Abstract
Rationale: Transforming growth factor (TGF)-β1 is involved in airway inflammation and remodeling, two key processes in asthma pathogenesis. Tobacco smoke and traffic emissions induce airway inflammation and modulate TGF-β1 gene expression. We hypothesized that the effects of functional TGF-β1 variants on asthma occurrence vary by these exposures.
Objectives: We tested these hypotheses among 3,023 children who participated in the Children's Health Study.
Methods: Tagging single-nucleotide polymorphisms rs4803457 C>T and C-509T (a functional promoter polymorphism) accounted for 94% of the haplotype diversity of the upstream region. Exposure to maternal smoking in utero was based on smoking by biological mother during pregnancy. Residential distance from nearest freeway was calculated based on residential address at study entry.
Measurements and Main Results: Children with the −509TT genotype had a 1.8-fold increased risk of early persistent asthma (95% confidence interval [CI], 1.11–2.95). This association varied marginally significantly by in utero exposure to maternal smoking. Compared with children with the −509CC/CT genotype with no in utero exposure to maternal smoking, those with the −509TT genotype with such exposure had a 3.4-fold increased risk of early persistent asthma (95% CI, 1.46–7.80; interaction, P = 0.11). The association between TGF-β1 C-509T and lifetime asthma varied by residential proximity to freeways (interaction P = 0.02). Children with the −509TT genotype living within 500 m of a freeway had over three-fold increased lifetime asthma risk (95% CI, 1.29–7.44) compared with children with CC/CT genotype living > 1500 m from a freeway.
Conclusions: Children with the TGF-β1 −509TT genotype are at increased risk of asthma when they are exposed to maternal smoking in utero or to traffic-related emissions.
Keywords: maternal smoking, traffic, asthma, genetics, gene–environment interaction, association study
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
The associations between transforming growth factor (TGF)-β1 C-509T promoter polymorphism and childhood asthma have been inconsistent, which could in part be explained by differences in environmental exposures (traffic and tobacco smoke) that affect TGF-β1 expression.
What This Study Adds to the Field
Children with the TGF-β1 −509TT genotype are at higher risk of asthma occurrence if they live near a freeway or were exposed to tobacco smoke in utero.
Airway inflammation and remodeling are critical pathophysiologic events in asthma. Genes involved in these processes are candidate genes for evaluation in association studies. Accumulating evidence indicates that the transforming growth factor (TGF)-β1 gene may play an important role in airway inflammation (1, 2) and remodeling (3, 4), suggesting that functional polymorphisms in the TGF-β1 gene that affect its expression may modulate asthma occurrence.
One such functional variant, a C-to-T base substitution at position −509 (i.e., C-509T) in the TGF-β1 gene promoter, increases TGF-β1 gene transcription (5) and plasma TGF-β1 concentrations (6). Although some epidemiologic studies found significant associations between C-509T and asthma occurrence in adults (5, 7, 8), data on childhood asthma are limited and are inconsistent (9–12). This promoter variant has been associated with wheeze during the first year of life due to respiratory syncytial virus infection (9) and with asthma in Mexican American children (10), but others have failed to find significant associations with asthma in children (11, 12). The inconsistency in the results could be due in part to differences in environmental exposures or to confounding by racial admixture.
Of special interest are environmental exposures that may interact with genetic variants involved in expression of TGF-β1. Two common childhood exposures, tobacco smoke and traffic-related air pollutants, cause oxidant stress–mediated airway inflammation and have been associated with asthma in children (13, 14). Data from experimental studies show that these exposures also modulate TGF-β1 gene expression in the airways (15, 16). In experimental model of rat tracheal explants, two- to three-fold increased TGF-β1 expression was found when the explants were exposed to urban air particles (15) and cigarette smoke (16). This up-regulation of TGF-β1 expression was mediated by oxidant stress because tetramethylthiourea (an oxidant scavenger) inhibited this overexpression. These investigators also found that TGF-β1 downstream signaling led to induction of other fibrogenic genes (e.g., connective tissue growth factor and procollagen) and increased deposition of extracellular matrix because these effects were inhibited by TGF-β1 antagonist fetuin. Based on these findings, we hypothesized that genetic variants in the TGF-β1 gene are associated with asthma risk and that the association may be influenced by environmental exposures that modulate TGF-β1 expression, such as tobacco smoke and traffic-related pollutants.
In this analysis, we aimed to test two hypotheses: (1) common genetic variants in the TGF-β1 promoter region are associated with childhood asthma, and (2) the association varies in children who were exposed in utero to maternal smoking, exposed to secondhand smoke at home, or lived near freeways (a surrogate for high exposure from traffic-related air pollutants). To better account for genetic variations in the TGF-β1 promoter region, we determined the pattern of linkage disequilibrium in the TGF-β1 locus using genotypic data from ethnically representative samples of the Multiethnic Cohort (MEC) (17). We assessed these hypotheses in a population-based study conducted among children who had participated in the Children's Health Study (CHS). Some of the results of this study have been previously reported in the form of an abstract (18).
METHODS
Study Design and Subject Enrollment
Children for this study participated in the CHS, which has been described previously (19, 20). In brief, fourth-, seventh-, and tenth-grade students (average ages 10, 13, and 16 years, respectively) from schools in 12 southern California communities were recruited in 1993 and 1996. The present analysis included 3,023 children who were Hispanic (n = 927) or non-Hispanic (n = 2,096) white (see online supplement for details). A parent or guardian provided informed consent. The University of Southern California Institutional Review Board approved the study.
Assessment of Exposures of Interest
A child's exposure to maternal smoking in utero was based on smoking by the biological mother during pregnancy. Secondhand exposure to smoking was based on exposure to smoking inside the house during childhood. Residential distance from the nearest freeway, major road, and traffic density within 1 to 50 m of residence were calculated based on residential address at study entry (see online supplement for details).
Asthma Definitions
Lifetime asthma status was defined by parental report of physician-diagnosed asthma at enrollment in the CHS. Because early transient wheezing episodes often do not represent asthma (21), we excluded children with early transient wheezing (n = 54; none had asthma symptoms or medication use in previous year to study entry). We divided subjects with asthma into two subgroups based on asthma-related phenotypes as described by Martinez and colleagues (21). We defined early persistent asthma as diagnosis before age 3 years with at least one episode of wheeze or asthma medication use after starting first grade. Children diagnosed with asthma after age 3 years were classified as having late-onset asthma. We validated the asthma diagnosis in a subset of the CHS cohort. Based on the medical records review of 172 children, 95.9% (n = 165) had a definite (physician diagnosis of asthma; n = 1 18) or a probable (physician report of wheeze and steroid and/or β2-adrenergic agonist use; n = 54) asthma diagnosis.
Determination of Haplotype Block Structure
We determined the haplotype block structure of the TGF-β1 gene using genotyping data on subjects from the MEC (see Table E1 of the online supplement for polymorphisms used to determine haplotype) (17). We estimated the TGF-β1 diplotype frequencies in non-Hispanic and Hispanic white children separately using SAS macro code written by Dr. Daniel Stram (available at http://www-rcf.usc.edu/∼stram/tagSNPs.html). This haplotype estimation technique provides the maximum likelihood estimates of the haplotype frequencies assuming Hardy-Weinberg equilibrium (22).
Genotyping
We used the TaqMan Allelic Discrimination assay for genotyping the rs4803457 C > T and C-509T single-nucleotide polymorphisms (SNPs). Details of the genotyping methods and quality control protocol are presented in the online supplement.
Statistical Analysis
We evaluated the associations of the variant genotypes and diplotypes with asthma outcomes for the study population overall and by ethnic group. Logistic regression models were fitted to compute odds ratios (ORs) and 95% confidence intervals (CIs) for genotype- and diplotype-based analyses. Age, sex, ethnicity, child's atopic status (determined based on parental report of allergy and/or hay fever), parental history of asthma, parent/guardian's education, in utero exposure to maternal smoking, exposure to secondhand smoke (number of smokers at home; e.g., none, one, two or more), residential distance from nearest freeway, health insurance coverage, and community of residence were considered as potential confounders.
The associations between the C-509T and asthma best fitted a recessive genetic model. Therefore, we compared the children with one or two copies of the rs4803457T/−509T diplotypes with those carrying no copy of the rs4803457T/−509T diplotype (reference group) in diplotype-based analyses. Subsequently, we examined whether environmental exposures (e.g., in utero exposure to maternal smoking, secondhand smoke exposure, and residential distance from freeways) and susceptibility factors (e.g., parental history of asthma, child's sex, and child's atopic status) modified the relationship between the C-509T genotypes and asthma outcomes using separate models for each factor. We used a recessive genetic model for C-509T genotype to test these modifying effects using likelihood ratio tests. To test the modifying effects of residential distance from nearest freeway and from a major road, we categorized the freeway distance into four categories (<500 m, 500–1,000 m, 1,001–1,500 m, and >1,500 m) and major road distance into two categories (<75 m and ⩾75 m), as were used previously in this cohort, and showed significant associations with lung function growth and asthma (14, 23). We used tertiles of traffic density (traffic volume/m2) within 150 m of residence to test the modifying effects of traffic density on the association between TGF-β1 C-509T genotype and asthma. All tests were two-sided at a 5% significance level. We used SAS version 9.1 (SAS Institute, Inc., Cary, NC) for all analyses.
RESULTS
Children with asthma were more likely to be boys than girls (54.7 vs. 45.2%; P < 0.0001) (Table 1), have a parental history of asthma (42.8 vs. 16.9%, p < 0.0001), and have health insurance (92.5 vs. 84.7%; P < 0.0001) compared with children without asthma. Atopy was positively associated with asthma (P < 0.0001). There were also significant differences in the distribution of parent/guardian's education between children with and without asthma. The prevalences of asthma in Hispanic and Non-Hispanic white children were comparable (14.1 vs. 12.2%; P = 0.16).
TABLE 1.
DESCRIPTIVE STATISTICS OF THE STUDY POPULATION
| No Asthma
|
Lifetime Asthma
|
||||
|---|---|---|---|---|---|
| n* | % | n* | % | P Value | |
| Sex | |||||
| Girls | 1,433 | 54.8 | 185 | 45.3† | 0.0004 |
| Boys | 1,182 | 45.2 | 223 | 54.7 | |
| Age, yr | |||||
| ⩽10 | 1,436 | 54.9 | 215 | 52.7 | 0.18 |
| 11–12 | 500 | 19.1 | 70 | 17.2 | |
| >12 | 679 | 26.0 | 123 | 30.1 | |
| Ethnicity | |||||
| Non-Hispanic white | 1,801 | 68.9 | 295 | 72.3 | 0.16 |
| Hispanic white | 814 | 31.1 | 113 | 27.7 | |
| Parental history of asthma | |||||
| No | 2,050 | 83.1 | 222 | 57.2† | <0.0001 |
| Yes | 418 | 16.9 | 166 | 42.8 | |
| Exposure to maternal smoking in utero | |||||
| No | 2,125 | 83.0 | 329 | 81.6 | 0.49 |
| Yes | 434 | 17.0 | 74 | 18.4 | |
| Number of smokers at home | |||||
| None | 1,800 | 71.2 | 273 | 68.8 | 0.46 |
| 1 | 488 | 19.3 | 79 | 19.9 | |
| 2 or more | 240 | 9.5 | 45 | 11.3 | |
| Child's atopic status‡ | |||||
| No | 1,663 | 68.3 | 94 | 26.4† | <0.0001 |
| Yes | 772 | 31.7 | 262 | 73.6 | |
| Annual family income | |||||
| <$15,500 | 307 | 13.6 | 42 | 11.7 | 0.12 |
| $15,000–$49,999 | 961 | 42.7 | 139 | 38.8 | |
| ⩾$50,000 | 983 | 43.7 | 177 | 49.4 | |
| Parent/guardian education | |||||
| <12th grade | 313 | 12.3 | 28 | 7.0† | 0.01 |
| 12th grade | 488 | 19.2 | 85 | 21.1 | |
| Some college | 1,147 | 45.0 | 206 | 51.2 | |
| College | 267 | 10.5 | 38 | 9.5 | |
| Some graduate | 330 | 13.0 | 45 | 11.2 | |
| Health insurance coverage | |||||
| No | 395 | 15.3 | 30 | 7.5† | <0.0001 |
| Yes | 2,180 | 84.7 | 370 | 92.5 | |
Numbers do not always add up because of missing data.
P values from Pearson chi-square tests comparing children with asthma with those without asthma.
Child's atopic status was determined based on parental report of allergy and hay fever.
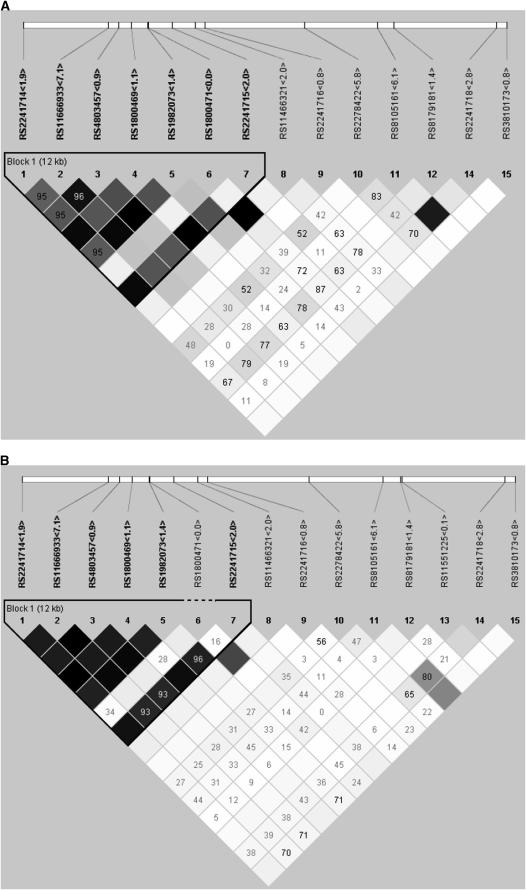
The rs4803457 C>T was located 1,063 bp upstream of the C-509T SNP. In analyses of the overall sample as in analyses restricted to non-Hispanic and Hispanic children, the -509 and the rs4803457 alleles were in Hardy-Weinberg equilibrium (Table 2). As tagging SNPs, these two SNPs accounted for almost 94% of the variability of the block that contains the promoter region in both ethnic groups. Although the allele and diplotype frequencies differed by ethnic group, the haplotype block structure that contained the promoter variant of the TGF-β1 gene did not substantially differ by ethnicity (Figures 1A and 1B). Based on these two SNPs, we determined four diplotypes (CC, CT, TC, and TT). In each ethnic group, the rs4803457C/−509C was the most common diplotype. The −509T variant allele was primarily carried with the rs4803457T/−509T diplotype because the rs4803457C/−509T diplotype frequency was very low (i.e., ∼0.5%) in each ethnic group. Based on the MEC genotyping data, we found that C-509T SNP was in tight linkage (D′ = 1.0) with the T869C (a leucine-to-proline substitution at codon 10; rs1982073) and G915C (an arginine-to-proline substitution at codon 25; rs1800471), and found nonsynonymous SNPs in the exon 1, with the −509T allele being carried in the −509T/869C/915G haplotype in each ethnic group.
TABLE 2.
ALLELE AND DIPLOTYPE FREQUENCIES IN THE STUDY POPULATION
| Allele Frequency
|
Diplotype Frequencies rs4803457 C>T − rs1800469 C>T
|
|||||||
|---|---|---|---|---|---|---|---|---|
| C | T | PHWE* | CC | CT | TC | TT | R2h† | |
| Overall sample (n = 3,023) | ||||||||
| rs4803457 | 0.57 | 0.43 | 0.24 | |||||
| 0.567 | 0.004 | 0.072 | 0.357 | 0.938 | ||||
| rs1800469 (C-509T) | 0.64 | 0.36 | 0.99 | |||||
| Non-Hispanic white (n = 2,096) | ||||||||
| rs4803457 | 0.60 | 0.40 | 0.17 | |||||
| 0.592 | 0.005 | 0.081 | 0.322 | 0.938 | ||||
| rs1800469 (C-509T) | 0.67 | 0.33 | 0.88 | |||||
| Hispanic white (n = 927) | ||||||||
| rs4803457 | 0.51 | 0.49 | 0.61 | |||||
| 0.509 | 0.004 | 0.052 | 0.435 | 0.939 | ||||
| rs1800469 (C-509T) | 0.56 | 0.44 | 0.22 | |||||
P values for Hardy-Weinberg equilibrium (from PROC Allele in SAS).
R2h, or coefficient of determination, is a measure that describes the extent of the haplotype diversity accounted for by rs4803457 C>T and rs1800469 C>T single-nucleotide polymorphisms.
Figure 1.
The linkage disequilibrium plot of the transforming growth factor-β1 gene for (A) whites (non-Hispanic white) and (B) Latinos (Hispanic white). The numbers in boxes represent the D′ in percentages if it is less than 1.
We found that children who were homozygous for the T allele at the −509 position were at increased risk of early persistent asthma (adjusted OR, 1.81; 95% CI, 1.11–2.95) (Table 3). The magnitude of the associations did not differ by ethnicity (Table E2). Late-onset asthma was not significantly associated with the C-509T genotypes. In diplotype-based analysis, children carrying two copies of the rs4803457T/−509T (TT) diplotypes were at 1.8-fold increased risk of early persistent asthma (95% CI, 1.12–2.98) compared with children with no copies of the TT diplotype.
TABLE 3.
ASSOCIATIONS BETWEEN TRANSFORMING GROWTH FACTOR-β1 C-509T AND rs4803457 POLYMORPHISMS AND RS4803457T/−509T DIPLOTYPES AND ASTHMA
| Lifetime Asthma
|
Early Persistent Asthma*
|
Late-Onset Asthma†
|
|||||
|---|---|---|---|---|---|---|---|
| No Asthma (n) | n | OR (95% CI) | n | OR (95% CI) | n | OR (95% CI) | |
| rs1800469 (C-509T) | |||||||
| CC | 1,065 | 168 | 1.0 (Ref.) | 65 | 1.0 | 103 | 1.0 (Ref.) |
| CT | 1,211 | 184 | 0.98 (0.77–1.26) | 74 | 1.01 (0.70–1.47) | 110 | 0.94 (0.69–1.27) |
| TT | 339 | 56 | 1.33 (0.93–1.91) | 31 | 1.81 (1.11–2.95) | 25 | 0.95 (0.59–1.55) |
| rs4803457 (C>T) | |||||||
| CC | 861 | 141 | 1.0 (Ref.) | 55 | 1.0 (Ref.) | 86 | 1.0 (Ref.) |
| CT | 1,267 | 182 | 0.83 (0.64–1.08) | 73 | 0.79 (0.54–1.18) | 109 | 0.80 (0.58–1.10) |
| TT | 487 | 85 | 1.21 (0.88–1.67) | 42 | 1.46 (0.92–2.30) | 43 | 0.99 (0.66–1.49) |
| No. of rs4803457T/−509T diplotypes | |||||||
| 0 | 1.0 (Ref.) | 1.0 (Ref.) | 1.0 (Ref.) | ||||
| 1 | 0.98 (0.76–1.25) | 1.01 (0.69–1.46) | 0.94 (0.69–1.27) | ||||
| 2 | 1.35 (0.94–1.93) | 1.83 (1.12–2.98) | 0.97 (0.60–1.58) | ||||
Definition of abbreviations: CI = confidence interval; Ref. = reference category; OR = odds ratio.
Diagnosis by 3 years of age.
Diagnosis by 3 years of age.
The associations between TGF-β1 C-509T and asthma varied by residential distance from freeways (Table 4). Children with the −509TT genotype who lived less than 500 m from a freeway had over threefold (95% CI, 1.29–7.44) increased risk of lifetime asthma (P for interaction = 0.02), whereas children with the −509TT genotype living between 500 m and 1000 m from a freeway had a marginally significant (almost twofold; P = 0.08) increased risk of asthma compared with children with the CC/CT genotype living more than 1,500 m from a freeway. When stratified by C-509T genotype, in children with the CC/CT genotype, living within 500 m of a freeway was not associated with asthma (OR, 0.89; 95% CI, 0.56–1.41) (Table E3). In contrast, in children with the TT genotype, living within 500 m was associated with an almost fivefold increased risk (OR, 4.85; 95% CI, 1.36–17.32) of lifetime asthma. This joint effect of residential distance less than 500 m from a freeway and TGF-β1 −509TT genotype seemed to be stronger for early persistent asthma than for all asthma cases, although the P value for interaction was not statistically significant. We found similar results with the diplotype-based analysis. Restricting the analyses to long-term resident (i.e., lived ⩾1 yr before asthma diagnosis) showed similar associations (Table E4). In general, children who lived near a freeway were also living close to a major road and had high traffic density near their homes (Table E5 ). When data were analyzed for residential distance from a major road (<75 vs. ⩾75 m) and traffic density within 150 m, the overall results for early persistent asthma were in agreement with the results obtained with freeway distance, although the P values for interaction were not statistically significant (Table E6).
TABLE 4.
JOINT EFFECTS OF TRANSFORMING GROWTH FACTOR-β1 C-509T GENOTYPES AND rs4803457T/−509T DIPLOTYPES AND RESIDENTIAL DISTANCE FROM FREEWAY ON ASTHMA
| Residential Distance from Nearest Freeway (m) | Lifetime Asthma
|
Early Persistent Asthma
|
||||
|---|---|---|---|---|---|---|
| No Asthma (n) | n | OR (95% CI) | n | OR (95% CI) | ||
| rs1800469 (C-509T) | ||||||
| CC/CT | >1,500 | 949 | 153 | 1.0 (Ref.) | 55 | 1.0 (Ref.) |
| CC/CT | 1,001–1,500 | 246 | 45 | 1.01 (0.67–1.54) | 25 | 1.71 (0.95–3.10) |
| CC/CT | 500–1,000 | 300 | 52 | 0.91 (0.60–1.38) | 25 | 1.20 (0.66–2.18) |
| CC/CT | <500 | 239 | 36 | 0.93 (0.59–1.48) | 12 | 0.90 (0.43–1.89) |
| TT | >1,500 | 151 | 15 | 0.79 (0.43–1.44) | 7 | 1.08 (0.46–2.56) |
| TT | 1,001–1,500 | 38 | 6 | 0.78 (0.29–2.11) | 6 | 2.53 (0.86–7.39) |
| TT | 500–1,000 | 52 | 14 | 1.95 (0.97–3.92) | 6 | 2.10 (0.75–5.92) |
| TT | <500 | 30 | 9 | 3.10 (1.29–7.44) | 5 | 5.48 (1.72–17.42) |
| P* = 0.02 | P* = 0.18 | |||||
| No. of rs4803457T/-509T diplotypes | ||||||
| 0 or 1 | >1,500 | 1.0 (Ref.) | 1.0 (Ref.) | |||
| 0 or 1 | 1,001–1,500 | 1.01 (0.67–1.54) | 1.71 (0.95–3.10) | |||
| 0 or 1 | 500–1,000 | 0.90 (0.60–1.36) | 1.19 (0.66–2.16) | |||
| 0 or 1 | <500 | 0.93 (0.59–1.48) | 0.90 (0.43–1.89) | |||
| 2 | >1,500 | 0.80 (0.44–1.45) | 1.09 (0.46–2.57) | |||
| 2 | 1,001–1,500 | 0.78 (0.29–2.11) | 2.53 (0.86–7.39) | |||
| 2 | 500–1,000 | 2.08 (1.03–4.19) | 2.25 (0.79–6.37) | |||
| 2 | <500 | 3.11 (1.30–7.46) | 5.49 (1.73–17.48) | |||
| P* = 0.02 | P* = 0.18 | |||||
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
ORs adjusted for age, sex, ethnicity, atopic status, parental history of asthma, annual family income, parental education, in utero exposure to maternal smoking, number of smokers at home, insurance status, and community of residence. Children living in Lake Arrowhead and Lompoc were excluded from the analysis. Models could not be fit for late-onset asthma phenotype.
P values for TGF-β1 C-509T by residential distance from nearest freeway were obtained from likelihood ratio test from nonstratified models with appropriate interaction terms and were based on 3 degrees of freedom.
Children homozygous for the −509T allele who were exposed in utero to maternal smoking were at greater risk of early persistent asthma compared with those with CC/CT genotype who were not exposed to maternal smoking in utero (Table 5). Specifically, relative to children with CC/CT genotypes who were unexposed to maternal smoking in utero, those with the TT genotype who were exposed to maternal smoking were at a 3.4-fold higher risk of early persistent asthma (95% CI, 1.46–7.80); however, the P value for interaction was marginally significant. When stratified by C-509T genotype, in children with CC/CT genotype, in utero exposure to maternal smoking was not associated with early persistent asthma (OR, 0.97; 95% CI, 0.57–1.66 (Table E7). In contrast, in children with the TT genotype, in utero exposure to maternal smoking was associated with over threefold increased risk (95% CI, 0.81–12.26) of early persistent asthma.
TABLE 5.
TRANSFORMING GROWTH FACTOR-β1 C-509T GENOTYPES AND rs4803457T/−509T DIPLOTYPES AND EXPOSURE TO MATERNAL SMOKING IN UTERO AND EARLY PERSISTENT ASTHMA
| In Utero Maternal Smoking | No Asthma (n) | Early Persistent Asthma
|
||||
|---|---|---|---|---|---|---|
| n | OR (95% CI) | |||||
| rs1800469 (C-509T) | ||||||
| CC/CT | No | 1,844 | 107 | 1.0 (Ref.) | ||
| TT | No | 281 | 22 | 1.47 (0.87–2.48) | ||
| CC/CT | Yes | 382 | 29 | 0.99 (0.59–1.66) | ||
| TT | Yes | 52 | 9 | 3.41 (1.46–7.80) | ||
| P = 0.11* | ||||||
| No. of rs4803457T/−509T diplotypes | ||||||
| 0 or 1 | No | 1.0 (Ref.) | ||||
| 2 | No | 1.49 (0.88–2.52) | ||||
| 0 or 1 | Yes | 0.99 (0.59–1.66) | ||||
| 2 | Yes | 3.43 (1.46–8.03) | ||||
| P = 0.12* | ||||||
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
ORs adjusted for child's age, sex, ethnicity, atopy, health insurance status, and community of residence, and parental education, parental asthma, annual family income, residential distance from nearest freeway, and number of smokers at home.
The P value for the in utero maternal smoking by TGF-β1–C-509T interaction was obtained from likelihood ratio tests from a nonstratified model with appropriate interaction terms and was based on 1 degree of freedom.
In the overall sample, 508 (17.1%) children had in utero exposure to maternal smoking. Of them, 222 (43.7%) were not exposed to maternal smoking during childhood. Among children (n = 2,454) who had no in utero exposure to maternal smoking, 138 (5.6%) were exposed to maternal smoking during childhood. To identify whether the effects were mediated by in utero exposure to maternal smoking and/or by childhood exposure to secondhand smoke, we conducted sensitivity analyses excluding children with certain exposure categories. First, we restricted the analysis to children who had no childhood exposure to smoking at home (n = 1,912). In this subgroup, we found that children with the −509TT genotype had over eightfold increased risk (95% CI, 2.22–30.63) of early persistent asthma when they were exposed to maternal smoking in utero (Table E8) compared with children with the −509 CC/CT genotype who had no in utero exposure to maternal smoking. The interaction by in utero maternal smoking was marginally statistically significant (P for interaction = 0.06). Then, we restricted the analysis to children who had no in utero exposure to maternal smoking (n = 2,254). In this subgroup, the number of smokers at home did not modify the association between TGF-β1 −509TT genotype and early persistent asthma (P for interaction = 0.57; not shown).
Although data were sparse to formally test for modifying effects of freeway distance and maternal smoking during pregnancy on the association between TGF-β1 C-509T and asthma, our data suggest that the two exposures may impart a synergistic effect (Table E9). Among children with exposure to in utero maternal smoking, those with the −509TT genotype living within 500 m from a freeway were at 10-fold increased risk of asthma (95% CI, 1.25–80.40) compared with children with the CC/CT genotype living more than 1500 m from a freeway.
The modifying effects of residential distance from freeway and in utero exposure to maternal smoking on the relationship of TGF-β1 with asthma were similar in diplotype-based analysis and by ethnic groups. Although the OR associated with the −509TT genotype for early persistent asthma was larger in children without atopy, the P value for interaction by atopy was not statistically significant (Table E10). Child's ethnicity, parental asthma, and sex did not confound or modify any of the genetic associations.
DISCUSSION
We found that children with the TGF-β1 −509TT genotype (a variant genotype that increases TGF-β1 gene expression) were at greater risk of asthma if they resided near freeways. Our results also suggest that maternal smoking during pregnancy affects the associations between TGF-β1 −509TT genotype and early persistent asthma. Although the allele frequencies of the two SNPs examined in this study and the diplotype frequencies based on these two SNPs differed by ethnicity, the haplotype block span that contained the functional promoter variant was similar between the two racial/ethnic groups, and the associations did not vary by ethnicity.
Previous studies showed that the −509T variant is a functional SNP because it affects the promoter activity and plasma TGF-β1 concentrations. Silverman and colleagues (5) demonstrated that the −509T SNP increases the TGF-β1 promoter affinity for the transcription factor Yin Yang 1 by 30% resulting in a 30% increase in basal promoter activity. Grainger and colleagues (6) reported that the C-509T SNP accounted for 8.2% of the additive genetic variance of plasma TGF-β1 concentrations. Taken together, findings from these studies indicate that the −509C SNP is likely to be a genetic determinant of levels of TGF-β1 and support involvement of this SNP in asthma pathogenesis.
In genotyping data from the MEC, we found that the −509T allele is present in the −509T/869C/915G haplotype, a finding consistent with the report by Pulleyn and colleagues (24). The T869C (a leucine-to-proline substitution at codon 10) and G915C (an arginine-to-proline substitution at codon 25) nonsynonymous SNPs are located in exon 1 and have been reported to be functional in epidemiologic and in in vitro studies with the 869CC and 915GG genotypes being associated with increased TGF-β1 levels (5, 6, 25–27). All three risk alleles (i.e., −509T, 869C, and 915G) reported in these studies are represented by the −509T/869C/915G haplotype. Because the two SNPs we genotyped in this study captured approximately 94% of the haplotype variation of the promoter and the entire exon 1 regions (i.e., they were in tight linkage disequilibrium with exon 1 SNPs), we did not genotype these SNPs because they would have provided redundant information.
Our finding of stronger associations between C-509T SNP and early persistent asthma in children who had in utero exposure to maternal smoking or lived near a freeway (a surrogate of increased likelihood to higher exposure to traffic emissions) is supported by the current understanding of the biology of TGF-β1. In the airways, similar to many tissues, TGF-β1 remains in an inactive form, bound with the latency-associated protein and latent TGF-β1–binding protein (28). Reactive oxygen species are major factors in the activation of latent TGF-β1 (29, 30). Smoking and traffic emissions (e.g., fine and ultrafine particles, diesel exhaust, polyaromatic hydrocarbons) induce oxidant stress and thereby are likely to be important activators of TGF-β1 expression in the airways. Therefore, it is plausible that children who are genetically predisposed to producing higher levels of TGF-β1 would be at greater risk of developing asthma in the presence of these exposures from early life, which augments TGF-β1 expression. This variation in TGF-β1 expression by these environmental factors may result in an earlier age at asthma development, especially in highly exposed children.
TGF-β1 is a pleiotropic, regulatory cytokine with pro- and antiinflammatory effects. It inhibits T-helper cell (Th) type 1 (Th1) (31) and Th2 differentiations (32) and is involved in immune tolerance with TGF-β1–secreting regulatory T cells (Th3) (33). A growing body of evidence indicates that in inflammatory milieu, TGF-β1 plays a critical role in the development of proinflammatory Th17 cells and inhibits Th1, Th2, and Th3 cell differentiation by inhibiting the expression of their master regulator genes TBX21 (T-box expressed in T cells), GATA3 (GATA binding protein 3), and FOXP3 (forkhead box P3), respectively (34–36). Because smoking and traffic exposures induce airway inflammation, it is plausible that increased expression of TGF-β1 due to these exposures and the C-509T functional polymorphisms promoted IL-17–mediated airway inflammation. Therefore, we speculate that the effect of the TGF-β1 C-509T polymorphism on asthma occurrence is mediated by its effect on airway inflammation and remodeling (e.g., deposition of extracellular matrix, fibroblast proliferation, and myofibroblast differentiation).
A number of studies suggest that the association of TGF-β1 C-509T with asthma could be due to effects of this polymorphism on atopy. We used questionnaire-based atopic status to assess variation in the gene variant association in children with and without atopy. Although questionnaire-based atopic status was not associated with C-509T and did not modify the observed genetic associations (not shown), we adjusted for atopic status in our analyses. Our finding that atopic status had no modifying role on the genetic associations is consistent with the findings by Mak and colleagues (8), who had used skin test results to define atopy. In addition, studies have found inconsistent associations between C-509T and atopic markers (e.g., serum IgE, eosinophil count, positive skin test to allergens) (5, 10, 11, 37). Therefore, it is unlikely that the relationship of TGF-β1 C-509T with asthma is primarily due to effects of this polymorphism on atopy.
We considered the potential effects of a number of sources of errors and biases in this study. Demographic factors (age, sex, ethnicity), socioeconomic factors (family income, parental education, and health insurance coverage), exposure to maternal smoking during pregnancy and secondhand smoke after birth, and asthma prevalences showed modest differences between participants and nonparticipants (Table E11). In addition, children living near freeways were more likely to be from low socioeconomic status (Table E12) than those living further away from freeways. To address any potential for selection bias or confounding from these modest differences, we compared models with and without each covariate of interest and evaluated the percent change in the ORs. None of the covariates changed the ORs by more than 5%. Concern in using parental report of physician-diagnosed asthma in epidemiologic studies has been raised because parental report may not reflect physician diagnosis and access to healthcare may lead to variation in likelihood of diagnosis. To investigate this potential bias, we reviewed medical records of children with asthma and found strong evidence that parental report reflected physician diagnosis. Restricting our analyses to children with health insurance yielded similar results (not shown).
Several studies have found that maternal recall of smoking during pregnancy is reliable even after 10 to 30 years (38–41). Parental report of smoking has been found to be reliable in some (42–44) and was found to be underreported in children with asthma (45).Therefore, although recall bias is possible for in utero and secondhand smoke exposures, this may have attenuated the risk estimates because children with asthma were more likely to be in the unexposed group. Because genotyping for the TGF-β1 SNPs is not used in diagnosing asthma, diagnostic bias or recall bias in reporting age at asthma onset and persistence of symptoms by first grade (used to determine asthma phenotypes) with respect to the studied SNPs is unlikely. Detail information on residential history was unavailable. However, restricting the analyses to long-term residents showed similar results. In addition, if the parents realized that living near freeways was one of the factors that resulted in asthma development in them or in their children, they would have been more likely to move to a home further from the freeways. In our sample, about 13% children lived within 500 m of a freeway, and this proportion did not vary by asthma status. The proportion of parents with and without asthma living within 500 m of a freeway was also similar (∼13%). Therefore, any misclassification in exposure assessment is likely to be nondifferential and unlikely to explain our findings.
In association studies involving multiethnic populations, population stratification could bias the results. To address this potential bias, we have excluded children who were African American or Asian or who belonged to a mixed ethnic background because of small sample sizes to conduct ethnic-specific analysis. Because our results for the genetic associations yielded similar results by ethnicity (see Table 3 and 4) and the joint effects of gene and environmental factors (traffic and smoking exposures) showed similar magnitude of associations by ethnicity (not shown), our analytic approach of conducting pooled analyses adjusting for ethnicity seem to be reasonable. In addition, restricting the analyses to non-Hispanic whites provided similar results.
Our findings are consistent with the earlier findings reported in white (5) and Hispanic (10) populations and add to the growing body of evidence that environmental exposures that result in oxidant stress modify genetic associations with asthma occurrence. The role of C-509T and environmental factors causing oxidant stress on TGF-β1 expression have been well documented, and the analyses were based on specified a priori hypotheses. In this setting, we do not consider it appropriate to disregard prior information on the biology of TGF-β1 expression and to adjust the P values for testing a set of selected a priori hypotheses. Some of the results were based on small sample sizes, and we conducted multiple tests based on a priori hypotheses. Therefore, these results should be interpreted with caution.
Internal and external replications of our findings could provide stronger support to the observed associations; however, replication of findings in different populations may not always be found because of genetic and environmental heterogeneities across populations. In our sample, we had two fourth-grade cohorts (average age, 10 yr in both groups) recruited in 1993 (n = 1,071) and 1996 (n = 1,148), which provided us with the opportunity to examine the associations in two comparable populations with similar genetic and environmental backgrounds (Table E13). The results (as shown in Tables E14, E15, and E16, which could be compared with Tables 3, 4, and 5, respectively) showed similar associations between two fourth-grade student groups and are consistent with the results found for the overall sample.
We conclude that the homozygous TT genotype at the TGF-β1 −509 locus is associated with increased risk of early persistent asthma in children. Although the −509 locus is functional, it also tags a common haplotype in TGF-β1 that includes two additional functional SNPs in exon 1. The effect of the TGF-β1 promoter variants on asthma in children was largest in children exposed to high oxidant burdens during critical developmental windows. Further studies of the TGF-β1 locus in multiethnic populations are needed to fully understand the role of TGF-β1 DNA sequence variations in childhood asthma occurrence and the mechanism by which it may interact with air pollutants.
Supplementary Material
Acknowledgments
The authors thank John Thomas Casagrande, David Van Den Berg, Christopher A. Haiman, Melissa Frasco, and Grace Young-Un Shim for providing the genotyping data on a sample of white and Latino participants of the Multiethnic Cohort for determining the haplotype block structures in these populations.
Supported by the National Heart, Lung and Blood Institute (grants 5R01HL61768 and 5R01HL76647); the Southern California Environmental Health Sciences Center (grant 5P30ES007048) funded by the National Institute of Environmental Health Sciences; the Children's Environmental Health Center (grants 5P01ES009581, R826708–01 and RD83 1861–01) funded by the National Institute of Environmental Health Sciences and the Environmental Protection Agency; the National Institute of Environmental Health Sciences (grant 5P01ES01 1627); and the Hastings Foundation.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200704-561OC on August 2, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.de Boer WI, van Schadewijk A, Sont JK, Sharma HS, Stolk J, Hiemstra PS, van Krieken JH. Transforming growth factor beta1 and recruitment of macrophages and mast cells in airways in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;158:1951–1957. [DOI] [PubMed] [Google Scholar]
- 2.Fong CY, Pang L, Holland E, Knox AJ. TGF-beta1 stimulates IL-8 release, COX-2 expression, and PGE(2) release in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2000;279:L201–L207. [DOI] [PubMed] [Google Scholar]
- 3.Kokturk N, Tatlicioglu T, Memis L, Akyurek N, Akyol G. Expression of transforming growth factor beta1 in bronchial biopsies in asthma and COPD. J Asthma 2003;40:887–893. [DOI] [PubMed] [Google Scholar]
- 4.Redington AE, Madden J, Frew AJ, Djukanovic R, Roche WR, Holgate ST, Howarth PH. Transforming growth factor-beta 1 in asthma: measurement in bronchoalveolar lavage fluid. Am J Respir Crit Care Med 1997;156:642–647. [DOI] [PubMed] [Google Scholar]
- 5.Silverman ES, Palmer LJ, Subramaniam V, Hallock A, Mathew S, Vallone J, Faffe DS, Shikanai T, Raby BA, Weiss ST, et al. Transforming growth factor-beta1 promoter polymorphism C-509T is associated with asthma. Am J Respir Crit Care Med 2004;169:214–219. [DOI] [PubMed] [Google Scholar]
- 6.Grainger DJ, Heathcote K, Chiano M, Snieder H, Kemp PR, Metcalfe JC, Carter ND, Spector TD. Genetic control of the circulating concentration of transforming growth factor type beta1. Hum Mol Genet 1999;8:93–97. [DOI] [PubMed] [Google Scholar]
- 7.Nagpal K, Sharma S, B-Rao C, Nahid S, Niphadkar PV, Sharma SK, Ghosh B. TGFbeta1 haplotypes and asthma in Indian populations. J Allergy Clin Immunol 2005;115:527–533. [DOI] [PubMed] [Google Scholar]
- 8.Mak JC, Leung HC, Ho SP, Law BK, Ho AS, Lam WK, Ip MS, Chan-Yeung MM. Analysis of TGF-beta(1) gene polymorphisms in Hong Kong Chinese patients with asthma. J Allergy Clin Immunol 2006;117:92–96. [DOI] [PubMed] [Google Scholar]
- 9.Hoffjan S, Ostrovnaja I, Nicolae D, Newman DL, Nicolae R, Gangnon R, Steiner L, Walker K, Reynolds R, Greene D, et al. Genetic variation in immunoregulatory pathways and atopic phenotypes in infancy. J Allergy Clin Immunol 2004;113:511–518. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Romieu I, Wu H, Sienra-Monge JJ, Ramirez-Aguilar M, Del Rio-Navarro BE, Del Lara-Sanchez IC, Kistner EO, Gjessing HK, London SJ. Genetic polymorphisms in transforming growth factor beta-1 (TGFB1) and childhood asthma and atopy. Hum Genet 2007;121:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hobbs K, Negri J, Klinnert M, Rosenwasser LJ, Borish L. Interleukin-10 and transforming growth factor-beta promoter polymorphisms in allergies and asthma. Am J Respir Crit Care Med 1998;158:1958–1962. [DOI] [PubMed] [Google Scholar]
- 12.Heinzmann A, Bauer E, Ganter K, Kurz T, Deichmann KA. Polymorphisms of the TGF-beta1 gene are not associated with bronchial asthma in Caucasian children. Pediatr Allergy Immunol 2005;16:310–314. [DOI] [PubMed] [Google Scholar]
- 13.Boulet LP, Lemiere C, Archambault F, Carrier G, Descary MC, Deschesnes F. Smoking and asthma: clinical and radiologic features, lung function, and airway inflammation. Chest 2006;129:661–668. [DOI] [PubMed] [Google Scholar]
- 14.McConnell R, Berhane K, Yao L, Jerrett M, Lurmann F, Gilliland F, Kunzli N, Gauderman J, Avol E, Thomas D, et al. Traffic, susceptibility, and childhood asthma. Environ Health Perspect 2006;114:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai J, Xie C, Vincent R, Churg A. Air pollution particles produce airway wall remodeling in rat tracheal explants. Am J Respir Cell Mol Biol 2003;29:352–358. [DOI] [PubMed] [Google Scholar]
- 16.Wang RD, Wright JL, Churg A. Transforming growth factor-beta 1 drives airway remodeling in cigarette smoke-exposed tracheal explants. Am J Respir Cell Mol Biol 2005;33:387–393. [DOI] [PubMed] [Google Scholar]
- 17.Kolonel LN, Henderson BE, Hankin JH, Nomura AMY, Wilkens LR, Pike MC, Stram DO, Monroe KR, Earle ME, Nagamine FS. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salam MT, Gauderman WJ, McConnell R, Lin PC, Wenten M, Gilliland FD. Transforming growth factor-beta 1, traffic, and childhood asthma. Am J Respir Crit Care Med 2007;175:A259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters JM, Avol E, Navidi W, London SJ, Gauderman WJ, Lurmann F, Linn WS, Margolis H, Rappaport E, Gong H, et al. A study of twelve Southern California communities with differing levels and types of air pollution: I. Prevalence of respiratory morbidity. Am J Respir Crit Care Med 1999;159:760–767. [DOI] [PubMed] [Google Scholar]
- 20.Peters JM, Avol E, Gauderman WJ, Linn WS, Navidi W, London SJ, Margolis H, Rappaport E, Vora H, Gong H Jr, et al. A study of twelve Southern California communities with differing levels and types of air pollution: II. Effects on pulmonary function. Am J Respir Crit Care Med 1999;159:768–775. [DOI] [PubMed] [Google Scholar]
- 21.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995;332:133–138. [DOI] [PubMed] [Google Scholar]
- 22.Stram DO, Haiman CA, Hirschhorn JN, Altshuler D, Kolonel LN, Henderson BE, Pike MC. Choosing haplotype-tagging SNPS based on unphased genotype data using a preliminary sample of unrelated subjects with an example from the Multiethnic Cohort Study. Hum Hered 2003;55:27–36. [DOI] [PubMed] [Google Scholar]
- 23.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, Lurmann F, Avol E, Kunzli N, Jerrett M, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet 2007;369:571–577. [DOI] [PubMed] [Google Scholar]
- 24.Pulleyn LJ, Newton R, Adcock IM, Barnes PJ. TGFbeta1 allele association with asthma severity. Hum Genet 2001;109:623–627. [DOI] [PubMed] [Google Scholar]
- 25.Awad MR, El-Gamel A, Hasleton P, Turner DM, Sinnott PJ, Hutchinson IV. Genotypic variation in the transforming growth factor-beta1 gene: association with transforming growth factor-beta 1 production, fibrotic lung disease, and graft fibrosis after lung transplantation. Transplantation 1998;66:1014–1020. [DOI] [PubMed] [Google Scholar]
- 26.Hinke V, Seck T, Clanget C, Scheidt-Nave C, Ziegler R, Pfeilschifter J. Association of transforming growth factor-beta1 (TGFbeta1) T29 → C gene polymorphism with bone mineral density (BMD), changes in BMD, and serum concentrations of TGF-beta1 in a population-based sample of postmenopausal German women. Calcif Tissue Int 2001;69:315–320. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y, Miyauchi A, Goto J, Takagi Y, Okuizumi H, Kanematsu M, Hase M, Takai H, Harada A, Ikeda K. Association of a polymorphism of the transforming growth factor-beta1 gene with genetic susceptibility to osteoporosis in postmenopausal Japanese women. J Bone Miner Res 1998;13:1569–1576. [DOI] [PubMed] [Google Scholar]
- 28.Sheppard D. Transforming growth factor beta: a central modulator of pulmonary and airway inflammation and fibrosis. Proc Am Thorac Soc 2006;3:413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 1996;10:1077–1083. [DOI] [PubMed] [Google Scholar]
- 30.Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, Taylor SE, Ledbetter S, Lawrence CM, Rifkin DB, et al. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res 2006;166:839–848. [DOI] [PubMed] [Google Scholar]
- 31.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor beta-induced inhibition of T helper type 1 differentiation. J Exp Med 2002;195:1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen CH, Seguin-Devaux C, Burke NA, Oriss TB, Watkins SC, Clipstone N, Ray A. Transforming growth factor beta blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med 2003;197:1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan YY, Flavell RA. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev 2006;212:114–130. [DOI] [PubMed] [Google Scholar]
- 34.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006;441:235–238. [DOI] [PubMed] [Google Scholar]
- 35.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 2006;441:231–234. [DOI] [PubMed] [Google Scholar]
- 36.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006;24:179–189. [DOI] [PubMed] [Google Scholar]
- 37.Buckova D, Izakovicova Holla L, Benes P, Znojil V, Vacha J. TGF-beta1 gene polymorphisms. Allergy 2001;56:1236–1237. [DOI] [PubMed] [Google Scholar]
- 38.Rice F, Lewis A, Harold G, van den Bree M, Boivin J, Hay DF, Owen MJ, Thapar A. Agreement between maternal report and antenatal records for a range of pre and peri-natal factors: the influence of maternal and child characteristics. Early Hum Dev 2007;83:497–504. [DOI] [PubMed] [Google Scholar]
- 39.Yawn BP, Suman VJ, Jacobsen SJ. Maternal recall of distant pregnancy events. J Clin Epidemiol 1998;51:399–405. [DOI] [PubMed] [Google Scholar]
- 40.Kesmodel U, Olsen SF. Smoking habits among pregnant Danish women: reliability of information recorded after delivery. J Epidemiol Community Health 1999;53:239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, Willett WC, Buka SL. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology 1999;10:774–777. [PubMed] [Google Scholar]
- 42.Gehring U, Leaderer BP, Heinrich J, Oldenwening M, Giovannangelo ME, Nordling E, Merkel G, Hoek G, Bellander T, Brunekreef B. Comparison of parental reports of smoking and residential air nicotine concentrations in children. Occup Environ Med 2006;63:766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brunekreef B, Leaderer BP, van Strien R, Oldenwening M, Smit HA, Koopman L, Kerkhof M. Using nicotine measurements and parental reports to assess indoor air: the PIAMA birth cohort study. Prevention and incidence of asthma and mite allergy. Epidemiology 2000;11:350–352. [DOI] [PubMed] [Google Scholar]
- 44.Marbury MC, Hammond SK, Haley NJ. Measuring exposure to environmental tobacco smoke in studies of acute health effects. Am J Epidemiol 1993;137:1089–1097. [DOI] [PubMed] [Google Scholar]
- 45.Clark SJ, Warner JO, Dean TP. Passive smoking amongst asthmatic children: questionnaire or objective assessment? Clin Exp Allergy 1994;24:276–280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.