Abstract
Symbiotic nitrogen fixation, the process whereby nitrogen-fixing bacteria enter into associations with plants, provides the major source of nitrogen for the biosphere. Nitrogenase, a bacterial enzyme, catalyzes the reduction of atmospheric dinitrogen to ammonium. In rhizobia-leguminous plant symbioses, the current model of nitrogen transfer from the symbiotic form of the bacteria, called a bacteroid, to the plant is that nitrogenase-generated ammonia diffuses across the bacteroid membrane and is assimilated into amino acids outside of the bacteroid. We purified soybean nodule bacteroids by a procedure that removed contaminating plant proteins and found that alanine was the major nitrogen-containing compound excreted. Bacteroids incubated in the presence of 15N2 excreted alanine highly enriched in 15N. The ammonium in these assays neither accumulated significantly nor was enriched in 15N. The results demonstrate that a transport mechanism rather than diffusion functions at this critical step of nitrogen transfer from the bacteroids to the plant host. Alanine may serve only as a transport species, but this would permit physiological separation of the transport of fixed nitrogen from other nitrogen metabolic functions commonly mediated through glutamate.
In 1956 (1), ammonium was shown to be the product of nitrogenase, the bacterial enzyme that reduces atmospheric dinitrogen. In free-living, nitrogen-fixing microorganisms, the nitrogenase-generated ammonium is assimilated into glutamate through the glutamine synthetase/glutamate synthase pathway. Various transaminases utilize glutamate to generate all of the other amino acids, which are then used to synthesize proteins, nucleic acids and other nitrogen-containing molecules.
In rhizobia-leguminous plant symbioses, the majority of the dinitrogen reduced by the microsymbionts, referred to as bacteroids, is transferred to the plant. Because the rhizobia are physically separated from the plant cytosol by a plant-derived symbiosome membrane, the ammonium must move across the bacteroid and symbiosome membranes. The ammonia diffusion hypothesis has been the prevalent model surmising the initial steps of leguminous nodule nitrogen transfer. In this model, the product of nitrogenase exists in two forms within the cell, ammonium [NH4+] and ammonia [NH3], which are readily interconvertible. The ammonium ion cannot diffuse across membranes, but ammonia is believed to diffuse freely out of the bacteroid. Once outside the bacteroid but within the symbiosome space, the ammonia may either diffuse or be transported across the symbiosome membrane (2) where it is assimilated by the host plant into glutamate by the concerted action of glutamine synthetase and glutamate synthase. Glutamate serves as the central nitrogen metabolite in the plant nodule cells for the synthesis of the other amino acids, nucleic acids, and other nitrogen-containing compounds such as ureides that are the principal nitrogen compounds transported from the nodules to the plant shoots and leaves of N2-fixing soybeans. Consistent with this hypothesis are the observations by several laboratories that the ammonium assimilation enzymes of bacteroids are repressed relative to the levels found in cultured cells (3–7).
In 1985, Kahn et al. (8) proposed a nutrient exchange model in which the nitrogenase-generated ammonium was first assimilated into a carbon skeleton and then carried across the bacteroid membrane. Glutamate and aspartate were suggested as candidates to participate in membrane transversing shuttles such as the malate-aspartate shuttle. Glutamate has been shown to be taken up and metabolized by bacteroids (9–11) but has not been found to be exported. However, alanine and aspartate have been shown to be exported from bacteroids of Rhizobium leguminosarum (12, 13) and Bradyrhizobium japonicum (14), but the amounts measured were not considered sufficient to support the operation of a nutrient exchange system. The ammonia diffusion hypothesis remains as the model for nitrogen movement from bacteroid to plant as there is no evidence to directly support a nitrogen-containing compound other than ammonia for this role (15). The results presented here demonstrate that alanine, not ammonia, serves as the carrier of nitrogenase-generated ammonium out of the bacteroid.
EXPERIMENTAL PROCEDURES
Soybeans (Glycine max L. cv. Williams 82) were inoculated with either B. japonicum strain 110 or 2143 and grown as described (16, 17). Bacteroids were isolated anaerobically under argon either as described by Bergersen and Turner (18) or by the sucrose density gradient procedure of Waters et al. (19). The bacteroids isolated as described by Waters et al. (19) were resuspended in 50 mM Tricine, 5 mM potassium phosphate, 0.5 mM MgCl2, and 0.1 mM EDTA (TMEP), pH 8.0, after washing once in the same buffer. Bacteroids equivalent to ≈8 mg dry weight were added to 14-ml assay vials containing 1 ml of TMEP (pH 8.0) with or without 2 mM d,l-malic acid and shaken at ≈80 rpm at room temperature. The gas phase contained 0.008 atmosphere of O2 in dinitrogen. At timed intervals, the assay vials were opened and the contents poured into microfuge tubes and immediately centrifuged at 11,000 × g for 20 sec. The clear supernatant was removed and frozen at −20°C until analyzed for amino acids and ammonium on Beckman 6300 amino acid analyzers. To determine the 15N contents of the nitrogen-containing components of the assay mixture other than ammonium, samples were prepared for gas chromatography-mass spectrometry (GC-MS) as described by Mawhinney et al. (20). GC-MS was performed by using a Carlo Erba model 4161 gas chromatograph interfaced with a Kratos MS25 double-focusing mass spectrometer (Kratos, Manchester, U.K.). A Kratos Mach 3 data system was used for data acquisition, data reduction, and ion monitoring. 15N-d,l-alanine was derivatized and used as a standard for GC-MS analyses. 15N-alanine, 15N2, and [15NH4]2SO4 were purchased from ISOTEC (Miamisburg, OH). 14N-alanine was purchased from Sigma. 15N-alanine and 14N-alanine were used as standards for GC-MS analyses. Traces of oxygen in 15N2 either purchased or prepared from [15NH4]2SO4 as described by Burris (21) were removed by storage over versine (22). The ammonium was collected from assays by microdiffusion and analyzed for 15N content by R. H. Burris at the University of Wisconsin by using isotope ratio mass spectrometry. Acetylene reduction assays were done as described (16, 17), except that ≈8 mg dry weight of bacteroids were incubated under 0.06 atmosphere of O2. Oxamate, carbonyl cyanide m-chlorophenylhydrazone (CCCP), reagents, buffer, and salts were purchased from Sigma.
RESULTS
The results reported by Bergersen and Turner (18) with a continuously monitored flow-through system were confirmed by using fixed time assays up to 80 min with washed suspensions of B. japonicum bacteroids isolated as described by these authors. Briefly, ammonium accumulation was observed in the assay medium in which the bacteroids were incubated in the absence of malate or succinate. In the presence of malate or succinate, ammonia release was reduced. Bacteroids prepared by this method had been shown earlier to be contaminated with enzymes from the plant nodule cytosol and also from ruptured mitochondria (19) that could be removed by a sucrose density gradient procedure (16, 17, 19). Ammonium accumulation in N2 reduction assays of sucrose density gradient-purified bacteroids was generally <0.1 ηmol/min × mg dry weight of bacteroids, compared with ≈0.5 ηmol/min × mg dry weight of bacteroids prepared without the purification procedure. Although most assays of sucrose density gradient-purified bacteroids showed markedly lower rates of ammonium accumulation, the remaining assays showed a decrease in assay ammonium content with time. More accurate estimates of ammonium release or uptake were difficult because the endogenous ammonium in assay solutions including bacteroids was typically of the order of 50 ηmol/ml.
The only compound that increased significantly and linearly with time in N2 fixation assays with sucrose density gradient-purified bacteroids was alanine (Fig. 1). Alanine excretion was linear for up to 80 min in the presence of malate. In the absence of malate, alanine was again the only compound that increased significantly with time, but the amounts were considerably diminished. All of the amino acids combined, except alanine, did not increase above background levels until 60 min.
Figure 1.
Excretion of alanine and other amino acids from sucrose density gradient-purified B. japonicum bacteroids. Assays were performed at 0.008 atmosphere of oxygen as described in Experimental Procedures, except that at 40 min, as indicated by the arrow, oxygen was increased to 0.1 atmosphere to the indicated assays. •, Alanine excretion in the presence of 2 mM d,l-malate under 0.008 atmosphere of oxygen; ○, alanine excretion when the oxygen partial pressure was increased to 0.1 atmosphere at 40 min; ▵, alanine excretion in the absence of 2 mM d,l-malate under 0.008 atmosphere of oxygen; and ▴, all other amino acids except alanine in the presence of 2 mM d,l-malate.
When the host cell cytosol was added back to the sucrose density gradient-purified bacteroids, alanine production was reduced and ammonium production increased. Addition of alanine, glutamate, or aspartate to the nodule cytosol fraction in the absence of bacteroids resulted in the generation of ammonium. The amount of ammonium produced from different preparations of nodule cytosol was quite variable. The mechanism of the ammonium formation was not determined. The nodule cytosol fraction from the sucrose density gradients also was assayed for the generation of alanine and other amino acids but none were found.
The mitochondrial fraction was collected from the sucrose density gradients and assayed similarly to the bacteroids with malate under 0.008 atmosphere of O2, but in the presence of added ammonium. The rationale for adding ammonium was to determine whether mitochondria were capable of the synthesis of alanine from nitrogenase-generated ammonium released from the bacteroids. Proline was the only amino acid released from the mitochondria in significant levels. Mitochondria incubated under 0.008 atmosphere O2 in the presence of ammonium and malate released proline at rates of up to 0.26 ηmol/min/mg dry weight.
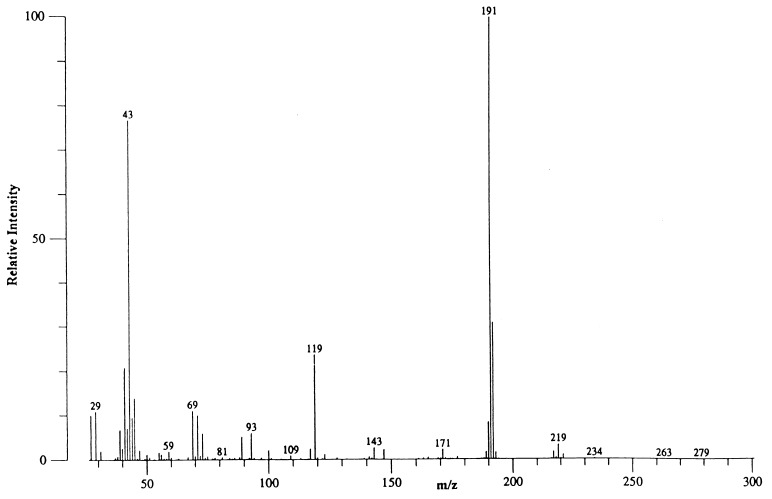
To determine whether the alanine excreted from bacteroids carried the ammonium generated via nitrogenase, sucrose density gradient-purified bacteroids were incubated under 15N2 with 0.008 atmosphere O2 and the compounds found in the assay medium were analyzed by GC-MS (Fig. 2). The assay solutions were collected at timed intervals from 10 to 40 min. Alanine was the only compound found that contained significant amounts of 15N. The N2 supplied to the bacteroids was 99.4 atom% enriched in 15N and the majority of alanine contained the isotopic label as can be seen by the signal at m/z 191 (base peak) that corresponded to cleavage of the 15N-alanine-pentafluoropropionylisopropyl (PF-IP) ester and elimination of the ester moiety (87 atomic mass units). A base peak ion at m/z 190 would result from the cleavage of the 14N-alanine–PF-IP ester. The 15N content of the ammonium obtained from the assay solutions typically contained <0.06 atom% excess.
Figure 2.
Mass spectral analysis of the alanine fraction of the sucrose density gradient-purified B. japonicum exudate after separation by gas chromatography. Bacteroids were incubated under 0.008 atmosphere of O2 and 0.5 atmosphere of 15N2 in argon for 12 min. The x-axis is mass/charge ratio of the measured ions and the y-axis is intensity relative to the largest signal (base peak) which was m/z 191. The molecular ion was not discernible (molecular weight 278 Da). The ion at m/z 219 resulted from cleavage of m-59 (isopropoxy moiety).
Oxamate, an inhibitor of B. japonicum bacteroid alanine dehydrogenase (23), reduced alanine excretion, and caused an increase in ammonium release (Table 1). The incorporation of 15N from 15N2 into ammonium by bacteroids in the presence of oxamate incubated under 0.008 atmosphere of O2 was >10 atom% excess. When the oxamate experiment was repeated in the presence of 0.6 atmosphere of O2, the 15N in ammonium was ≈0.9 atom% excess. The energy uncoupler, CCCP, which inhibits nitrogenase activity because of its effect on the energy status of the cell dramatically inhibited alanine excretion (Table 1). Addition of ammonium at levels of 100 μM to the incubation medium increased the level of alanine excreted by ≈30% (Table 1).
Table 1.
Effect of several compounds on alanine and ammonium release from B. japonicum bacteroids
| Addition | Alanine excreted* | Ammonium excreted* |
|---|---|---|
| None | 0.42 | 0.03 |
| Oxamate 10 mM | <0.01 | 0.28 |
| CCCP 100 μM | <0.01 | 0.07 |
| Ammonium 100 μM | 0.55 | ND† |
Data given as ηmol/min/mg dry weight; values are average of three replicates
Not determined because of the presence of exogenous ammonium.
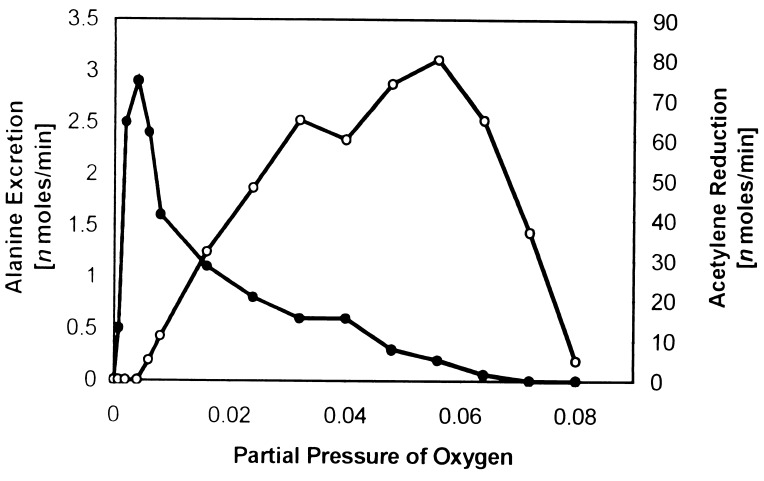
The optimal oxygen partial pressure for alanine excretion was markedly different than that of acetylene reduction (Fig. 3). Little acetylene reduction activity was found at the lower partial pressures of oxygen where alanine excretion was optimal, and likewise little alanine excretion was found at the higher partial pressures of oxygen where acetylene reduction was optimal. Assays containing both C2H2 (0.2 atmosphere) and N2 (0.8 atmosphere) and 0.008 atmosphere of O2 produced primarily alanine and low levels of ethylene, whereas those containing both substrates, but with a 0.06 atmosphere of O2 produced primarily ethylene and small amounts of alanine. If acetylene was added 20 or 40 min after the assays were begun in the presence of N2 and 0.008 atmosphere of O2, there was little, if any, inhibition of alanine excretion nor was significant ethylene produced. Conversely, if N2 was added 20 or 40 min after the assay were begun in the presence of C2H2 and 0.06 atmosphere of O2, ethylene production was not noticeably inhibited nor was significant alanine excreted. When oxygen at ≥0.1 atmospheres was added during assays of alanine excretion initially containing 0.008 atmosphere O2, for the purpose of irreversibly inactivating nitrogenase, alanine excretion ceased (Fig. 1). Likewise, when oxygen at ≥0.1 atmosphere was added during assays of acetylene reduction initially containing 0.06 atmosphere O2, again for the purpose of irreversibly inactivating nitrogenase, acetylene reduction activity ceased. Ammonium release was <0.1 ηmol/min × mg dry weight of bacteroids at all partial pressures of O2.
Figure 3.
Effect of oxygen on alanine excretion and acetylene reduction by sucrose density gradient-purified B. japonicum bacteroids. Assay conditions were as described in Experimental Procedures. Filled symbols, alanine excretion; open symbols, acetylene reduction.
The results reported above were the same with either of two different strains of B. japonicum, 110 and 2143, inoculated onto plants grown in growth chambers, greenhouses, and the field. The results shown here were done in the absence of leghemoglobin, but when it was added, identical results were obtained. Leghemoglobin was not used routinely as purified preparations contained small peptides, amino acids, and ammonium. To avoid the nuisance of these contaminants, leghemoglobin was not normally included in the assays. The assay pH for the experiments reported here were done at pH 8 because initially this appeared to be the optimal pH. As the assay conditions were optimized, equivalent rates of alanine excretion were found between pH 7 and 8 with activity decreasing at lower and high pH values.
DISCUSSION
The ammonia diffusion hypothesis is the current model of nitrogen transfer between the bacteroid and the plant host. However, the results presented here clearly demonstrate that alanine rather than ammonia is the principal nitrogen-containing compound released from N2-fixing B. japonicum bacteroids. Alanine was the only nitrogen-containing compound excreted from sucrose density gradient-purified bacteroids in significant quantities and it accumulated linearly with time. Alanine excretion was dependent on nitrogenase activity as bacteroids exposed to levels of oxygen to inactivate nitrogenase, or to CCCP to inhibit energy generation for N2 fixation, did not excrete alanine. Oxamate, an inhibitor of alanine dehydrogenase, reduced alanine excretion but enhanced ammonium release by N2-fixing bacteroids. The release of ammonium from sucrose density gradient-purified bacteroids in the presence of oxamate, but not in its absence, demonstrated that alanine was the preferred excretion product. Furthermore, exogenous ammonium increased alanine excretion. If bacteroids do not possess an ammonium uptake system (15), then exogenous ammonia can only enter by diffusion. Because exogenous ammonia can diffuse into bacteroids to be assimilated into alanine, intracellular ammonia should be able to diffuse out of the cell. However, ammonia was not released from sucrose density gradient-purified bacteroids, indicating that the intracellular, nitrogenase-generated ammonium was efficiently incorporated into alanine. The 15N2 labeling experiments demonstrated that the recently formed ammonium from nitrogenase was incorporated into alanine that was then transported from the bacteroid. Individual samples for GC-MS analysis were collected over intervals from 10 to 40 min, and the alanine from each was highly labeled. Very little 15N was found in the ammonium released from the bacteroids, except when oxamate was present. The atom% excess found in the ammonium released by bacteroids in the presence of oxamate was lower than that found in alanine, but this was due largely to the dilution of labeled ammonium by endogenous ammonium. Alanine excretion was markedly stimulated by the presence of malate, which is consistent with a large number of reports demonstrating the significance of dicarboxylic acids to symbiotic nitrogen fixation (24). Malate has been shown to be one of the more abundant organic acids within soybean nodules (25).
The rate of alanine released from the bacteroids as shown in Fig. 1 was ≈0.35 ηmol/min × mg dry weight of bacteroids in the presence of malate. Bergersen and Turner (18) reported rates of ammonium accumulation near zero if malate was present at the start of the assay and 0.55 ηmol/min × mg dry weight of bacteroids if malate was added up to 100 min after the start of the assay. The rates of malate-dependent alanine production presented here and malate-dependent ammonium production reported by Bergersen and Turner (18) after extended assay intervals are similar. The difference in the identity of the nitrogen product from the bacteroids was due to the method of bacteroid isolation as (i) there was no significant ammonia from sucrose density gradient-purified bacteroids, but there was from nonpurified bacteroids, and (ii) addition of plant nodule cytosol to sucrose density purified bacteroids resulted in the reduction of alanine and the increase of ammonium. The sucrose density purification procedure has been shown not to affect the metabolic activities, including nitrogen fixation, of the bacteroids (16, 17). Furthermore, if the sucrose density gradient purification procedure disrupted the bacteroid membrane, it should have increased rather than decreased ammonium/ammonia transfer across the membrane.
Alanine was formed via alanine dehydrogenase, which is a member of a class of enzymes usually considered as having catabolic functions; that is, they release ammonium from amino acids during periods of nutrient deficiency. However, we previously purified and characterized the alanine dehydrogenase from B. japonicum bacteroids and found that the kinetic mechanism overwhelmingly favored alanine formation (23, 26). B. japonicum bacteroid alanine dehydrogenase possessed a Theorell–Chance mechanism in the direction of alanine formation with a rapid-equilibrium addition of ammonium. The in vivo catalytic activity of alanine dehydrogenase was apparently greater than that of nitrogenase as exogenous ammonium increased alanine excretion (Table 1). This finding demonstrated that alanine dehydrogenase efficiently assimilated nitrogenase-generated ammonium into alanine. Furthermore, Werner et al. (27) reported soybean bacteroid alanine dehydrogenase had a developmental profile that coincided with the development of nitrogenase activity. This result is in contrast to the other ammonium assimilatory enzymes that are repressed during symbiotic development (3–7). Thus, both the kinetic mechanism and the developmental profile of B. japonicum alanine dehydrogenase are consistent with a role for this enzyme in alanine formation for export from bacteroids.
In 1980, Hageman and Burris (28), using purified nitrogenase components, demonstrated that the electron flux determined the substrate reduction preference of the enzyme complex. At high electron flux, dinitrogen was the preferred substrate, whereas at low electron flux, acetylene was the preferred substrate (28). The preferred excretion of alanine by bacteroids at low partial pressures of oxygen (Fig. 3) indicated a high in vivo electron flux to nitrogenase, whereas the preferred reduction of acetylene at greater partial pressures of oxygen indicated a lower in vivo electron flux. In addition, Hageman and Burris (28) found that the Km for acetylene declines as the electron flux declines (28). Thus, the differences in the electron flux for optimal reduction of each substrate plus the variability of the Km values for acetylene as a function of electron flux mean that numerical comparisons of alanine production and acetylene reduction have no relevance. Furthermore, the use of conditions optimal for acetylene reduction rather than for a nitrogen-carrying compound is the primary reason why alanine excretion was not observed previously.
In summary, our results clearly demonstrate that alanine was the primary compound exported from bacteroids carrying nitrogenase-generated ammonium, and that a transport mechanism rather than diffusion functions at this critical step of nitrogen transfer from the bacteroid to the plant host. Our results are consistent with the role of malate in bacteroid metabolism, and they also provide additional reservations about the use of the acetylene reduction technique for measuring nitrogen fixation activity of nodulated leguminous plants, especially ex planta bacteroids. These results have salient implications regarding nodule nitrogen metabolism and whole nodule physiology. The most obvious question is the metabolic fate of alanine after it leaves the bacteroid. Alanine is most likely only a transport species as glutamate is the central compound of nitrogen metabolism in most organisms. In 1954, Aprison et al. (29) reported that glutamate was the predominately labeled compound in soybean nodules after a 2-h exposure to 15N2, an observation subsequently confirmed by many other researchers. The use of alanine as a transport species would functionally and spatially separate and distinguish bacteroid nitrogen transport from bacteroid, symbiosome, and plant host cellular nitrogen metabolism, thereby facilitating the rapid movement of fixed nitrogen from the bacteroid to the plant.
Acknowledgments
We acknowledge the following contributions: R. H. Burris for 15N analysis of ammonium samples; M. T. Smith, D. G. Blevins, and D. D. Randall for many helpful discussions, H. J. Evans, B. A. McClure, and S. L. Albrecht for review of manuscript drafts; and N. J. Emerich for technical assistance. This work was supported by the University of Missouri Research Board.
ABBREVIATIONS
- GC-MS
gas chromatography-mass spectrometry
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
References
- 1. Burris R H. In: Inorganic Nitrogen Metabolism. McElroy W D, Glass B B, editors. Baltimore: John Hopkins Univ. Press; 1956. pp. 316–343. [Google Scholar]
- 2.Tyerman S D, Whitehead L F, Day D A. Nature (London) 1995;378:629–632. [Google Scholar]
- 3.Bishop P E, Guevara J G, Engelke J A, Evans H J. Plant Physiol. 1976;57:542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown C M, Dilworth M J. J Gen Microbiol. 1975;86:39–48. doi: 10.1099/00221287-86-1-39. [DOI] [PubMed] [Google Scholar]
- 5.Kurz W G W, Rokosh D A, LaRue T A. Can J Microbiol. 1975;21:1009–1012. doi: 10.1139/m75-149. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig R A, Signer E R. Nature (London) 1977;267:245–248. doi: 10.1038/267245a0. [DOI] [PubMed] [Google Scholar]
- 7.Tate R, Riccio A, Merrick M, Patriarca E J. Mol Plant-Microbe Interact. 1998;11:188–198. doi: 10.1094/MPMI.1998.11.3.188. [DOI] [PubMed] [Google Scholar]
- 8.Kahn M L, Kraus J, Somerville J E. In: Nitrogen Fixation Research Progress. Evans H J, Bottomley P J, Newton W E, editors. Dordrecht, The Netherlands: Nijhoff; 1985. pp. 193–199. [Google Scholar]
- 9.Bergersen F J, Turner G L. J Gen Microbiol. 1988;134:2441–2448. [Google Scholar]
- 10.Salminen S O, Streeter J G. J Gen Microbiol. 1990;136:2119–2126. [Google Scholar]
- 11.Udvardi M K, Salom C L, Day D A. Mol Plant-Microbe Interact. 1988;1:250–254. [Google Scholar]
- 12.Appels M A, Haaker H. Plant Physiol. 1991;95:740–747. doi: 10.1104/pp.95.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosendahl L, Dilworth M J, Glenn A R. J Plant Physiol. 1992;139:635–638. [Google Scholar]
- 14.Kouchi H, Fukai K, Katagari K, Minamisawa H, Tajima S. Physiol Plant. 1988;73:327–334. [Google Scholar]
- 15.Udvardi M K, Day D A. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:493–523. doi: 10.1146/annurev.arplant.48.1.493. [DOI] [PubMed] [Google Scholar]
- 16.Karr D B, Emerich D W. Plant Physiol. 1988;86:693–699. doi: 10.1104/pp.86.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karr D B, Suzuki F, Waters J K, Emerich D W. J Plant Physiol. 1990;136:659–663. [Google Scholar]
- 18.Bergersen F J, Turner G L. Proc R Soc London B. 1990;238:295–320. [Google Scholar]
- 19.Waters J K, Karr D B, Emerich D W. Biochemistry. 1985;24:6479–6486. [Google Scholar]
- 20.Mawhinney T P, Robinett R S R, Atalay A, Madson M A. J Chromatogr. 1986;358:231–242. doi: 10.1016/s0021-9673(01)90333-4. [DOI] [PubMed] [Google Scholar]
- 21.Burris R H. Aust J Plant Physiol. 1976;3:41–51. [Google Scholar]
- 22.Pugh L H, Umbreit W W. Arch Biochem Biophys. 1966;115:122–128. doi: 10.1016/s0003-9861(66)81047-0. [DOI] [PubMed] [Google Scholar]
- 23.Smith M T, Emerich D W. J Biol Chem. 1993;268:10746–10753. [PubMed] [Google Scholar]
- 24.Vance C P, Heichel G H. Annu Rev Plant Physiol Mol Biol. 1991;42:373–392. [Google Scholar]
- 25.Stumpf D K, Burris R H. Anal Biochem. 1979;95:311–315. doi: 10.1016/0003-2697(79)90221-5. [DOI] [PubMed] [Google Scholar]
- 26.Smith M T, Emerich D W. Arch Biochem Biophys. 1993;304:379–385. doi: 10.1006/abbi.1993.1365. [DOI] [PubMed] [Google Scholar]
- 27.Werner D, Moerschel E, Stripf R, Winchenbach B. Planta. 1980;147:320–329. doi: 10.1007/BF00379840. [DOI] [PubMed] [Google Scholar]
- 28.Hageman R V, Burris R H. Biochim Biophys Acta. 1980;591:63–75. doi: 10.1016/0005-2728(80)90220-0. [DOI] [PubMed] [Google Scholar]
- 29.Aprison M H, McGee W E, Burris R H. J Biol Chem. 1954;208:29–39. [PubMed] [Google Scholar]