Abstract
Rationale: Basic science research suggests a causal role for endothelial dysfunction in chronic obstructive pulmonary disease (COPD). Clinical studies examining endothelial function are lacking, particularly early in the disease. Flow-mediated dilation (FMD) is a physiologic measure of endothelial reactivity to endogenous nitric oxide.
Objectives: We hypothesized that lower FMD among former smokers would be associated with lower post-bronchodilator FEV1, higher percentage of emphysema using computed tomography (CT) and lower diffusing capacity.
Methods: We measured FMD, pulmonary function, and CT percentage of emphysema in a random sample of 107 cotinine-confirmed former smokers in the ongoing EMCAP study. FMD was defined as percentage change in the brachial artery diameter with reactive hyperemia. Generalized additive models were used to adjust for potential confounders and assess linearity.
Measurements and Main Results: Mean age of participants was 71 ± 5 years, 46% were female, and pack-years averaged 48 ± 26. Mean FMD was 3.8 ± 3.1%; mean post-bronchodilator FEV1, 2.3 ± 0.8 L; and mean CT percentage of emphysema, 26 ± 10%. A 1 SD decrease in FMD was associated with a 132-ml (95% confidence interval, 16–248 ml; P = 0.03) decrement in post-bronchodilator FEV1 and a 2.6% (95% confidence interval, 0.5–4.7%; P = 0.02) increase in CT percentage of emphysema in fully adjusted models. These associations were linear across the spectrum from normality to disease, independent of smoking history, and also significant among participants without COPD. Associations with diffusing capacity were consistent but nonsignificant (P = 0.09). The FMD–FEV1 association was entirely attributable to percentage of emphysema.
Conclusions: Impaired endothelial function, as measured by FMD, was associated with lower FEV1 and higher CT percentage of emphysema in former smokers early in COPD.
Keywords: pulmonary disease, chronic obstructive; bronchitis, chronic; pulmonary emphysema; endothelial dysfunction
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Basic science research suggests a potentially causal role for endothelial dysfunction in chronic obstructive pulmonary disease (COPD). Clinical studies examining endothelial function are lacking, particularly early in the disease.
What This Study Adds to the Field
Endothelial dysfunction, measured by flow-mediated dilation, was associated with lower post-bronchodilator FEV1 and higher computed tomography percentage of emphysema in former smokers across a spectrum from normal lung function and anatomy to moderate to severe COPD and emphysema.
Recent research on the pathogenesis of chronic obstructive pulmonary disease (COPD) suggests that perturbations in the vasculature and, specifically, endothelial health may occur early in COPD (1). Decreased expression of vascular endothelial growth factor (VEGF) causes endothelial apoptosis, epithelial apoptosis, and emphysema (2–4). The second messenger lipid ceramide mediates VEGF blockade–induced apoptosis in COPD and ceramide instillation acutely triggers apoptosis in pulmonary endothelial cells, causing emphysema in mice (5).
Apoptotic endothelial cells are present in the lungs of smokers with COPD (6, 7), and there are significant morphologic differences in the endothelium of smokers with mild, moderate, and severe COPD compared with smokers without COPD (8). Furthermore, nitric oxide (NO)-mediated, endothelium-dependent relaxation provoked by adenosine diphosphate and acetylcholine is attenuated in the excised pulmonary arteries of patients with COPD compared with those of smoking and nonsmoking control subjects (9, 10). Published studies assessing in vivo endothelial function in COPD are, however, lacking.
Measurement of endothelial function in the pulmonary vascular bed in vivo requires invasive catheterization and is not feasible in large numbers of relatively healthy patients. A noninvasive measure of endothelial function is flow-mediated dilation (FMD) of the brachial artery, which measures endothelium-dependent, NO-mediated vasodilation (11, 12).
We tested the hypothesis that endothelial dysfunction, as assessed by brachial artery FMD, would be associated with lower post-bronchodilator FEV1, higher computed tomography (CT) percentage of emphysema, and lower diffusing capacity for carbon monoxide (DlCO) among an unselected sample of former smokers in an ongoing cohort study, the Emphysema and Cancer Action Project (EMCAP) study. We chose this design over a case-control study because we were interested in examining the continuum of changes in FMD in early and preclinical COPD, rather than those that may occur in late, severe COPD. We restricted the sample to former smokers because current cigarette smoking causes acute reductions in FMD (13, 14).
Preliminary results from this study were previously presented in abstract form (15).
METHODS
Study Sample
The EMCAP study recruited 557 participants at one site of a lung cancer CT screening research program (16) in 2001–2002. Inclusion criteria were current and former smoking with 10 or more pack-years, age 60 years and older, willingness to undergo CT screening for lung cancer, and no cancer history other than nonmelanoma skin cancer. Of 557 EMCAP participants, 313 (56%) denied current smoking at baseline and follow-up and were hence eligible for the FMD study.
We invited a random sample of 196 of these former smokers to participate in the FMD study, of whom 71 declined the FMD measurement, 6 met FMD exclusion criteria of systolic blood pressure greater than 180 mm Hg, Raynaud's phenomenon, or prior total mastectomy, and 119 completed the FMD measurement (61% of those invited). The Columbia University Institutional Review Board approved all study activities.
Measures
Participants underwent the following measures, each of which was performed at approximately the same time in the morning and in the following order.
FMD of the Brachial Artery
FMD was measured in 2005–2006 using a standardized protocol adapted from a large multicenter study (17), consistent with published guidelines (18). Subjects fasted for 12 hours, avoided exercise for 6 hours, and rested in the supine position for 15 minutes (18). Regular medications were not withheld. Brachial artery diameter was measured 6 cm above the antecubital crease of the right arm using B-mode ultrasound with a 15-MHz linear array transducer (SONOS 5500; Philips, Andover, MA). A blood pressure cuff was inflated to at least 50 mm Hg above systolic blood pressure to occlude arterial flow to the forearm for 5 minutes (18), then brachial artery diameter was remeasured during reactive hyperemia 1 minute after cuff deflation (19, 20). End-diastolic images were digitized by a frame grabber (model LG3; Scion Corporation, Frederick, MD).
A single reader blinded to clinical status measured all brachial artery diameters off-line using analysis software for three consecutive cardiac cycles at rest and 1 minute postdeflation for each participant. FMD was expressed as percentage change from rest (100 × [brachial artery diameter during reactive hyperemia – diameter at rest]/diameter at rest). Absolute intraobserver variability for FMD measurements was 1.3%.
Spirometry
Pre- and post-bronchodilator spirometry was performed according to American Thoracic Society (ATS) guidelines (21) using the EasyOne portable spirometer (ndd Medical Technologies, Chelmsford, MA), which we previously validated against a dry seal, rolling-barrel spirometer (22). Assessment of spirometry quality followed ATS/European Respiratory Society (ERS) recommendations (23).
COPD and COPD severity were defined according to ATS/ERS COPD criteria using post-bronchodilator measures (24).
CT Emphysema Index
All participants underwent low-dose, noncontrast, full-lung CT scanning on a Siemens 16 multidetector scanner (120 kVp, 169 mAs, 6:1 pitch, 5-mm thickness, single breath-hold, contiguous slices from the thoracic inlet to the adrenal glands). The CT percentage of emphysema in the lungs was assessed as the proportion of lung volume below a threshold attenuation of −910 Hounsfield units (HU) compared with the total volume of the lung. This measure is also commonly referred to as the emphysema index or density mask (25). We modified the base threshold to correct for interscan variations using the attenuation of tracheal air. We first determined the average attenuation of a cylindrical sample of air in the trachea taken above the bronchial branch point. The attenuation of the sampled air was then compared with an average attenuation of −970 HU, with the difference used to correct the baseline index attenuation of −910 HU.
DlCO
Single-breath DlCO was measured with a Sensormedics Autobox 220 Series instrument (Viasys Healthcare, Yorba Linda, CA) following ATS/ERS guidelines (26). The participant exhaled to residual volume, then was switched in to a gas mixture of 0.3% CO, 10% helium, 21% oxygen, and balance nitrogen. The participant then inhaled rapidly to total lung capacity, held his/her breath for 10 seconds, then exhaled to residual volume. The average of all acceptable tests (minimum of two tests) was reported. Breath-hold time was assessed by the method of Jones and Meade (27).
Covariate Data
Information on age, sex, race/ethnicity, educational attainment, and physician diagnoses of cardiovascular diseases was recorded by interviewer-administered questionnaire. Use of medications and supplements was assessed by validated medication inventory (28). Blood pressure was measured with mercury sphygmomanometers and cuffs of appropriate size. Anthropometric measurements of height and weight were determined by the use of calibrated scales. Body mass index was calculated as weight (kg) divided by height squared (m2).
Current smoking status was verified by urinary cotinine levels, which were ascertained using enzyme-linked immunosorbent assay (Orasure Technologies, Inc., Bethlehem, PA), according to the manufacturer's instructions. Current smoking was defined as urinary cotinine levels above 500 ng/ml.
Statistical Analysis
Means and SDs were calculated. Age- and sex-adjusted associations of FMD and potential confounders, such as statin use, were estimated in linear regression models. The multivariate association of lung measures and FMD was assessed in linear regression models that included age, sex, race/ethnicity, height, pack-years of smoking, educational attainment, asthma, body mass index, diabetes, hypertension, hypercholesterolemia, and use of statins, aspirin, β-blockers, omega-3 supplements, and postmenopausal hormones (see Table 3). The statistical significance of departures for linearity was tested in nested generalized additive models with and without lowess (locally-weighted polynomial regression) smoother functions. We decided a priori to adjust lung measures for age, sex, race/ethnicity, and height rather than use percent-predicted FEV1 and FVC due to the sizable proportion of participants in this cohort for whom accurate prediction equations are currently unavailable (U.S. Hispanics of non-Mexican origin and Asian/Pacific Islanders). All P values were two-tailed with P < 0.05 considered statistically significant. Analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC) and S-plus 6.2 (Insightful Corp., Seattle, WA).
TABLE 3.
MULTIVARIATE MEAN DIFFERENCES IN FEV1, PERCENTAGE OF EMPHYSEMA, AND DIFFUSING CAPACITY PER 1 SD CHANGE IN FLOW-MEDIATED DILATION OF THE BRACHIAL ARTERY IN FORMER SMOKERS
| Former Smokers (n = 107)
|
Former Smokers without COPD (n = 63)
|
|||
|---|---|---|---|---|
| Difference in Lung Measure per 1 SD Change in FMD (95% CI) | P Value | Difference in Lung Measure per 1 SD Change in FMD (95% CI) | P Value | |
| Post-bronchodilator FEV1, ml | ||||
| Model 1* | 147 (31, 264) | 0.015 | 148 (55, 241) | 0.003 |
| Model 2† | 148 (34, 263) | 0.013 | 151 (57, 245) | 0.003 |
| Model 3‡ | 132 (16, 248) | 0.029 | 107 (7, 207) | 0.042 |
| CT percentage of emphysema | ||||
| Model 1* | −2.5 (−4.8, −0.1) | 0.041 | −2.3 (−5.3, 0.7) | 0.144 |
| Model 2† | −2.5 (−4.9, −0.1) | 0.046 | −2.4 (−5.3, 0.6) | 0.128 |
| Model 3‡ | −2.6 (−4.7, −0.5) | 0.018 | −3.5 (−6.1, −0.8) | 0.017 |
| DlCO, ml/min/mm Hg | ||||
| Model 1* | 0.708 (−0.088, 1.505) | 0.085 | 0.399 (−0.304, 1.103) | 0.271 |
| Model 2† | 0.712 (−0.087, 1.511) | 0.084 | 0.387 (−0.330, 1.104) | 0.295 |
| Model 3‡ | 0.708 (−0.088, 1.504) | 0.088 | 0.461 (−0.319, 1.240) | 0.254 |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; CT = computed tomography; FMD = flow-mediated dilation.
Model 1 adjusted for age, sex, race/ethnicity, and height.
Model 2 adjusted for age, sex, race/ethnicity, height, and pack-years.
Model 3 adjusted for age, sex, race/ethnicity, height, pack-years, educational attainment, asthma, body mass index, diabetes, hypertension, hypercholesterolemia, and use of statins, aspirin, β-blockers, omega-3 supplements, and postmenopausal hormones.
RESULTS
Participant Characteristics
FMD was measured in a random sample of 119 participants in the EMCAP study who reported no current smoking. Of these, eight with cotinine levels consistent with active smoking and four with brachial artery images of insufficient quality for analysis were excluded. The remaining 107 participants included in analyses were similar to former smokers in the overall cohort with respect to age, sex, race/ethnicity, smoking history, and prevalences of diagnoses of hypertension, hypercholesterolemia, and COPD, but had a lower prevalence of diagnosed diabetes (13 vs. 17%, P = 0.01).
Participants in the study sample were elderly, were evenly divided between men and women, were 17% nonwhite, and had moderately heavy smoking histories (Table 1). A majority had hypercholesterolemia and 42% took a statin. Hypertension was reported commonly, but mean blood pressures were within the normal range and relatively few participants took a β-blocker or angiotensin converting enzyme (ACE) inhibitor. A history of cardiovascular disease was uncommon. Lung function in this sample of former smokers ranged from completely normal to severely impaired. Of the total sample of 107 participants, a majority did not have COPD (defined as post-bronchodilator FEV1/FVC ratio < 0.70), 12 (11%) had mild COPD, 21 (20%) had moderate COPD, and 9 (9%) had severe or very severe COPD.
TABLE 1.
CHARACTERISTICS OF COTININE-CONFIRMED FORMER SMOKERS WITH VALID FLOW-MEDIATED DILATION MEASURES IN THE EMCAP STUDY AND THE SUBSET WHO DID NOT HAVE CHRONIC OBSTRUCTIVE PULMONARY DISEASE
| Former Smokers (n = 107) | Former Smokers without COPD* (n = 63) | |
|---|---|---|
| Age (mean ± SD), yr | 71 ± 5 | 70 ± 5 |
| Female, % | 46 | 46 |
| Ethnicity, % | ||
| White | 83 | 79 |
| African American | 6 | 6 |
| Hispanic | 8 | 10 |
| Asian/Pacific Islander | 3 | 5 |
| Education, % | ||
| Graduate degree | 28 | 24 |
| Four-year college | 32 | 41 |
| High school/some college | 35 | 32 |
| No high school degree | 6 | 3 |
| Cotinine level (mean ± SD), ng/ml | 14 ± 39 | 16 ± 49 |
| Pack-years, mean ± SD | 48 ± 26 | 44 ± 24 |
| Height (mean ± SD), cm | 168 ± 9 | 167 ± 9 |
| BMI (mean ± SD), kg/m2 | 28 ± 4 | 28 ± 4 |
| Physician diagnosis of the following, % | ||
| Hypercholesterolemia | 67 | 67 |
| Hypertension | 46 | 51 |
| Diabetes | 12 | 16 |
| Coronary heart disease | 3 | 3 |
| Cerebrovascular disease | 4 | 3 |
| Peripheral vascular disease | 6 | 3 |
| Congestive heart failure | 2 | 3 |
| Sleep apnea | 6 | 10 |
| Cardiovascular medications, % | ||
| Aspirin | 63 | 63 |
| HMG-CoA reductase inhibitor (statin) | 42 | 41 |
| β-blocker | 12 | 14 |
| ACE inhibitor | 12 | 16 |
| Omega-3 supplement | 35 | 33 |
| Postmenopausal hormones† | 22 | 16 |
| Blood pressure (mean ± SD), mm Hg | ||
| Systolic | 128 ± 15 | 128 ± 16 |
| Diastolic | 73 ± 9 | 73 ± 9 |
| Physician-diagnosed asthma before age 45 yr, % | 9 | 8 |
| MRC-defined chronic bronchitis, % | 19 | 16 |
| COPD medications, % | ||
| Short-acting β-agonists | 13 | 5 |
| Long-acting β-agonists | 14 | 6 |
| Short-acting anticholinergic | 6 | 2 |
| Long-acting anticholinergic | 7 | 0 |
| Theophylline | 0 | 0 |
| Inhaled corticosteroids | 8 | 5 |
| Systemic corticosteroids | 1 | 0 |
| Prebronchodilator FVC (mean ± SD), L | 3.17 ± 0.91 | 3.16 ± 0.77 |
| Prebronchodilator FEV1 (mean ± SD), L | 2.20 ± 0.71 | 2.38 ± 0.62 |
| Prebronchodilator FEV1/FVC ratio, mean ± SD | 0.69 ± 0.11 | 0.72 ± 0.08 |
| Post-bronchodilator FVC (mean ± SD), L | 3.25 ± 0.95 | 3.27 ± 0.77 |
| Post-bronchodilator FEV1 (mean ± SD), L | 2.31 ± 0.76 | 2.52 ± 0.59 |
| Post-bronchodilator FEV1/FVC ratio, mean ± SD | 0.71 ± 0.11 | 0.77 ± 0.04 |
| Bronchodilator response (mean ± SD), % | 7.6 ± 33 | 10.9 ± 31 |
| COPD,* % | ||
| None | 60 | 100 |
| Mild | 11 | |
| Moderate | 20 | |
| Severe or very severe | 9 | |
| DlCO (mean ± SD), ml/min/mm Hg | 17.8 ± 5.1 | 18.4 ± 4.1 |
| DlCO/Va, mean ± SD | 3.9 ± 0.7 | 4.0 ± 0.6 |
| CT emphysema index (mean ± SD), % | 26.1 ± 10.5 | 24.0 ± 8.6 |
Definition of abbreviations: ACE = angiotensin converting enzyme; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CT = computed tomography; DlCO = carbon monoxide diffusing capacity; EMCAP = Emphysema and Cancer Action Project; HMG-CoA = 3-hydroxy-3-methyl-glutaryl-coenzyme A; MRC = Medical Research Council; Va = alveolar volume.
Defined as post-bronchodilator FEV1/FVC ratio < 0.70.
Among women only.
Table 1 also shows the characteristics of the former smokers in the sample who did not have COPD. There were no significant differences between the overall sample and those without COPD, other than for pulmonary function and emphysema measures.
FMD
The mean diameter of the brachial artery before cuff inflation in the whole study sample was 3.8 ± 0.7 mm. The mean increase in brachial artery diameter during reactive hyperemia after cuff deflation was 0.2 ± 0.1 mm. The corresponding mean FMD, or percentage of change in the brachial artery diameter, was 3.8 ± 3.1%.
Table 2 shows mean differences in FMD and standard errors by relevant covariates after adjustment for age and sex. There were no large differences in FMD with respect to age and sex, although Asian/Pacific Islanders had a larger response than other race/ethnic groups. Cotinine levels among these former smokers were not related to FMD, nor was obesity or height. FMD was lower among patients with hypercholesterolemia, hypertension, diabetes, and cardiovascular disease, although these differences were large only for cerebrovascular disease and congestive heart failure. Use of aspirin, statins, ACE inhibitors, β-blockers, and omega-3 supplements were associated with lower FMD, and postmenopausal hormone use was associated with higher FMD. None of these associations approached even a nominal level of statistical significance. Use of COPD medications, particularly steroids and anticholinergics, were associated with relatively large differences in FMD.
TABLE 2.
AGE- AND SEX-ADJUSTED MEAN DIFFERENCES IN FLOW-MEDIATED DILATION OF THE BRACHIAL ARTERY (PERCENTAGE OF CHANGE IN ARTERY DIAMETER DURING REACTIVE HYPEREMIA) IN FORMER SMOKERS
| Former Smokers (n = 107)
|
Former Smokers without COPD* (n = 63)
|
|||||
|---|---|---|---|---|---|---|
| Mean Difference | Standard Error | Mean Difference | Standard Error | |||
| Age (per 10 yr) | −0.390 | 0.636 | −0.436 | 0.789 | ||
| Gender, female | −0.074 | 0.613 | −0.294 | 0.786 | ||
| Ethnicity | ||||||
| White | Reference | Reference | ||||
| African American | 0.371 | 1.400 | 0.473 | 1.717 | ||
| Hispanic | −0.187 | 1.114 | −0.0979 | 1.358 | ||
| Asian/Pacific Islander | 1.455 | 1.911 | 1.102 | 1.959 | ||
| Education | ||||||
| Graduate degree | Reference | Reference | ||||
| Four-year college | 0.029 | 0.812 | −1.023 | 1.011 | ||
| High school/some college | −0.097 | 0.790 | −1.139 | 1.058 | ||
| No high school degree | −0.482 | 1.433 | −3.081 | 2.324 | ||
| Cotinine level, ng/ml | −0.006 | 0.008 | −0.007 | 0.008 | ||
| Pack-years (per 10 pack-years) | 0.001 | 0.012 | 0.016 | 0.016 | ||
| Height (per 10 cm) | −0.18 | 0.052 | −0.043 | 0.063 | ||
| BMI (per 1 kg/m2) | 0.012 | 0.072 | −0.080 | 0.093 | ||
| Physician diagnosis of the following | ||||||
| Hypercholesterolemia | −0.838 | 0.672 | −0.626 | 0.874 | ||
| Hypertension | −0.555 | 0.616 | −0.658 | 0.779 | ||
| Diabetes | −0.384 | 0.938 | −0.675 | 1.066 | ||
| Coronary heart disease | −0.905 | 1.896 | 0.791 | 2.290 | ||
| Cerebrovascular disease | −1.429 | 1.642 | −2.279 | 2.2278 | ||
| Peripheral vascular disease | −0.276 | 1.335 | 2.560 | 2.240 | ||
| Congestive heart failure | −2.067 | 2.329 | −2.808 | 2.340 | ||
| Sleep apnea | 0.174 | 1.329 | 0.387 | 1.357 | ||
| Medications | ||||||
| Aspirin | −0.447 | 0.637 | 1.009 | 0.807 | ||
| HMG-CoA reductase inhibitor (statin) | −0.250 | 0.650 | −0.445 | 0.836 | ||
| β-blocker | −0.640 | 0.948 | −0.324 | 1.138 | ||
| ACE inhibitor | −0.441 | 0.950 | −0.145 | 1.125 | ||
| Omega-3 supplement | −0.962 | 0.646 | −1.056 | 0.866 | ||
| Postmenopausal hormones | 0.782 | 1.171 | 0.398 | 1.934 | ||
| Blood pressure (per 10 mm Hg) | ||||||
| Systolic | −0.032 | 0.021 | −0.0343 | 0.025 | ||
| Diastolic | 0.048 | 0.034 | 0.0242 | 0.043 | ||
| Physician-diagnosed asthma before age 45 yr | −0.783 | 1.074 | −0.102 | 1.454 | ||
| MRC-defined chronic bronchitis | −0.278 | 0.793 | 0.267 | 1.082 | ||
| COPD medications | ||||||
| Short-acting β-agonists | −1.050 | 0.903 | 2.389 | 1.860 | ||
| Long-acting β-agonists | −1.134 | 0.875 | −1.179 | 1.643 | ||
| Short-acting anticholinergic | −2.825 | 1.315 | ||||
| Long-acting anticholinergic | −1.451 | 1.240 | ||||
| Inhaled corticosteroids | −2.386 | 1.083 | −2.546 | 1.811 | ||
| Systemic corticosteroids | −3.247 | 3.190 | ||||
See Table 1 for definition of abbreviations.
Defined as post-bronchodilator FEV1/FVC ratio < 0.70.
FMD and FEV1
The association of FMD with post-bronchodilator FEV1 is show in Table 3. A 1SD decrease in FMD was associated with a 147-ml (95% confidence interval [CI], 31–264 ml; P = 0.01) decrement in post-bronchodilator FEV1 after adjustment for age, sex, height, and race/ethnicity (model 1). This association was essentially unchanged after additional adjustment for pack-years of smoking (model 2) and only slightly attenuated after additional adjustment for educational attainment, body mass index, asthma, hypercholesterolemia, hypertension, and diabetes, and use of statins, aspirin, β-blockers, omega-3 supplements, and postmenopausal hormones (model 3).
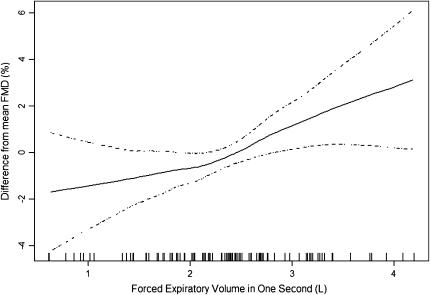
Because the association of FMD and FEV1 in this study spanned a range of post-bronchodilator lung function from normal to severe COPD, we examined the linearity of this association to test if endothelial dysfunction measured by FMD occurred predominantly late in the progression of COPD or if it was linear across the spectrum of lung dysfunction. Figure 1 shows that the multivariate association of FMD and post-bronchodilator FEV1 was approximately linear across this range of lung function. There was no graphical suggestion of a stronger relationship in severe COPD manifest by a “hockey stick” shape rather than a straight line. The multivariate relationship between FMD and post-bronchodilator FEV1 in this flexible model was significant (P = 0.02) and there was no evidence for a departure from linearity (P = 0.30). We confirmed these graphical analyses by testing for effect modification of the FMD–FEV1 association by COPD status in the full multivariate model and found no evidence of an interaction (P = 0.95).
Figure 1.
Multivariate association of the post-bronchodilator FEV1 and flow-mediated dilation (FMD) of the brachial artery in former smokers. Solid line = smoothed regression line adjusted for covariates listed in Table 3, model 3; dashed lines = 95% confidence intervals; vertical lines = rug plot of data points. The multivariate relationship between FEV1 and FMD was statistically significant (P = 0.02). The smoothed curve did not differ significantly from a straight line (P = 0.30), suggesting a linear relationship between FEV1 and FMD.
Given the apparent linear relationship of FMD and post-bronchodilator FEV1, we evaluated whether FMD was significantly related to post-bronchodilator FEV1 among former smokers who had not developed COPD. Among 63 participants without COPD, the magnitude of the association of FMD and post-bronchodilator FEV1 was the same as in the overall sample; standard errors were smaller such that statistical significance was retained in all models for FEV1 (Table 3). Nonlinear analyses yielded similar results as those for the overall sample.
In contrast, the association of FMD with post-bronchodilator FVC was of borderline statistical significance in the whole sample (multivariate difference in FVC, 131 ml [95% CI, 2–259 ml] per 1 SD change in FMD; P = 0.050) and was not statistically significant in those without COPD (112 ml [95% CI, −22 to 245 ml]; P = 0.11). FMD was positively but not significantly associated with the post-bronchodilator FEV1/FVC ratio in both the whole sample (multivariate difference in FEV1/FVC ratio, 1.3% [95% CI, −0.9 to 3.5%] per 1 SD change in FMD; P = 0.25) and in those without COPD (0.6% [95% CI, −0.6 to 1.9%]; P = 0.11).
Analyses of FMD and prebronchodilator spirometry measures yielded similar results to post-bronchodilator results. For example, a 1-SD decrease in FMD was associated with a 153-ml (95% CI, 48–257 ml; P = 0.005) decrement in prebronchodilator FEV1 in the full multivariate model among all participants. There was no association of FMD with bronchodilator response (−0.75% [95% CI, −2.3 to 0.76%]; P = 0.33).
FMD and CT Percentage of Emphysema
Findings for CT percentage of emphysema (Table 3) were similar to those for FEV1. A 1-SD decrease in FMD was associated with a 2.5% (95% CI, 0.1–4.8%; P = 0.04) absolute increase in CT percentage of emphysema after adjustment for age, sex, height, and race/ethnicity. The association was unchanged after additional adjustment for pack-years of smoking and in the full multivariate model.
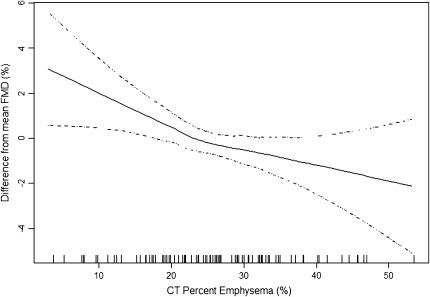
Figure 2 shows that the multivariate association of FMD and CT percentage of emphysema was approximately linear across the range of emphysema; there was no suggestion of a stronger relationship in severe COPD. The multivariate relationship between FMD and CT percentage of emphysema in this flexible model was highly significant (P = 0.005), and there was no evidence for a departure from linearity (P = 0.24). Furthermore, there was no effect modification of the FMD–emphysema association by COPD (P = 0.96).
Figure 2.
Multivariate association of the computed tomography (CT) percentage of emphysema and flow-mediated dilation (FMD) of the brachial artery in former smokers. Solid line = smoothed regression line adjusted for covariates listed in Table 3, model 3; dashed lines = 95% confidence intervals; vertical lines = rug plot of data points. The multivariate relationship between CT percentage of emphysema and FMD was statistically significant (P = 0.005). The smoothed curve did not differ significantly from a straight line (P = 0.24), suggesting a linear relationship between CT percentage of emphysema and FMD.
In analyses restricted to participants without COPD, the magnitude of the association between FMD and CT percentage of emphysema was similar to the overall sample in models 1 and 2 (Table 3), and the relationship was statistically significant in the full multivariate model (model 3).
FMD and DlCO
Associations of FMD for DlCO were consistent with those for FEV1 and CT percentage of emphysema but did not attain statistical significance in the whole sample (Table 3, P = 0.09). Unlike for FEV1 and percentage of emphysema, three values for DlCO were highly influential outliers in the multivariate models (Cook's distance > 6%). Exclusion of these three values improved the model fit appreciably and yielded a multivariate difference in DlCO of 0.89 ml/minute/mm Hg (95% CI, 0.02–1.77; P = 0.049) per 1 SD change in FMD. Restriction to participants without COPD yielded consistent, nonsignificant results (Table 3).
Additional Analyses
We explored the joint association of FMD with post-bronchodilator FEV1 and CT percentage of emphysema in post hoc analyses. The association between FMD and CT percentage of emphysema was independent of and unchanged after adjustment for post-bronchodilator FEV1 (P = 0.015). In contrast, the association between FMD and post-bronchodilator FEV1 was entirely explained by adjustment for CT percentage of emphysema (P = 0.99).
When the main analyses were repeated with the inclusion of the eight current smokers, results were very similar. The full multivariate models yielded a significance of P = 0.027 for FEV1 and P = 0.013 for CT percentage emphysema. Adjustment for use of COPD medications did not change associations appreciably (P = 0.039 and P = 0.019 for FEV1 and CT percentage of emphysema, respectively), nor did exclusion of the small number of participants with sleep apnea (P = 0.020 and P = 0.017, respectively), or cardiovascular disease and congestive heart failure (P = 0.021 and P = 0.014, respectively).
DISCUSSION
We observed a strong, linear association of endothelial function as measured by FMD with both post-bronchodilator FEV1 and CT percentage of emphysema in former smokers across the spectrum of disease from normal lung function to moderately severe COPD. These associations were independent of smoking and other major causes of endothelial dysfunction and COPD, and were present among participants without COPD. The relationship between FMD and post-bronchodilator FEV1 was explained entirely by percentage of emphysema, which suggests that changes in percentage of emphysema may mediate the relationship of FMD and FEV1. These results provide in vivo evidence in humans to support basic research studies (1–5) that suggest that endothelial dysfunction might be involved in the pathogenesis of emphysema and, secondarily, COPD.
To our knowledge, published studies on FMD in patients with COPD are lacking. However, two case-control studies of more severe COPD were recently presented in abstract form. Eickhoff and colleagues found appreciably lower FMD in patients with moderate to severe COPD compared with smoking control subjects and a strong correlation of FMD and FEV1 in patients with COPD (29). Teramoto and coworkers found similar relationships in severe COPD (30). Both of these studies, however, used a case-control design and hence were unable to assess associations early in the disease or separately for FEV1 and CT percentage of emphysema.
Other studies have found abnormalities in systemic vascular function in COPD. Sabit and colleagues recently reported greater aortic pulse wave velocity among patients with COPD compared with unmatched controls (31), and Zureik and colleagues previously demonstrated an inverse association between carotid-femoral pulse wave velocity and FEV1 (32). These studies are generally consistent with our findings but are not specific for endothelial function because pulse wave velocity is a measure of arterial stiffness. Abnormal vascular stiffness in COPD may result from diffuse subclinical atherosclerosis or primary abnormalities in elastin in both the lung parenchyma and in the adventitia of large vessels such as the aorta. FMD, in contrast, is a marker of endothelial function and measures NO-mediated, endothelium-dependent relaxation. A limitation of the current study is the lack of a measure of endothelium-independent vasodilation, which was not made for reasons relating to participant burden in this ongoing cohort study. Abnormal vascular stiffness, however, would not explain the results of a study that showed markedly reduced endothelial progenitor cells in severe COPD (33).
The hypothesis of a primary vascular insult in COPD is not new. The pathologist A. A. Liebow postulated almost 50 years ago that changes in the local vascular milieu modulate alveolar destruction in COPD (34). More recent research has shown that smoking causes acute endothelial dysfunction (13, 14). The mechanisms by which this occurs are not clear, but one potential causal agent is acrolein (35), which triggers endothelial apoptosis of human pulmonary microvascular endothelial cells via suppression of prostaglandin (PG) I2 gene expression (36). Endothelial apoptosis may in turn lead to microvascular damage in the lungs and subsequent emphysema.
An alternative explanation for our findings is that aspects of COPD and emphysema may cause endothelial dysfunction, because the cross-sectional design of this study is not well suited to determination of the direction of causality. Hypoxemia, infection, inflammation, system stress, and pulmonary hypertension may all impair FMD, may be caused by severe COPD, and were not measured directly in this study. They are generally likely, however, to be mild or absent in smokers without COPD, in whom associations between FMD and post-bronchodilator FEV1 and CT percentage of emphysema were present. At a minimum, the linear associations of FMD with lung function and lung density document endothelial dysfunction in mild and “pre”-COPD and emphysema.
Confounding by factors known to lower FMD and cause COPD, such as cigarette smoking, might also have led to our findings. Current smokers were excluded using cotinine levels. Additional adjustment for cotinine levels as a measure of environmental tobacco smoke did not alter results (data not shown). Medications such as statins and postmenopausal hormones improve endothelial function, but adjustment for these medications assessed via a validated medication inventory (28) did not alter findings appreciably. Other potential confounders including age, sex, race/ethnicity, socioeconomic status, and obesity were adjusted for with flexible multivariate models. Nonetheless, FMD is affected by a large number of factors and behaviors (18), so it remains possible that unmeasured confounding contributed to some of the observed associations.
We did not include clinical cardiovascular disease in the multivariate models because endothelial dysfunction is a cause of myocardial infarctions and stroke, not an effect of them, and hence, clinical cardiovascular disease is not a causal confounder of the observed associations. Secondary analyses in which patients with clinical cardiovascular disease were excluded showed consistent, significant results. We found relatively strong associations between FMD and medications for COPD in age- and sex-adjusted analyses; however, it is unclear if COPD medications affect FMD or if they were just prescribed for patients with moderate to severe COPD who had low FMD. FMD is not impaired by elevations in plasma epinephrine in experimental settings (37). Intravenous administration of acetylcholine and cortisol antagonists do affect FMD (38); however, the doses of inhaled anticholinergics that are absorbed and circulate to the brachial artery are low. Additional adjustment of COPD medications did not explain associations of FMD and lung measures, and associations were present in patients without COPD who were not administered these medications.
FMD of the brachial artery has been validated against intracoronary endothelial reactivity (11, 12). We did not validate it, however, against endothelial reactivity in the pulmonary circulation. The systemic nature of endothelial function makes it likely, although not certain, that changes in endothelial function in the brachial and coronary circulations also occur in the pulmonary circulation.
FMD was measured in this study at 1 minute after cuff deflation. This approach has previously been related to cardiovascular outcomes and risk (39, 40) but differs slightly from peak FMD, which can occur before or after 1 minute after cuff deflation. Both approaches are consistent with published FMD guidelines (18).
An additional limitation of the study was its relatively small size to assess associations for DlCO and FEV1/FVC ratio, which are prone to more measurement error than, for example, FEV1 (23, 26). The association of FMD with DlCO was consistent with the study's overall hypotheses but was not statistically significant in the main analysis. The single-breath assessment of DlCO used in this study may have resulted in an underestimation of the true FMD–DlCO association because single-breath Va underestimates true TLC in emphysema (41) and single-breath DlCO correlates poorly with gas exchange in COPD (42). Exclusion of extreme outliers of DlCO, however, resulted in a significant association with FMD.
In conclusion, we found that endothelial dysfunction measured by FMD was independently associated with FEV1 and CT percentage of emphysema in former smokers. These associations were linear across a spectrum of disease from normal lung function and anatomy to moderate to severe COPD and emphysema. This novel finding of endothelial dysfunction early in COPD and emphysema requires replication in larger and longitudinal cohorts, but it is consistent with basic science research hypothesizing a primary vascular role in the pathogenesis of COPD.
Acknowledgments
The authors thank Sean Mota for extensive assistance with database management.
Supported by grants HL075476, HL077612, RR024156, and ES09089 from the National Institutes of Health.
Originally Published in Press as DOI: 10.1164/rccm.200707-980OC on August 29, 2007
Conflict of Interest Statement: None of the authors has a commercial relationship with a financial entity that has an interest in the subject of this manuscript.
References
- 1.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Possible effects of vascular endothelial growth factor in the pathogenesis of chronic obstructive pulmonary disease. Am J Med 2003;114:354–358. [DOI] [PubMed] [Google Scholar]
- 2.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 3.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006;290:L209–L221. [DOI] [PubMed] [Google Scholar]
- 4.Tuder RM, Yoshida T, Fijalkowka I, Biswal S, Petrache I. Role of lung maintenance program in the heterogeneity of lung destruction in emphysema. Proc Am Thorac Soc 2006;3:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tudor RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med 2005;11:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henson PM, Vandivier RW, Douglas I. Cell death, remodeling, and repair in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:713–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demedts IK, Demoor T, Bracke KR, Joos GF, Brusselle GG. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos S, Peinado VI, Ramirez J, Morales-Blanhir J, Bastos R, Roca J, Rodriguez-Roisin R, Barbera JA. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with moderate chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003;167:1250–1256. [DOI] [PubMed] [Google Scholar]
- 9.Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, Gimferrer JM, Rodriguez-Roisin R. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol 1998;274:L908–L913. [DOI] [PubMed] [Google Scholar]
- 10.Dinh-Xuan AT, Pepke-Zaba J, Butt AY, Cremona G, Higenbottam TW. Impairment of pulmonary-artery endothelium-dependent relaxation in chronic obstructive lung disease is not due to dysfunction of endothelial cell membrane receptors nor to L-arginine deficiency. Br J Pharmacol 1993;109:587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol 1995;26:1235–1241. [DOI] [PubMed] [Google Scholar]
- 12.Takase B, Uehata A, Akima T, Nagai T, Nishioka T, Hamabe A, Satomura L, Ohsuzu F, Kurita A. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol 1998;82:1535–1539, A7–A8. [DOI] [PubMed] [Google Scholar]
- 13.Celermajer DS, Sorensen KE, Georgakopoulos DE, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilatation in healthy young adults. Circulation 1993;88:2149–2155. [DOI] [PubMed] [Google Scholar]
- 14.Wiesmann F, Petersen SE, Leeson PM, Francis JM, Robson MD, Wang Q, Choudhury R, Channon KM, Neubauer S. Global impairment of brachial, carotid, and aortic vascular function in young smokers: direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol 2004;44:2056–2064. [DOI] [PubMed] [Google Scholar]
- 15.Barr RG, Mesia Vela S, Austin JHM, Keller B, Reeves A, Shimbo D, Stevenson L. Impaired flow-mediated dilatation of the brachial artery is associated with low lung function and emphysema in ex-smokers: the EMCAP study [abstract]. Am J Respir Crit Care Med 2007;175:A515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763–1771. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs Jr DR, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 18.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, et al.; International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 19.Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol 2003;41:1769–1775. [DOI] [PubMed] [Google Scholar]
- 20.Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol 2000;86:207–210. [DOI] [PubMed] [Google Scholar]
- 21.American Thoracic Society. Standardization of spirometry: 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 22.Barr RG, Stemple KJ, Mesia-Vela S, Basner R, Derk S, Henneberger P, Milton DK, Taveras B. Reproducibility and validity of a portable spirometer. Respir Care 2007; in press. [PMC free article] [PubMed]
- 23.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, van der Grinten CP, Gustafsson P, et al. General considerations for lung function testing. Eur Respir J 2005;26:153–161. [DOI] [PubMed] [Google Scholar]
- 24.Celli BR, MacNee W; American Thoracic Society/European Respiratory Society Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]
- 25.Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”: an objective method to quantitate emphysema using computed tomography. Chest 1988;94:782–787. [DOI] [PubMed] [Google Scholar]
- 26.MacIntyre N, Crapo RO, Viegi G, et al. Standardisation of the single breath determination of carbon monoxide uptake in the lung. Eur Respir J 2005;26:720–735. [DOI] [PubMed] [Google Scholar]
- 27.Jones RS, Meade F. A theoretical and experimental analysis of anomalies in the estimation of pulmonary diffusion capacity by the single breath method. Q J Exp Physiol Cogn Med Sci 1961;46:131–143. [DOI] [PubMed] [Google Scholar]
- 28.Psaty BM, Lee M, Savage PJ, Rutan GH, German PS, Lyles M. Assessing the use of medications in the elderly: method and initial results in the Cardiovascular Health Study. J Clin Epidemiol 1992;45:683–692. [DOI] [PubMed] [Google Scholar]
- 29.Eickhoff P, Kiss D, Schreder M, Kohansal R, Valipour A, Geyer K, Burghuber OC. Systemic inflammation and endothelial dysfunction in COPD [abstract]. Am J Respir Crit Care Med 2007;175:A516. [Google Scholar]
- 30.Teramoto S, Yamamoto H, Yamaguchi Y, Hanaoka Y, Ishii M, Ouchi Y. Impaired endothelial function in patients with severe COPD [abstract]. Am J Respir Crit Care Med 2007;175:A515. [Google Scholar]
- 31.Sabit R, Bolton CE, Edwards PH, Pettit RJ, Evans WD, McEniery CM, Wilkinson IB, Cockcroft JR, Shale DJ. Arterial stiffness and osteoporosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;2007:1259–1265. [DOI] [PubMed] [Google Scholar]
- 32.Zureik M, Benetos A, Neukirch C, Courbon D, Bean K, Thomas F, Ducimetiere P. Reduced pulmonary function is associated with central arterial stiffness in men. Am J Respir Crit Care Med 2001;164:2181–2185. [DOI] [PubMed] [Google Scholar]
- 33.Palange P, Testa U, Huertas A, Calabro L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, et al. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J 2006;27:529–541. [DOI] [PubMed] [Google Scholar]
- 34.Leibow AA. Pulmonary emphysema with special reference to vascular change. Am Rev Respir Dis 1959;80:67–93. [DOI] [PubMed] [Google Scholar]
- 35.Hoffman D, Hecht S. Handbook of experimental pharmacology. Heidelberg, Germany: Springer; 1990.
- 36.Nana-Sinkam SP, Lee JD, Sotto-Santiago S, Stearman RS, Keith RL, Choudhury Q, Cool C, Parr J, Moore MD, Bull TM, et al. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med 2007;175:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyson KS, Shoemaker JK, Hughson RL. Effect of acute sympathetic nervous system activation on flow-mediated dilation of brachial artery. Am J Physiol Heart Circ Physiol 2006;290:H1446–H1453. [DOI] [PubMed] [Google Scholar]
- 38.Broadley AJ, Korszun A, Abdelaal E, Moskvina V, Jones CJ, Nash GB, Ray C, Deanfield J, Frenneaux MP. Inhibition of cortisol production with metyrapone prevents mental stress-induced endothelial dysfunction and baroreflex impairment. J Am Coll Cardiol 2005;46:344–350. [DOI] [PubMed] [Google Scholar]
- 39.Shimbo D, Grahame-Clarke C, Miyake Y, Rodriguez C, Sciacca R, Di Tullio M, Boden-Albala B, Sacco R, Homma S. The association between endothelial dysfunction and cardiovascular outcomes in a population-based multi-ethnic cohort. Atherosclerosis 2007;192:197–203. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JFJ, Lehman BT, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 2004;109:613–619. [DOI] [PubMed] [Google Scholar]
- 41.Milite FM, Lederer DJ, DeMercado G, Fernandez BL, Fani P, Basner RC. Concurrent measurement of single breath, rebreathe and plethysmographic lung volume in emphysema [abstract]. Am J Respir Crit Care Med 2007;175:A607. [Google Scholar]
- 42.Weingarten JA, Lederer DJ, Milite FM, Basner RC. Predictors of gas exchange abnormality in emphysema [abstract]. Am J Respir Crit Care Med 2007;175:A609. [Google Scholar]