothed plot shows a very slight increase (Figure 2E). Specific IgG antibodies to all conjugates except HDI–ImmunoCAP were positively associated with exposure. Especially strong associations were found for IgG to HDIV–HSA and N100–HSA.
For IgG measured by ImmunoCAP (P interaction term ⩽ 0.1), IgG to N3300–HSA (P interaction term ⩽ 0.05) and to N100–HSA (P interaction term ⩽ 0.05), stronger associations were seen in atopic subjects (adjusted PR [95% CI]: 2.5 [0.99–6.4], 2.8 [1.6–4.8], and 3.5 [2.1–5.8], respectively) than in nonatopic subjects for whom none of the associations was significant. Exclusion of workers with a high IgG background reaction to HSA did not alter any of these associations (data not shown).
Glove use during paint-related tasks, which varies among workers, did not affect exposure–response associations in this study. The use of respiratory protection during spray painting is compulsory and was always observed during the fieldwork. Therefore, the effect of respiratory protection could not be investigated.
Physiological Testing
Individuals with asthmalike symptoms were more likely to have BHR (adjusted PR [95% CI], 2.2 [1.5–3.2]). These individuals also had lower baseline FEV1, FEV1/FVC, and MMEF between 90 and 96% compared with symptom-free workers. For COPD-like symptoms, the association with BHR was less strong than for asthmalike symptoms and only borderline statistically (P = 0.07) significant (adjusted PR [95% CI], 1.6 [1.0–2.5]). In addition, none of the lung function parameters was significantly associated with COPD-like symptoms. Individuals with work-related symptoms were more likely to be hyperresponsive, but this was statistically significant only in those with rhinitis symptoms (PRs ⩾ 1.8). No clear associations between work-related symptoms and lung function were found.
DISCUSSION
The results of this study provide evidence for exposure–response relationships for exposure to complex mixtures of isocyanates and both work-related and non–work-related respiratory symptoms and specific sensitization.
Exposure to diisocyanate monomers has been assessed in various epidemiologic studies. In the majority of these studies, mean or maximum exposure levels are reported for a population in which a measure of disease frequency is investigated (19–25). However, few studies have considered the issue of quantitative exposure response in isocyanate asthma (26). Two case-control studies demonstrated that higher exposure levels were more likely to be found in companies at which there were workers with a successful claim for occupational asthma (27) or in doctor-diagnosed asthma cases (26) than in control companies or matched control subjects from the same company, respectively. Differences in study design complicate the comparison of these studies with the present study.
The use of product formulations containing complex mixtures of oligomer isocyanates is increasing (4). Currently, oligomers are the major contributor to isocyanate exposure worldwide. Several studies have shown respiratory symptoms or asthma in workers exposed to oligomeric aromatic isocyanates (28–30). Oligomers of aliphatic HDI are widely used in the spray-painting industry. Decreased lung function parameters (31, 32) and high asthma symptom prevalences have been reported in this industry (33–38). Only one study has incorporated exposure assessment. That study demonstrated a relation between peak exposure and reduced lung function in car painters who smoke (32). However, the population size was too small (n = 36) to be conclusive.
This is the first study performed in an end-user industry in which complex exposure patterns of isocyanates were assessed. Over 500 task-based exposure measurements were taken using a state-of-the-art method (12) and used to estimate monthly cumulative personal exposure.
A working day of a spray painter consists of cycles of short tasks, and even exposure during spray painting is highly variable for all workers (12). Therefore, isocyanate exposure in this study consists of a series of peaks, which is highly correlated with average exposure through the duration of the tasks. Consequently, it is not possible to differentiate between cumulative and peak exposure.
Although HDI oligomers were the major exposure factor, product formulations also contained trace amounts of monomeric HDI leading to detectable but very low monomer exposure levels. Personal task-based HDI levels up to 29 μg NCO/m3 were found, which did not exceed the Dutch short-term exposure limit for HDI (70 μg NCO/m3). In contrast, HDI oligomer levels ranged up to 3,760 μg NCO/m3. Therefore, despite the high correlation between oligomer and monomer levels, it seems unlikely that these monomer levels contributed significantly to the observed associations with symptoms.
Animal studies indicate that relative potencies of different isocyanate compounds are variable (39–42). Theoretically, this kind of information might be used to calculate a weighted total NCO concentration. However, for many of the measured isocyanate compounds, this information is not available, which limits the possibilities to use the information on oligomer levels for calculation of overall NCO levels weighted by toxic properties. Moreover, because exposure to HDI and its individual oligomers correlated highly, this would practically only have led to a rescaling of the exposure variable.
The company participation rate of this study was low (10–30%), whereas the mean worker participation rate within the companies of 67% was acceptable. Control measures are very similar among car body repair shops in the Netherlands and spray-booths and ventilation are always present. Yet, working practices may vary, and it cannot be ruled out that more compliant companies were more likely to participate. The negative association between atopy and exposure may point toward another type of selection bias. Possibly, atopic workers are more likely to develop symptoms and leave the industry or atopic workers with preexisting conditions may avoid seeking work as a spray painter. This warrants further attention in follow-up studies because it may result in a healthy worker effect.
Regardless of a possible healthy worker effect, a high prevalence of reported symptoms was noted in spray painters but also in other workers. Positive associations with exposure were found for asthmalike and COPD-like symptoms, work-related chest tightness, and work-related conjunctivitis. Smoothed spline plots corroborated these associations and confirmed that the log-linear models describe the relation with asthmalike symptoms in a satisfactory way. For work-related chest tightness, a steeper increase at high exposure levels was suggested. The surprisingly stronger association for work-related conjunctivitis in nonatopic individuals seems to be explained by the underrepresentation of atopic workers in the highest exposure range.
The significance of asthmalike symptoms found in this study was corroborated by the BHR results and lung function testing. Asthmalike symptoms were associated with BHR and lung function parameters indicative of obstruction. These associations were weaker or did not exist for COPD-like symptoms, indicating that these symptoms may be due to other respiratory conditions.
The low prevalence of specific IgE antibodies in this population of workers who were actively working at the time of the study complicates the assessment of its association with exposure as well as with health effects. Nevertheless, an association between specific IgE to N100–HSA and work-related chest tightness as well as exposure to isocyanates was indicated. The results suggest that, at most, specific IgE plays a role in a minority of individuals with symptoms. Thus, other mechanisms, like cell-mediated allergic reactions or pulmonary irritation (4, 43), are likely to be involved. The association between IgE to each of the isocyanate conjugates and work-related rhinitis in the absence of a statistically significant association with exposure is remarkable and needs to be further explored.
IgG antibodies are usually considered an effect of exposure. The observed relationship between IgG and exposure can therefore be regarded as an external validation of the exposure assessment in this study. In addition, it shows that, despite the low prevalence of specific IgE, the conjugates used are suitable reagents for the detection of isocyanate-specific immune responses. The significantly stronger association between specific IgG and exposure in atopic subjects, despite their lower exposure levels, suggests that they are immunologically more responsive to isocyanates than nonatopic individuals. A remarkable high prevalence of IgG to HDIL–HSA was found in office workers. A recent study demonstrated specific IgG to HDI–HSA in 13% of 139 individuals without known exposure to isocyanates (44). Whether specific IgG antibodies to HDIL–HSA in office workers represents actual exposure needs to be further explored.
Taken together, despite a possible healthy worker effect, exposure–response relationships were demonstrated for respiratory symptoms and sensitization in this population of spray painters exposed mainly to oligomers of HDI. Specific IgG antibodies seem to be primarily a marker of exposure. The association between specific IgE to N100–HSA and symptoms on one hand and exposure on the other hand is suggestive of an IgE-mediated mechanism in only a small proportion of the symptomatic individuals. A more detailed evaluation of immunologic and physiological endpoints is needed to gain insight in the nature of symptoms induced by isocyanates and the role of specific antibodies in this population.
Supplementary Material
Acknowledgments
The authors thank the following individuals for their contribution to this study: all company owners and workers who cooperated and made this study possible; Sjaak de Vreede, Willeke Munneke, Mirian Boeve, Siegfried de Wind, Mischa Zengeni, and Jos Rooijackers for their support in the fieldwork and laboratory analyses; Frieke Kuper and Peter Thorne for their useful comments on the manuscript. In addition, they thank the Dutch branch organization for car body repair shops (FOCWA) for their contribution to the enrollment of companies.
Supported by a grant from the Cefic Long Research Initiative; by the Dutch Ministry of Social Affairs and Employment; and by National Institutes of Health grant R01-HL62622.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200702-215OC on July 26, 2007
Conflict of Interest Statement: A.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.R.-H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.C.L.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.-W.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. I.M.W. was a speaker in a scientific course on occupational asthma organized and financed by a pharmaceutical company (GlaxoSmithKline). G.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.V.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.H. received a grant of €370,000 from the Long Range Research Initiative (LRI) Program for the research described in this paper. LRI is an initiative from the chemical industry associations ACC (American Chemistry Council), JCIA (Japan Chemical Industry Association; Japan), and Cefic (European Chemical Industry Council; Europe) to address emerging and existing health and environmental issues (www.cefic.be).
References
- 1.Vandenplas O, Malo JL, Saetta M, Mapp CE, Fabbri LM. Occupational asthma and extrinsic alveolitis due to isocyanates: current status and perspectives. Br J Ind Med 1993;50:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein JA. Overview of diisocyanate occupational asthma. Toxicology 1996;111:181–189. [DOI] [PubMed] [Google Scholar]
- 3.Wisnewski AV, Redlich CA. Recent developments in diisocyanate asthma. Curr Opin Allergy Clin Immunol 2001;1:169–175. [DOI] [PubMed] [Google Scholar]
- 4.Wisnewski AV, Redlich C, Mapp C, Bernstein DI. Polyisocyanates and their prepolymers. In: Bernstein IL, Chan-Yeung M, Malo JL, Bernstein DI, editors. Asthma in the workplace. New York: Taylor & Francis Group; 2006. pp. 481–504.
- 5.Lesage J, Goyer N, Desjardins F, Vincent JY, Perrault G. Workers' exposure to isocyanates. Am Ind Hyg Assoc J 1992;53:146–153. [DOI] [PubMed] [Google Scholar]
- 6.Ott MG. Occupational asthma, lung function decrement, and toluene diisocyanate (TDI) exposure: a critical review of exposure-response relationships. Appl Occup Environ Hyg 2002;17:891–901. [DOI] [PubMed] [Google Scholar]
- 7.Bello D, Woskie SR, Streicher RP, Liu Y, Stowe MH, Eisen EA, Ellenbecker MJ, Sparer J, Youngs F, Cullen MR, et al. Polyisocyanates in occupational environments: a critical review of exposure limits and metrics. Am J Ind Med 2004;46:480–491. [DOI] [PubMed] [Google Scholar]
- 8.McDonald JC, Keynes HL, Meredith SK. Reported incidence of occupational asthma in the UK, 1989–97. Occup Environ Med 2000;57:823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karjalainen A, Kurppa K, Virtanen S, Keskinen H, Nordman H. Incidence of occupational asthma by occupation and industry in Finland. Am J Ind Med 2000;37:451–458. [DOI] [PubMed] [Google Scholar]
- 10.Ameille J, Pauli G, Calastreng-Crinquand A, Vervloet D, Iwatsubo Y, Popin E, Bayeux-Dunglas MC, Kopferschmitt-Kubler MC. Reported incidence of occupational asthma in France, 1996–99: the ONAP programme. Occup Environ Med 2003;60:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medical Research Council Committee on the Aetiology of Chronic Bronchitis. Instructions for the use of the questionnaire on respiratory symptoms. Dawlish, UK: Holman Ltd.; 1966.
- 12.Pronk A, Tielemans E, Skarping G, Bobeldijk I, van Hemmen J, Heederik D, Preller L. Inhalation exposure to isocyanates of car body repair shop workers and industrial spray painters. Ann Occup Hyg 2006;50:1–14. [DOI] [PubMed] [Google Scholar]
- 13.Dewair MA, Baur X. Studies on antigens useful for detection of IgE antibodies in isocyanate-sensitized workers. J Clin Chem Clin Biochem 1982;20:337–340. [DOI] [PubMed] [Google Scholar]
- 14.Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, Redlich CA. Isocyanate vapor-induced antigenicity of human albumin. J Allergy Clin Immunol 2004;113:1178–1184. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 16.Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness: standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993;16:53–83. [PubMed] [Google Scholar]
- 17.Skov T, Deddens J, Petersen MR, Endahl L. Prevalence proportion ratios: estimation and hypothesis testing. Int J Epidemiol 1998;27:91–95. [DOI] [PubMed] [Google Scholar]
- 18.Hastie T, Tibshirani RJ. Generalized additive models. New York: Chapman & Hall; 1990.
- 19.Bodner KM, Burns CJ, Randolph NM, Salazar EJ. A longitudinal study of respiratory health of toluene diisocyanate production workers. J Occup Environ Med 2001;43:890–897. [DOI] [PubMed] [Google Scholar]
- 20.White WG, Morris MJ, Sugden E, Zapata E. Isocyanate-induced asthma in a car factory. Lancet 1980;1:756–760. [DOI] [PubMed] [Google Scholar]
- 21.Clark RL, Bugler J, McDermott M, Hill ID, Allport DC, Chamberlain JD. An epidemiology study of lung function changes of toluene diisocyanate foam workers in the UK. Int Arch Occup Environ Health 1998;71:169–179. [DOI] [PubMed] [Google Scholar]
- 22.Jones RN, Rando RJ, Glindmeyer HW, Foster TA, Hughes JM, O'Neil CE, Weill H. Abnormal lung function in polyurethane foam producers: weak relationship to toluene diisocyanate exposures. Am Rev Respir Dis 1992;146:871–877. [DOI] [PubMed] [Google Scholar]
- 23.Grammer LC, Eggum P, Silverstein M, Shaughnessy MA, Liotta JL, Patterson R. Prospective immunologic and clinical study of a population exposed to hexamethylene diisocyanate. J Allergy Clin Immunol 1988;82:627–633. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein DI, Korbee L, Stauder T, Bernstein JA, Scinto J, Herd ZL, Bernstein IL. The low prevalence of occupational asthma and antibody-dependent sensitization to diphenylmethane diisocyanate in a plant engineered for minimal exposure to diisocyanates. J Allergy Clin Immunol 1993;92:387–396. [DOI] [PubMed] [Google Scholar]
- 25.Ott MG, Klees JE, Poche SL. Respiratory health surveillance in a toluene di-isocyanate production unit, 1967–97: clinical observations and lung function analyses. Occup Environ Med 2000;57:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meredith SK, Bugler J, Clark RL. Isocyanate exposure and occupational asthma: a case-referent study. Occup Environ Med 2000;57:830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarlo SM, Liss GM, Dias C, Banks DE. Assessment of the relationship between isocyanate exposure levels and occupational asthma. Am J Ind Med 1997;32:517–521. [DOI] [PubMed] [Google Scholar]
- 28.Ulvestad B, Melbostad E, Fuglerud P. Asthma in tunnel workers exposed to synthetic resins. Scand J Work Environ Health 1999;25:335–341. [DOI] [PubMed] [Google Scholar]
- 29.Simpson C, Garabrant D, Torrey S, Robins T, Franzblau A. Hypersensitivity pneumonitis-like reaction and occupational asthma associated with 1,3-bis(isocyanatomethyl) cyclohexane pre-polymer. Am J Ind Med 1996;30:48–55. [DOI] [PubMed] [Google Scholar]
- 30.Petsonk EL, Wang ML, Lewis DM, Siegel PD, Husberg BJ. Asthma-like symptoms in wood product plant workers exposed to methylene diphenyl diisocyanate. Chest 2000;118:1183–1193. [DOI] [PubMed] [Google Scholar]
- 31.Glindmeyer HW, Lefante JJ Jr, Rando RJ, Freyder L, Hnizdo E, Jones RN. Spray-painting and chronic airways obstruction. Am J Ind Med 2004;46:104–111. [DOI] [PubMed] [Google Scholar]
- 32.Tornling G, Alexandersson R, Hedenstierna G, Plato N. Decreased lung function and exposure to diisocyanates (HDI and HDI-BT) in car repair painters: observations on re-examination 6 years after initial study. Am J Ind Med 1990;17:299–310. [DOI] [PubMed] [Google Scholar]
- 33.Talini D, Monteverdi A, Benvenuti A, Petrozzino M, Di Pede F, Lemmi M, Carletti A, Macchioni P, Serretti N, Viegi G, et al. Asthma-like symptoms, atopy, and bronchial responsiveness in furniture workers. Occup Environ Med 1998;55:786–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mastrangelo G, Paruzzolo P, Mapp C. Asthma due to isocyanates: a mail survey in a 1% sample of furniture workers in the Veneto region, Italy. Med Lav 1995;86:503–510. [PubMed] [Google Scholar]
- 35.Cullen MR, Redlich CA, Beckett WS, Weltmann B, Sparer J, Jackson G, Ruff T, Rubinstein E, Holden W. Feasibility study of respiratory questionnaire and peak flow recordings in autobody shop workers exposed to isocyanate-containing spray paint: observations and limitations. Occup Med (Lond) 1996;46:197–204. [DOI] [PubMed] [Google Scholar]
- 36.Eifan AO, Derman O, Kanbur N, Sekerel BE, Kutluk T. Occupational asthma in apprentice adolescent car painters. Pediatr Allergy Immunol 2005;16:662–668. [DOI] [PubMed] [Google Scholar]
- 37.Sari-Minodier I, Charpin D, Signouret M, Poyen D, Vervloet D. Prevalence of self-reported respiratory symptoms in workers exposed to isocyanates. J Occup Environ Med 1999;41:582–588. [DOI] [PubMed] [Google Scholar]
- 38.Ucgun I, Ozdemir N, Metintas M, Metintas S, Erginel S, Kolsuz M. Prevalence of occupational asthma among automobile and furniture painters in the center of Eskisehir (Turkey): the effects of atopy and smoking habits on occupational asthma. Allergy 1998;53:1096–1100. [DOI] [PubMed] [Google Scholar]
- 39.Pauluhn J. Acute inhalation toxicity of polymeric diphenyl-methane 4,4′-diisocyanate in rats: time course of changes in bronchoalveolar lavage. Arch Toxicol 2000;74:257–269. [DOI] [PubMed] [Google Scholar]
- 40.Pauluhn J. Pulmonary irritant potency of polyisocyanate aerosols in rats: comparative assessment of irritant threshold concentrations by bronchoalveolar lavage. J Appl Toxicol 2004;24:231–247. [DOI] [PubMed] [Google Scholar]
- 41.Pauluhn J, Eidmann P, Mohr U. Respiratory hypersensitivity in guinea pigs sensitized to 1,6-hexamethylene diisocyanate (HDI): comparison of results obtained with the monomer and homopolymers of HDI. Toxicology 2002;171:147–160. [DOI] [PubMed] [Google Scholar]
- 42.Lee CT, Friedman M, Poovey HG, Ie SR, Rando RJ, Hoyle GW. Pulmonary toxicity of polymeric hexamethylene diisocyanate aerosols in mice. Toxicol Appl Pharmacol 2003;188:154–164. [DOI] [PubMed] [Google Scholar]
- 43.Raulf-Heimsoth M, Baur X. Pathomechanisms and pathophysiology of isocyanate-induced diseases–summary of present knowledge. Am J Ind Med 1998;34:137–143. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein DI, Ott MG, Woolhiser M, Lummus Z, Graham C. Evaluation of antibody binding to diisocyanate protein conjugates in a general population. Ann Allergy Asthma Immunol 2006;97:357–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.